Abstract
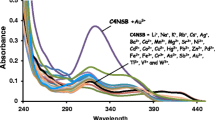
The present work describes a study of complexation efficiency of calix[4]arenes bearing benzoimidazolyl, benzothiazolyl, and benzoxazolyl heterocycles (5–7) towards several anions. The binding ability of calixarene derivatives 5–7 towards selected anions of different molecular geometries such as: F−, HSO4 −, I−, N3 −, NO3 −, NO2 −, SCN−, ClO4 −, Br−, CN−, Cl−, CH3COO− CF3SO3 − in methanol, has been investigated by fluorescence spectroscopic techniques, all anions were used as tetrabutylammonium salts to avoid possible complexation of cationic species by the derivative calix[4]arenes. Fluorescent chemosensor ability of these three calixarene derivatives was highly selective for iodide in contrast with other anions studied. The best chemosensor found, corresponds to compound 7, with an association constant of 2.01 × 104 mol−1 L and a detection limits of 0.22 ppm for iodide.







Similar content being viewed by others
References
Beer, P., Gale, P.: Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed. Engl. 40, 486–516 (2001)
Amendola, V., Bonizzoni, M., Esteban-Gomez, D., Fabbrizzi, L., Licchelli, M., Sancenon, F., Taglietti, A.: Some guidelines for the design of anion receptors. Coord. Chem. Rev. 250, 1451–1470 (2006)
Kuswandi, B., Verboom, W., Reinhoudt, D.: Tripodal receptors for cation and anion sensors. Sensors 6, 978–1017 (2006)
Prados, P., Quesada, R.: Recent advances in macrocyclic and macrocyclic-based anion receptors. Supramol. Chem. 20, 201–216 (2008)
Kubik, S.: Amino acid containing anion receptors. Chem. Soc. Rev. 38, 585–605 (2009)
Gale, P., Busschaert, N., Haynes, C., Karagiannidis, L., Kirby, I.: Anion receptor chemistry: highlights from 2011 and 2012. Chem. Soc. Rev. 43, 205–241 (2014)
Asfari, Z., Böhmer, V., Harrowfield McB, M., Vicens, J.: Calixarenes. Kluwer Academic, Dordrecht (2001)
Mokhtari, B., Pourabdollah, K., Dalali, N.: Analytical applications of calixarenes from 2005 up-to-date. J. Incl. Phenom. Macrocycl. Chem. 69, 1–55 (2011)
Kim, J.S., Quang, D.T.: Calixarene-derived fluorescent probes. Chem. Rev. 107, 3780–3799 (2007)
Kim, J.S., Lee, S.Y., Yoon, J., Vicens, J.: Hyperbranched calixarenes: synthesis and applications as fluorescent probes. Chem. Commun. 32, 4791–4802 (2009)
Stoddart, J.F. (ed.): Calixarenes Revisited. Royal Society of Chemistry, Cambridge (1998)
Gutsche, C.D., Iqbal, M.: p-tert-Butylcalix[4]arene. Org. Synth 8, 75–77 (1993)
Ludwig, R., Kim Dzung, N.T.: Calixarene-based molecules for cation recognition. Sensors 2, 397–416 (2002)
Arora, V., Chawla, H.M., Singh, S.P.: Calixarene as sensor materials for recognition and separation of metal ions. Arkivoc (ii). 172–200 (2007)
Sliwa, W., Girek, T.: Calixarene complexes with metal ions. J. Incl. Phenom. Macrocyclic. Chem. 66, 15–41 (2010)
Joseph, R., Rao, C.P.: Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors. Chem. Rev. 111(8), 4658–4702 (2011)
Shokova, E.A., Kovalev, V.V.: Calixarene-based anionic receptors. Russ. J. Org. Chem. 45(9), 1275–1314 (2009)
Matthews, S.E., Beer, P.D.: Calixarene-based anion receptors. Supramol. Chem. 17, 411–435 (2005)
Lhoták, P.: Anion receptors based on calixarenes. Top. Curr. Chem. 255, 65–95 (2005)
Edwards, N.Y., Possanza, A.L.: Calixarene-based anionic receptors: highlights from 2011. Supramol. Chem. 25(7), 446–463 (2013)
Rodriguez-Docampo, Z., Pascu, S.I., Kubik, S., Otto, S.: Noncovalent interactions within a synthetic receptor can reinforce guest binding. J. A. Chem. Soc. 128, 11206–11210 (2006)
Kumari, N., Hasan, M.A., Ward, B.D., Mishra, L.: Reactivity of tetrabutylammonium iodide with a heteronuclear 6Copper(II)-4Na(I) complex: selective recognition of iodide ion. Ind. Eng. Chem. Res. 52, 15007–15014 (2013)
Mendy, J.S., Saeed, M.A., Fronczek, F.R., Powell, D.R., Hossain Md, A.: Anion recognition and sensing by a new macrocyclic dinuclear copper(II) complex: a selective receptor for iodide. Inorg. Chem. 49, 7223–7225 (2010)
Liu, S.-Y., Xu, K.-X., He, Y.-B., Qin, H.-J., Meng, L.-Z.: New neutral receptors for fluoride based on Calix[4]arene bearing thiourea and amide. Chinese J. Chem. 23, 321–325 (2005)
López, M.V., Bermejo, M.R., Vasquéz, M.E., Taglietti, A., Zaragoza, G., Pedrido, R., Martínez-Calvo, M.: Sulfonamide-imines as selective fluorescent chemosensors for the fluoride anion. Org. Biomol. Chem. 8, 357–362 (2010)
Sakai, R., Okade, S., Barasa, E., Kakuchi, R., Ziabka, M., Umeda, S., Tsuda, K., Satoh, T., Kakuchi, T.: Efficient colorimetric anion detection based on positive allosteric system of urea-functionalized poly(phenylacetylene) receptor. Macromolecules 43, 7406–7411 (2010)
Bonizzoni, M., Fabbrizzi, L., Taglietti, A., Tiengo, F.: (Benzylidenamino)thioureas-chromogenic interactions with anions and N-H deprotonation. Eur. J. Org. Chem. 16, 3567–3574 (2006)
Singh, N., Jang, D.O.: Benzimidazole-based tripodal receptor: highly selective fluorescent chemosensor for iodide in aqueous solution. Org. Lett. 9(10), 1991–1994 (2007)
Ghosh, K., Saha, I.: A new benzimidazolium receptor for fluorescence sensing of iodide. Supramol. Chem. 22(5), 311–317 (2010)
Ghosh, K., Kar, D.: Fluorometric recognition of both dihydrogen phosphate and iodide by a new flexible anthracene linked benzimidazolium-based receptor. Beilstein J. Org. Chem. 7, 254–264 (2011)
Ma, B., Zeng, F., Zheng, F., Wu, S.: A fluorescent turn-on sensor for iodide based on a thymine–Hg(II)–thymine complex. Chem. Eur. J. 17, 14844–14850 (2011)
Tayade, K., Gallucci, J., Sharma, H., Attarde, S., Patil, R., Singh, N., Kuwar, A.: Exploration of selective recognition of iodide with dipodal sensor: 2, 2′-[ethane-1,2-diylbis(iminoethane-1,1-diyl)]diphenol. Dalton Trans. 43, 3584–3588 (2014)
Cheng, H.-S., Yan, H., Sun, Y.-L., Lu, C.-L., Huang, T.-Y., Chen, S.-J., Hu, C.-H., Wu, Y.-Y., Wu, A.-T.: A simple and highly selective receptor for iodide in aqueous solution. Analyst 137, 571–574 (2012)
Lee, D.Y., Singh, N., Kim, M.J., Jang, D.O.: Chromogenic and fluorescent recognition of iodide with benzoimidazole-based tripodal receptor. Org. Lett. 13(12), 3024–3027 (2011)
Suresh, V., Ahmed, N., Youn, I.S., Kim, K.S.: An imidazolium-based fluorescent cyclophane for the selective recognition of iodide. Chem. Asian J. 7, 658–663 (2012)
Kim, J.S., Park, S.Y., Kim, S.H., Thuéry, P., Souane, R., Matthews, S.E., Vicens, J.: A pyrenyl-appended triazole-based calix[4]arene as a fluorescent sensor for iodide ion. Bull. Korean Chem. Soc. 31(3), 624–629 (2010)
Joseph, R., Chinta, J.P., Rao, C.P.: Benzothiazole appended lower rim 1,3-diamido-derivative of calix[4]arene: synthesis, structure, receptor properties towards Cu2+, iodide recognition and computational modeling. Inorg. Chim. Acta 363, 2833–2839 (2010)
Joseph, R., Gupta, A., Ali, A., Rao, C.P.: Fluorescence and absorption studies on the selective recognition of iodide by lower rim 1,3-bis(aminoethoxy)-p-t-butyl-calix[4]arene derivate. Indian J. Chem. 46A, 1095–1100 (2007)
Quiroga-Campano, C., Gómez-Machuca, H., Jullian, C., De la Fuente, J., Pessoa-Mahana, H., Saitz, C.: Study by fluorescence of calix[4]arenes bearing heterocycles with divalents metals: highly selective detection of Pb(II). J. Incl. Phenom. Macrocyclic. Chem. 79, 161–169 (2014)
Zhang, W.C., Huang, Z.T.: Synthesis of 4-tert-Butylcalix[4]arenes bearing two schiff-base units at lower rim. Synthesis 1997, 1073–1076 (1997)
Santoyo-Gonzalez, F.A., Torres-Pinedo, A., Saitz-Barría, C.: An efficient synthesis of bis(calix[4]arenes), bis(crown-ether) substituted calix[4]arenes, aza-crown calix[4]arenes and thiaza-crown calix[4]arenes. Eur. J. Org. Chem. 3587–3593 (2000)
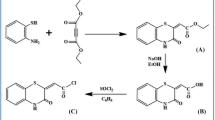
Saitz, C., De La Fuente, J., Jullian, C., Pessoa, H., Olea, C.: Synthesis of calix[4]arenes bearing benzothiazolyl, benzoxazolyl and benzoimidazolyl heterocycles. J. Chem. Res. (S) 6, 324–326 (2008)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Amendola, V., Boioocchi, M., Fabbrizzi, L., Fusco, N.: Putting the anion into the cage-fluoride. Inclusion in the smallest tris-imidazolium macrotricycle. Eur. J. Org. Chem. 32, 6434–6444 (2011)
Vasquez, M., Vasquez, M., Fabrizzi, Taglietti, A., Pedrido, R.M., González Noya, A.M., Bermejo, M.R.: A colorimetric approach to anion sensing: a selective chemosensor of fluoride Ions, in which color is generated by anion-enhanced π delocalization. Angew. Chem. Int. Ed. 43, 1962–1965 (2004)
Connors, K.A.: Binding Constant: The Measurement of Molecular Complex Stability. Wiley, New York (1987)
Qazi, M.A., Ocak, U., Ocak, M., Memon, S., Solangi, I.B.: Bifunctional calix[4]arene sensor for Pb(II) and Cr2O7 2− ions. J. Fluoresc. 23, 575–590 (2013)
Lee, J.L., Kim, S.K., Jung, J.H., Kim, J.S.: Bifunctional fluorescent calix[4]arene chemosensor for both a cation and anion. J. Org. Chem. 70, 1643–1646 (2005)
Chang, K.-C., Su, I.-H., Wang, Y.-Y., Chung, W.-S.: A bifunctional chromogenic calix[4]arene chemosensor for both cations and anions: a potential Ca2+ and F-switched INHIBIT logic gate with a YES logic function. Eur. J. Org. Chem. 4700–4704 (2010)
Acknowledgments
We thank FONDECYT (Grant 1100906).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gómez-Machuca, H., Quiroga-Campano, C., Jullian, C. et al. Study by fluorescence of calix[4]arenes bearing heterocycles with anions: highly selective detection of iodide. J Incl Phenom Macrocycl Chem 80, 369–375 (2014). https://doi.org/10.1007/s10847-014-0418-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0418-2