Abstract
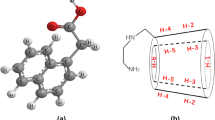
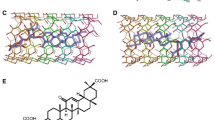
This study investigated inclusion formation and the physicochemical properties of naringin/cyclodextrin through a combined computational and experimental approach. Molecular dynamics simulations were applied to investigate the thermodynamics and geometry of naringin/cyclodextrin cavity docking. The complexes were investigated by UV, FT-IR, DSC, XRD, SEM, 2D-NOSEY and 1H-NMR analyses. Clearly visible protons belonging to naringin and chemical shift displacements of the H3 and H5 protons in cyclodextrin were anticipated in the formation of an inclusion complex. Naringin solubility increased linearly with increasing cyclodextrin concentration (displaying an AL profile). The simulations indicated that the phenyl group of naringin was located deep within the cyclodextrin cavity, while the glycoside group of naringin was on the plane of the wider rim of cyclodextrin. The simulation and molecular modeling results indicate that (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD) provided the more stable inclusion complex. This result was also in good concordance with the stability constants that had been determined by the phase solubility method. The consistency of the computational and experimental results indicates their reliability.










Similar content being viewed by others
References
Ji, Y., Wang, L., Watts, D.C., Qiu, H., You, T., Deng, F., Wu, X.: Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent. Mater. 30, 1263 (2014)
Leem, E., Nam, J.H., Jeon, M., Shin, W., Won, S., Park, S., Choi, M., Jin, B.K., Jung, U.J., Kim, S.R.: Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. J. Nutr. Biochem. 25, 801 (2014)
Özyürek, M., Akpınar, D., Bener, M., Türkkan, B., Güçlü, K., Apak, R.: Novel oxime based flavanone, naringin-oxime: synthesis, characterization and screening for antioxidant activity. Chem-Biol. Interact. 212, 40 (2014)
Cao, X., Lin, W., Liang, C., Zhang, D., Yang, F., Zhang, Y., Zhang, X., Feng, J., Chen, C.: Naringin rescued the TNF-α-induced inhibition of osteogenesis of bone marrow-derived mesenchymal stem cells by depressing the activation of NF-кB signaling pathway. Immunol. Res. 62, 357 (2015)
Shpigelman, A., Shoham, Y., Israeli-Lev, G., Livney, Y.D.: β-Lactoglobulin–naringenin complexes: nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocolloid 40, 214 (2014)
Sangpheak, W., Kicuntod, J., Schuster, R., Rungrotmongkol, T., Wolschann, P., Kungwan, N., Viernstein, H., Mueller, M., Pongsawasdi, P.: Physical properties and biological activities of hesperetin and naringenin in complex with methylated beta-cyclodextrin. Beilstein. J. Org. Chem. 11, 2763 (2015)
Lee, S.J., Kim, J., Kim, M.J., Kitaoka, M., Park, C.S., Lee, S.Y., Ra, M., Moon, T.W., Robyt, J.F., Park, K.H.: Transglycosylation of naringin by bacillus stearothermophilus maltogenic amylase to give glycosylated naringin. J. Agric. Food. Chem. 47, 3669 (1999)
Felton, L.A., Popescu, C., Wiley, C., Esposito, E.X., Lefevre, P., Hopfinger, A.J.: Experimental and computational studies of physicochemical properties influence NSAID-cyclodextrin complexation. AAPS PharmSciTech 15, 872 (2014)
Fernandes, A., Ivanova, G., Bras, N.F., Mateus, N., Ramos, M.J., Rangel, M., de Freitas, V.: Structural characterization of inclusion complexes between cyanidin-3-O-glucoside and beta-cyclodextrin. Carbohydr. Polym. 102, 269 (2014)
Kicuntod, J., Khuntawee, W., Wolschann, P., Pongsawasdi, P., Chavasiri, W., Kungwan, N., Rungrotmongkol, T.: Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J. Mol. Graph. Model. 63, 91 (2016)
Sangpheak, W., Khuntawee, W., Wolschann, P., Pongsawasdi, P., Rungrotmongkol, T.: Enhanced stability of a naringenin/2,6-dimethyl beta-cyclodextrin inclusion complex: molecular dynamics and free energy calculations based on MM- and QM-PBSA/GBSA. J. Mol. Graph. Model. 50, 10 (2014)
Whang, H.S., Vendeix, F.A., Gracz, H.S., Gadsby, J., Tonelli, A.: NMR studies of the inclusion complex of cloprostenol sodium salt with beta-cyclodextrin in aqueous solution. Pharm. Res. 25, 1142 (2008)
Savic, I.M., Savic-Gajic, I.M., Nikolic, V.D., Nikolic, L.B., Radovanovic, B.C., Milenkovic-Andjelkovic, A.: Enhencemnet of solubility and photostability of rutin by complexation with β-cyclodextrin and (2-hydroxypropyl)-β-cyclodextrin. J. Incl. Phenom. Macro. 86, 33 (2016)
Savic-Gajic, I., Savic, I.M., Nikolic, V.D., Nikolic, L.B., Popsavin, M.M., Kapor, A.J.: Study of the solubility, photostability and structure of inclusion complexes of carvedilol with β-cyclodextrin and (2-hydroxypropyl)-β-cyclodextrin. J. Incl. Phenom. Macro. 86, 7 (2016)
Savic, I.M., Nikolic, V.D., Savic-Gajic, I., Nikolic, L.B., Radovanovic, B.C., Mladenovic, J.D.: Investigation of properties and structural characterization of the quercetin inclusion complex with (2-hydroxypropyl)-β-cyclodextrin. J. Incl. Phenom. Macro. 82, 383 (2015)
Tačić, A., Savić, I., Nikolić, V., Savić, I., Ilić-Stojanović, S., Ilić, D., Petrović, S., Popsavin, M., Kapor, A.: Inclusion complexes of sulfanilamide with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macro. 80, 113 (2014)
Xiao, C., Li, K., Huang, R., He, G., Zhang, J., Zhu, L., Yang, Q., Jiang, K., Jin, Y., Lin, J.: Investigation of inclusion complex of epothilone A with cyclodextrins. Carbohydr. Polym. 102, 297 (2014)
Zhang, J., Li, K., Jiang, K., Cong, Y., Pu, S., Xie, X., Jin, Y., Lin, J.: Development of an oral satraplatin pharmaceutical formulation by encapsulation with cyclodextrin. RSC Adv. 6, 17074 (2016)
Jiao, A., Zhou, X., Xu, X., Jin, Z.: Molecular dynamics simulations of cyclodextrin–cumene hydroperoxide complexes in water. Comput. Theor. Chem. 1013, 1 (2013)
Boonyarattanakalin, K., Wolschann, P., Toochinda, P., Lawtrakul, L.: Molecular dynamics simulations of UC781-cyclodextrins inclusion complexes in aqueous solution. Eur. J. Pharm. Sci. 47, 752 (2012)
Dandawate, P., Vemuri, K., Khan, E.M., Sritharan, M., Padhye, S.: Synthesis, characterization and anti-tubercular activity of ferrocenyl hydrazones and their beta-cyclodextrin conjugates. Carbohydr. Polym. 108, 135 (2014)
Higuchi, T., Connor, K.A. (1965) Phase-solubility techniques. In: Reilly, C.N. (ed.) Advances in Analytical Chemistry and Instrumentation. Wiley, New York
Madan, J., Baruah, B., Nagaraju, M., Abdalla, M.O., Yates, C., Turner, T., Rangari, V., Hamelberg, D., Aneja, R.: Molecular cycloencapsulation augments solubility and improves therapeutic index of brominated noscapine in prostate cancer cells. Mol. Pharm. 9, 1470 (2012)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, J.T., Kudin, K.N., Burant, J.C. (eds.): Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford (2004)
Rajendiran, N., Siva, S.: Inclusion complex of sulfadimethoxine with cyclodextrins: preparation and characterization. Carbohydr. Polym. 101, 828 (2014)
Siva, S., Kothai Nayaki, S., Rajendiran, N.: Fabrication of cyclodextrins-procainamide supramolecular self-assembly: shape-shifting of nanosheet into microtubular structure. Carbohydr. Polym. 122, 123 (2015)
Boonyarattanakalin, K., Viernstein, H., Wolschann, P., Lawtrakul, L.: Influence of ethanol as a co-solvent in cyclodextrin inclusion complexation: a molecular dynamics study. Sci. Pharm. 83, 387 (2015)
Chiang, P.C., Shi, Y., Cui, Y.: Temperature dependence of the complexation mechanism of celecoxib and hydroxyl-beta-cyclodextrin in aqueous solution. Pharmaceutics. 6, 467 (2014)
Wood, D.J., Hruska, F.E., Saenger, W.: 1 H NMR study of the inclusion of aromatic molecules in α-cyclodextrin. J. Am. Chem. Soc. 99 (1977)
Liu, M., Chen, A., Wang, Y., Wang, C., Wang, B., Sun, D.: Improved solubility and stability of 7-hydroxy-4-methylcoumarin at different temperatures and pH values through complexation with sulfobutyl ether-beta-cyclodextrin. Food Chem. 168, 270 (2015)
Khuntawee, W., Wolschann, P., Rungrotmongkol, T., Wong-ekkabut, J., Hannongbua, S.: Molecular dynamics simulations of the interaction of beta cyclodextrin with a lipid bilayer. J. Chem. Inf. Model. 55, 1894 (2015)
Koehler, J.E., Saenger, W., van Gunsteren, W.F.: On the occurrence of three-center hydrogen bonds in cyclodextrins in crystalline form and in aqueous solution: comparison of neutron diffraction and molecular dynamics results. J. Biomol. Struct. Dyn. 6, 181 (1988)
Tekpinar, M., Yildirim, A., Wassenaar, T.A.: Molecular dynamics study of the effect of active site protonation on Helicobacter pylori 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase. Eur. Biophys. J. 44, 685 (2015)
Lee, S.J., Kim, J.C., Kim, M.J., Kitaoka, M., Park, C.S., Lee, S.Y., Ra, M.J., Moon, T.W., Robyt, J.F., Park, K.H.: Transglycosylation of naringin by Bacillus stearothermophilus maltogenic amylase to give glycosylated naringin. J. Agric. Food. Chem. 47, 3669 (1999)
Acknowledgements
This work was supported by the Program for Chang jiang Scholars and Innovative Research Team in University (No. IRT13095), the National Natural Science Foundation of China (Nos. 21442006, 21262043 and 20902079). The authors thank the High Performance Computing Center at Yunnan University for use of the high performance computing platform.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hui-Huan Yan, Jian-Qiang Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, HH., Zhang, JQ., Ren, SH. et al. Experimental and computational studies of naringin/cyclodextrin inclusion complexation. J Incl Phenom Macrocycl Chem 88, 15–26 (2017). https://doi.org/10.1007/s10847-017-0704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0704-x