Abstract
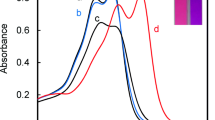
The commercially available natural organic dye, carminic acid (CA), an anthraquinone derivative bearing hydroxyl and carboxyl groups as recognition sites was found to be a colorimetric probe for Pb2+ in perfect aqueous solution under neutral conditions with specific selectivity and high sensitivity. Upon addition of Pb2+, the absorption maximum of CA showed a large red shift, and the resulted color change from red to purple could be easily identified even by the naked eye. The chemical stoichiometric ratio between CA and Pb2+ was determined to be 1:2 through Job plot, Pb2+ titration, and kinetic experiments. Moreover, other environmental relevant metal ions induced no or minimal spectral and color changes. The reversibility of Pb2+ to CA with EDTA even through several cycles was established for practical applications. The results indicated that CA can be a good candidate for simple, convenient and reversible colorimetric detection of Pb2+ in aqueous solution even though it was hard to be applied to determine Pb2+ on the water testing by US EPA.









Similar content being viewed by others
References
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210–3244
Meadows-Oliver M (2012) Environmental toxicants: lead and mercury. J Pediatr Health Care 26:213–215
Sobin C, Parisi N, Schaub T, Gutierrez M, Ortega AX (2011) δ-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 and peptide transporter 2*2 haplotype may differentially mediate lead exposure in male children. Arch Environ Contam Toxicol 61:521–529
Mudipalli A (2007) Lead hepatotoxicity & potential health effects. Indian J Med Res 126:518–527
Zhu J, Yu UQ, Li JJ, Zhao JW (2016) Colorimetric detection of lead(II) ions based on accelerating surface etching of gold nanorods to nanospheres: the effect of sodium thiosulfate. RSC Adv 6:25611–25619
Liu HW, Jiang SJ, Liu SH (1999) Determination of cadmium, mercury and lead in seawater by electrothermal vaporization isotope dilution inductively coupled plasma mass spectrometry. Spectrochim Acta B 54:1367–1375
Feldman BJ, Osterloh JD, Hata BH, D’Alessandro A (1994) Determination of lead in blood by square wave anodic stripping voltammetry at a carbon disk ultramicroelectrode. Anal Chem 66:1983–1987
Elfering H, Andersson JT, Poll JT (1998) Determination of organic lead in soils and waters by hydride generation inductively coupled plasma atomic emission spectrometry. Analyst 123:669–674
Liu Y, Liu Z, Wang Y, Dai J, Gao J, Xie J, Yan Y (2011) A surface ion-imprinted mesoporous sorbent for separation and determination of Pb(II) ion by flame atomic absorption spectrometry. Microchim Acta 172:309–317
He Q, Miller EW, Wong AP, Chang CJ (2006) A selective fluorescent sensor for detecting lead in living cells. J Am Chem Soc 128:9316–9317
Ranyuk E, Douaihy CM, Bessmertnykh A, Denat F, Averin A, Beletskaya I, Guilard R (2009) Diaminoanthraquinone-linked polyazamacrocycles: efficient and simple colorimetric sensor for lead ion in aqueous solution. Org Lett 11:987–990
Liu J, Lu Y (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125:6642–6643
Wang Z, Lee JH, Lu Y (2008) Label-free colorimetric detection of lead ions with a nanomolar detection limit and tunable dynamic range by using gold nanoparticles and DNAzyme. Adv Mater 20:3263–3267
Mazumdar D, Liu J, Lu G, Zhou J, Lu Y (2010) Easy-to-use dipstick tests for detection of lead in paints using non-cross-linked gold nanoparticle-DNAzyme conjugates. Chem Commun 46:1416–1418
Gil ES, Oliveira SC, Oliveira-Brett AM (2012) Hydroxyanthraquinones carminic acid and chrysazin anodic oxidation. Electroanalysis 24:2079–2084
Sun W, Han Y, Jiao K (2006) Voltammetric albumin quantification based on its interaction with carminic acid. J Serb Chem Soc 71:385–396
Comanici R, Gabel B, Gustavsson T, Markovitsi D, Cornaggia C, Pommeret S, Rusu C, Kryschi C (2006) Femtosecond spectroscopic study of carminic acid–DNA interactions. Chem Phys 325:509–518
Göktürk S (2005) Effect of hydrophobicity on micellar binding of carminic acid. J Photochem Photobiol A 169:115–121
Nevado JJB, Cabanillas CG, Salcedo AMC (1995) Simultaneous spectrophotometric determination of three food dyes by using the first derivative of ratio spectra. Talanta 42:2043–2051
Cabrera RB, Fernandez-Lahore HM (2007) Primary recovery of acid food colorant. Int J Food Sci Technol 42:1315–1326
Manzoori JL, Sorouraddin MH, Amjadi M (2000) Spectrophotometric determination of osmium based on its catalytic effect on the oxidation of carminic acid by hydrogen peroxide. Talanta 53:61–68
Manzoori JL, Sorouraddin MH, Haji-Shabani AM (1998) Spectrophotometric determination of nitrite based on its catalytic effect on the oxidation of carminic acid by bromate. Talanta 46:1379–1386
Callicoat D, Wolszon JD (1959) Carminic acid procedure for determination of boron. Anal Chem 31:1434–1437
Kaur P, Gupta VK (1989) Spectrophotometric determination of beryllium with carminic acid. Fresenius Z Anal Chem 334:447–449
Wanga F, Huanga W, Li K, Li A, Gao W, Tang B (2011) Study on the fluorescence enhancement in lanthanum(III)–carminic acid–cetyltrimethylammonium bromide system and its analytical application. Spectrochim Acta Part A 79:1946–1951
López-Martinez L, Guzman-Mar JL, Lopez-de Alba PL (2001) Simultaneous determination of uranium(VI) and thorium(IV) ions with carminic acid by bivariate calibration. J Radioanal Nucl Chem 247:413–417
Job P (1928) Formation and stability of inorganic complexes in solution. Ann Chim Puris 9:113–203
Irving HMNH, Freiser H, West TS (1978) IUPAC compendium of analytical nomenclature, definitive rules. Pergamon Press, Oxford
Benesi AH, Hildebrand HJ (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Hossain MD, Pandey RK, Rana U, Higuchi M (2015) Nano molar detection of cd(II) ions by luminescent metallo-supramolecular polymer formation. J Mater Chem C 3:12186–12191
Favaro G, Miliani C, Romani A, Vagnini M (2002) Role of protolytic interactions in photo-aging processes of carminic acid and carminic lake in solution and painted layers. J Chem Soc Perkin Trans 2:192–197
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakamaki, M., Aikawa, S. & Fukushima, Y. Colorimetric Determination of Pb2+ in Perfect Aqueous Solution Using Carminic Acid as a Selective Chemosensor. J Fluoresc 27, 1929–1935 (2017). https://doi.org/10.1007/s10895-017-2131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2131-1