Abstract
Purpose
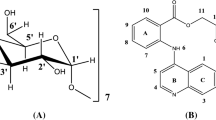
Cloprostenol sodium salt (referred as cloprostenol) may be used for the synchronization of estrous cycles in farm animal species. Cyclodextrins (CDs) have potential as drug delivery systems through the formation of inclusion complexes between CDs and drugs. This is the first study of the inclusion complex of cloprostenol with β-cyclodextrin (β-CD) in aqueous solution using NMR and 3D molecular dynamics simulations.
Methods
1D proton NMR spectra of β-CD, a complex of cloprostenol with β-CD, and cloprostenol in D2O were assigned and confirmed. The cross relaxation interactions from ROESY were used as constraints for 3D molecular modeling studies.
Results
In the 2D ROESY of the complex, cross-peaks were observed between the aromatic protons of cloprostenol and protons of the β-CD as well as between aliphatic protons and protons of the β-CD. The stoichiometry of the complex was found that β-CD forms a 1:1 inclusion complex with cloprostenol. The association constant K was 968 ± 120 M−1 at 298 K.
Conclusions
Aromatic side and/or aliphatic side chains of the cloprostenol is included in the β-CD while aliphatic side and/or aromatic side chains wraps around β-CD, respectively. The molecular modeling also confirms that β-CD forms a 1:1 inclusion complex with cloprostenol.








Similar content being viewed by others
REFERENCES
J. Szejtli. Cyclodextrin Technology. Kluwer, Boston, 1988.
M. Masson, B.V. Sigurdardottir, K. Matthiasson, and T. Loftsson. Investigation of drug-cyclodextrin complexes by a phase-distribution method: some theoretical and practical considerations. Chem. Pharm. Bull 53(8):8–964 (2005).
I. J. Oh, H. M. Song, and K. C. Lee. Effect of 2-hydroxypropyl-β-cyclodextrin on the stability of prostaglandin E2 in solution. Int. J. Pharm 106(20):135–140 (1994).
M. Yamaoto, F. Hirayama, and K. Uekama. Improvement of stability and dissolution of prostaglandin E1 by maltosyl β-cyclodextrin in lyophilized formulation. Chem. Pharm. Bull 40(3):747–751 (1992).
M. Wiese, H. P. Cordes, H. Chi, J. K. Seydel, T. Backensfeld, and B. W. Mueller. Interaction of prostaglandin E1 with α-cyclodextrin in aqueous systems: stability of the inclusion complex. J. Pharm. Sci 80(2):153–6 (1991).
K. Uekama, M. Kurihara, and F. Hirayama. Release control of prostaglandin E1 by utilizing hydrophilic and hydrophobic β-cyclodextrin derivatives. Nippon Kagaku Kaishi 10:1195–1199 (1990).
K. Uekama, A. Fujise, F. Hirayama, M. Otagiri, and K. Inaba. Improvement of dissolution characteristics and chemical stability of prostaglandin E1 by γ-cyclodextrin complexation. Chem. Pharm. Bull 32(1):275–9 (1984).
F. Ayama. Development and pharmaceutical evaluation of hydrophobic cyclodextrin derivatives as modified-release drug careers. Yakugaku Zasshi 113(6):425–437 (1993).
K. Inaba, T. Wakuda, K. Ichioka, and K. Uekama. Inclusion complexation of prostaglandin \(F_{2\alpha }\)with α- and γ-cyclodextrins in aqueous solution. Yakuzaigaku 48(3):209–14 (1988).
F. Hirayama, K. Uekama, and K. Hideomi. Molecular dynamics of prostaglandin \(F_{2\alpha }\)-cyclodextrin complexes in aqueous solution. Chem. Pharm. Bull 28(7):1975–80 (1980).
K. Uekama, and F. Hirayama. Inclusion complexation of prostaglandin \(F_{2\alpha }\)with α- and β-cyclodextrins in aqueous solution. Chem. Pharm. Bull 26(4):1195–2000 (1978).
C. Pean, C. Creminon, A. Wijkhuisen, B. Perly, and F. Djedaini-Pilard. Reliable NMR experiments for the study of β-cyclodextrin/prostaglandin E2 inclusion complex. Journal De Chimie Physique Et De Physico-Chimie Biologique 96:1486–1493 (1999).
K. Uekama, F. Hirayama, A. Fujise, M. Otagiri, K. Inaba, and H. Saito. Inclusion complexation of prostaglandin \(F_{2\alpha }\)with γ-cyclodextrin in solution and solid phases. J. Pharm. Sci 73(3):382–384 (1984).
B. Steffan, W. Fischer, G. Cordes, I. Habon, and R. Muller. H1-Nuclear Magnetic Resornance (NMR) studies on the inclusion complex of prostaglandin E1(PGE1) with γ-cyclodextrin. Pharm. Res 9(4):575–577 (1992).
K. Wuthrich. NMR of Proteins and Nucleic Acids, Wiley, New York, 1986.K.
J. Cavanagh, J. W. Fairbrother, A. Palmer, N. SkeltonM. Rance. Protein NMR Spectroscopy: Principles and Practice, Academic, San Diego, 2007.
M. Sotiris, and A. Ekaterini. The contribution of simple NMR spectroscopy techniques in the study of supramolecular interactions of cyclodextrins with various drugs. Pharmakeutike 12(3–4):79–89 (1999).
C. J. Christopher, T. A. Frenkiel, and A. N. Lane. A comparison of the ROESY and NOESY experiments for large molecules, with application to nucleic acids. J. Magn. Reson 87(1):144–152 (1990).
A. I. Popov, and K. Hallenga. Modern NMR Techniques and their Application in Chemistry, Marcel Dekker, Inc, 1991, pp. 604–606.
A. T. Brunger, P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D. Biol. Crystallogr 54:905–921 (1988).
A. W. Schuettelkopf, and D. M. F. van Aalten. PRODRG—a tool for high-throughput crystallography of protein-ligand complexes. Acta. Crystallogr. D. Biol. Crystallogr 60:1355–1363 (2004).
D.A. Pearlman, D.A. Case, J.W. Caldwell, W.S. Ross, T.E. Cheatham III, S. DeBolt, D. Ferguson, G. Seibel, and P. Kollman. AMBER, A package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comp. Phys. Commun 91:1–41 (1995).
A. Jakallan, D. B. Jack, and C. I. Bayly. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem 23(16):1623–1641 (2002).
E. G. Stein, L. M. Rice, and A. T. Brunger. Torsion-angle molecular dynamics as a new efficient tool for NMR structure calculation. J. Magn Reson 124:154–164 (1997).
HyperChem(TM) Professional 7.5, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA.
M. L. Bender, and M. Komiyama. Cyclodextrin Chemistry. Springer, Berlin, 1978.
T. Ramstad, C. E. Hadden, G. E. Martin, S. M Speaker, D. L. Teagarden, and T. J. Thamann. Determination by NMR of the binding constant for the molecular complex between alprostadil and α-cyclodextrin. Implications for a freeze-dried formulation. Int. J. Pharm 296:55–63 (2005).
ACKNOWLEDGEMENTS
This research was supported by USDA Animal Health Formula Funds/State of North Carolina.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1-A
Expanded aromatic region of 2D heteronuclear multiple quantum coherence (HMQC) (1H–13C one bond) spectrum of the cloprostenol for assigning the H18, H20, H22 peaks (DOC 558 kb)
Figure S1-B
Expanded region of 2D heteronuclear multiple bond correlation (HMBC) coherence (1H–13C long range) spectrum of cloprostenol for assigning the H5 proton peak (DOC 403 kb)
Rights and permissions
About this article
Cite this article
Whang, H.S., Vendeix, F.A.P., Gracz, H.S. et al. NMR Studies of the Inclusion Complex of Cloprostenol Sodium Salt with β-cyclodextrin in Aqueous Solution. Pharm Res 25, 1142–1149 (2008). https://doi.org/10.1007/s11095-007-9493-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9493-z