Abstract
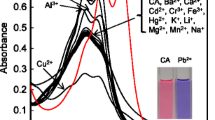
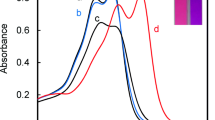
A colorimetric chemosensor is reported for Cu2+ by a simple mixture of two commercially available reagents, ponceau 6R (P6R) and poly(diallyldimethylammonium chloride) (PDADMAC) in an aqueous solution. P6R interacted electrostatically with PDADMAC to form the self-aggregate of P6R and exhibit a different sensing capability from P6R alone. The mixture showed high sensitivity and specific selectivity for detection of Cu2+ compared with various physiological and environmentally important metal ions. The addition of Cu2+ caused a hypsochromic shift with a distinct color change from red to yellow. While, upon adding Cu2+ to P6R alone, the solution underwent no distinct color change. The binding mode of P6R with Cu2+ was determined to be a 2:1 stoichiometry from Job plot and Cu2+ titration experiments. The results provide an easy method for developing new colorimetric sensors for metal ions by a combination of anionic dyes and cationic polyelectrolytes.









Similar content being viewed by others
References
Saleem, M., Lee, K.H.: Optical sensor: a promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv. 5, 72150–72287 (2015)
Quang, D.T., Kim, J.S.: Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 110, 6280–6301 (2010)
Kim, H.N., Ren, W.X., Kim, J.S., Yoon, J.: Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 41, 3210–3244 (2012)
Sakamaki, M., Aikawa, S., Fukushima, Y.: Colorimetric determination of Pb2+ in perfect aqueous solution using carminic acid as a selective chemosensor. J. Fluoresc. 27, 1929–1935 (2017)
Plastino, J., Green, E.L., Sanders-Loehr, J., Klinman, J.P.: An unexpected role for the active site base in cofactor orientation and flexibility in the copper amine oxidase from hansenula polymorpha. Biochemistry 38, 8204–8216 (1999)
Harris, E.D.: Copper as a cofactor and regulator of copper, zinc superoxide dismutase. J. Nutr. 122, 636–640 (1992)
Gaggelli, E., Kozlowski, H., Valensin, D., Valensin, G.: Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 106, 1995–2044 (2006)
Que, E.L., Domaille, D.W., Chang, C.J.: Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008)
Andreini, C., Banci, L., Bertini, I., Rosato, A.: Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome Res. 7, 209–216 (2008)
Bruijn, L.I., Miller, T.M., Cleveland, D.W.: Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 (2004)
Multhaup, G., Schlicksupp, A., Hesse, L., Beher, D., Ruppert, T., Masters, C.L., Beyreuther, K.: The amyloid precursor protein of Alzheimer’s disease in the reduction of copper(II) to copper(I). Science 271, 1406–1409 (1996)
Donnelly, P.S., Xiao, Z., Wedd, A.G.: Copper and Alzheimer’s disease. Curr. Opin. Chem. Biol. 11, 128–133 (2007)
Ninomiya, R., Koizumi, N., Murata, K.: Concentrations of cadmium, zinc, copper, iron, and metallothionein in liver and kidney of nonhuman primates. Biol. Trace Elem. Res. 87, 95–111 (2002)
Jonas, R.B.: Acute copper and cupric ion toxicity in an estuarine microbial community. Appl. Environ. Microbiol. 55, 43–49 (1989)
WHO: Guidelines for Drinking Water Quality. WHO, Geneva (2008)
Wang, R., Wang, W., Ren, H., Chae, J.: Detection of copper ions in drinking water using the competitive adsorption of proteins. Biosens. Bioelectron. 57, 179–185 (2014)
Xiong, S., Ye, S., Hu, X., Xie, F.: Electrochemical detection of ultra-trace Cu(II) and interaction mechanism analysis between amine-groups functionalized CoFe2O4/reduced graphene oxide composites and metal ion. Electrochim. Acta 217, 24–33 (2016)
Teodoro, M.T.F., de Dias, F.S., da Silva, D.G., Bezerra, M.A., Dantas, A.F., Teixeira, L.S.G., Pereira, A.L.C.: Determination of copper total and speciation in food samples by flame atomic absorption spectrometry in association with solid-phase extraction with bamboo (Bambusa vulgaris) fiber loaded with bathocuproine. Microchem. J. 132, 351–357 (2017)
Li, J., Zeng, Y., Hu, Q., Yu, X., Guo, J., Pan, Z.: A fluorescence “turn-on” chemodosimeter for Cu2+ in aqueous solution based on the ion promoted oxidation. Dalton Trans. 41, 3623–3626 (2012)
Meng, Q.T., Zhang, R., Jia, H.M., Gao, X., Wang, C.P., Shi, Y., Everest-Dass, A.V., Zhang, Z.Q.: A reversible fluorescence chemosensor for sequentially quantitative monitoring copper and sulfide in living cells. Talanta 143, 294–301 (2015)
Li, G., Tao, F., Wang, H., Wang, L., Zhang, J., Ge, P., Liu, L., Tong, Y., Sun, S.: A novel reversible colorimetric chemosensor for the detection of Cu2+ based on a water-soluble polymer containing rhodamine receptor pendants. RSC Adv. 5, 18983–18989 (2015)
Wu, X., Gong, X., Dong, W., Ma, J., Chao, J., Li, C., Wang, L., Dong, C.: A novel fluorescein-based colorimetric probe for Cu2+ detection. RSC Adv. 6, 59677–59683 (2016)
Tavallali, H., Deilamy-Rad, G., Ali Karimi, M., Rahimy, E.: A novel dye-based colorimetric chemosensors for sequential detection of Cu2+ and cysteine in aqueous solution. Anal. Biochem. 583, 113376 (2019)
Butler, G.B., Angelo, R.J.: Preparation and polymerization of unsaturated quaternary ammonium compounds VIII a proposed alternating intramolecular-intermolecular chain propagation. J. Am. Chem. Soc. 79, 3128–3131 (1957)
Assem, Y., Chaffey-Millar, H., Barner-Kowollik, C., Wegner, G., Agarwal, S.: Controlled/living ring-closing cyclopolymerization of diallyldimethylammonium chloride via the reversible addition fragmentation chain transfer process. Macromolecules 40, 3907–3913 (2007)
Wang, Y., Chen, J., Jiao, H., Chen, Y., Li, W., Zhang, Q., Yu, C.: Polymer-templated perylene-probe noncovalent self-assembly: a new strategy for label-free ultrasensitive fluorescence turn-on biosensing. Chem. Eur. J. 19, 12846–12852 (2013)
Dubas, S.T., Limsavarn, L., Iamsamai, C., Potiyaraj, P.J.: Assembly of polyelectrolyte multilayers on nylon fibers. J. Appl. Polym. Sci. 101, 3286–3290 (2006)
Sakamaki, M., Aikawa, S., Fukushima, Y.: Colorimetric chemosensor for Zn2+ based on pyrogallol red and poly(diallyldimethylammonium chloride) in aqueous solution. Polym. Bull. 75, 1667–1680 (2018)
Inoue, K., Aikawa, S., Sakamaki, M., Fukushima, Y.: Colorimetric chemosensor for Fe2+ and Fe3+ based on a ternary mixture of an anionic dye, a cationic polyelectrolyte, and a metal chelator in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 91, 171–177 (2018)
Inoue, K., Aikawa, S., Sakamaki, M., Fukushima, Y.: Colorimetric Co2+ sensor based on an anionic pyridylazo dye and a cationic polyelectrolyte in aqueous solution. Polym. Int. 67, 1589–1594 (2018)
Inoue, K., Aikawa, S., Fukushima, Y.: Colorimetric detection of Hg2+ using a mixture of an anionic azo dye and a cationic polyelectrolyte in aqueous solution. Polym. Int. 67, 755–760 (2018)
Inoue, K., Aikawa, S., Fukushima, Y.: Colorimetric chemosensor for Ni2+ based on alizarin complexone and a cationic polyelectrolyte in aqueous solution. J. Appl. Polym. Sci. 136, 47496 (2019)
Fukushima, Y., Aikawa, S.: Colorimetric detection of Ni2+ based on an anionic triphenylmethane dye and a cationic polyelectrolyte in aqueous solution. Tetrahedron Lett. 60, 675–680 (2019)
Fukushima, Y., Aikawa, S.: Colorimetric detection of Hg2+ based on nuclear fast red and a cationic polyelectrolyte in aqueous solution. J. Fluoresc. 30, 175–180 (2020)
Fukushima, Y., Aikawa, S.: Colorimetric detection of Mn(II) based on a mixture of an anionic pyridylazo dye and a cationic polyelectrolyte in aqueous solution. Color Technol. 136, 450–456 (2020)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Grynkiewicz, G., Poenie, M., Tsien, R.Y.: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukushima, Y., Aikawa, S. Colorimetric detection of Cu2+ using of a mixture of ponceau 6R and a cationic polyelectrolyte in aqueous solution. J Incl Phenom Macrocycl Chem 100, 143–148 (2021). https://doi.org/10.1007/s10847-021-01064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-021-01064-8