Abstract
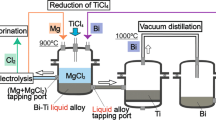
A new smelting process of Ti comprising magnesiothermic reduction of TiCl4 into liquid Bi and separation of Ti from Bi by electrorefining was proposed by our group. In this study, electrorefining of Ti from Bi–Ti alloys was investigated at 700 °C using a Bi–Ti alloy anode and a Ni cathode in equimolar NaCl–KCl to which 1–5 mol% of TiCl2 was added. On the alloy anode, the oxidation of Ti occurred at more negative potentials than that of Bi, which allowed selective dissolution of Ti in the alloy. At the Ni cathode, metallic Ti powder was obtained without significant Bi contamination (below 180 ppm). In addition, the amount of Ti deposits decreased gradually by further electrorefining, eventually reaching an almost constant value. This phenomenon is attributed to an increase in the ratio of Ti3+ to Ti2+ in the electrolyte, and it was concluded that Ti in the alloy was oxidized mainly to Ti3+. For this reason, the current efficiency for Ti deposition was calculated to be 91–97 % assuming that Ti3+ was reduced to Ti on the cathode.
Graphical Abstract
Electrorefining of Ti using Bi–Ti alloy anode was carried out in equimolar NaCl–KCl at 700 °C, and highly pure Ti powder (Bi cont.: <180 ppm) was obtained.










Similar content being viewed by others
References
Kroll W (1940) The production of ductile titanium. J Electrochem Soc 78:35–47. doi:10.1149/1.3071290
Chen GZ, Fray DJ, Farthing TW (2000) Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407:361–364. doi:10.1038/35030069
Ono K, Suzuki RO (2002) A new concept for producing Ti sponge: calciothermic reduction. JOM 54:59–61. doi:10.1007/BF02701078
Okabe TH, Oda T, Mitsuda Y (2004) Titanium powder production by preform reduction process (PRP). J Alloys Compd 364:156–163. doi:10.1016/S0925-8388(03)00610-8
Gleave WW, Quin JP (1956) Electrolytic manufacture of titanium. US patent 2,757,135, 31 July 1956
Hard RA, Prieto MA (1983) Process for making titanium metal from titanium ore. US patent 4,390,365, 28 June 1983
Megy JA (1984) Process for making titanium metal from titanium ore. US patent 4,468,248, 28 Aug 1984
Megy JA (1987) Process for making zero valent titanium from an alkali metal fluotitanate. US patent 4,668,286, 26 May 1987
Kimura E, Ogi K, K Sato (1986) Japan patent Sho 61–37338, 23 Aug 1986
Sato K, Kimura E (1989) Basic study on a continuous reduction process for titanium production. Shigen-to-Sozai 105:623–626
Kado Y, Kishimoto A, Uda T (2013) Electrolysis of TiO2 or TiCl2 using Bi liquid cathode in molten CaCl2. J Electrochem Soc 160:139–142. doi:10.1149/2.102310jes
Kado Y, Kishimoto A, Uda T (2015) New smelting process for titanium: magnesiothermic reduction of TiCl4 into liquid Bi and subsequent refining by vacuum distillation. Metall Mater Trans B 46:57–61. doi:10.1007/s11663-014-0164-2
Bard AJ (1976) Encyclopedia of electrochemistry of the elements, vol 10. Marcel Dekker Inc., New York
Janz GJ (1967) Molten salt handbook. Academic press Inc., New York
Sekimoto H, Nose Y, Uda T, Uehara A, Yamana H, Sugimura H (2011) Revaluation of equilibrium quotient between titanium ions and metallic titanium in NaCl–KCl equimolar molten salt. J Alloys Compd 509:5477–5482. doi:10.1016/j.jallcom.2011.02.090
Maruyama S, Kado Y, Uda T (2013) Phase diagram investigation of the Bi–Ti system. J Phase Equilib Diffus 34:289–296. doi:10.1007/s11669-013-0243-0
Bykov PA, Gol’dshtein LS, Raspopin PS (1985) Determination of the temperature dependence of the diffusion coefficient of titanium in liquid bismuth by anodic potential stabilization in a chloride melt. Elektrokhimiya 21:277–288
Weeks JR (1965) Liquidus curves of nineteen dilute binary alloys of bismuth. Trans Am Soc Met 58:302–322
Kishimoto A, Kado Y, Uda T. Investigation on Bi–Fe–Ti phase diagram. to be submitted
Ferry DM, Picard GS, Trémillon BL (1988) Pulse and AC impedance studies of the electrochemical systems of titanium in LiCl–KCl eutectic melt at 743 K. J Electrochem Soc 135:1443–1447. doi:10.1149/1.2096019
Flengas SN (1960) Solubilities of titanium tetrachloride in mixtures of potassium chloride and sodium chloride, and the electrode potentials of the titanium chlorides in 1/1 (mole) KCl–NaCl solutions. Ann N Y Acad Sci 79:853–872. doi:10.1111/j.1749-6632.1960.tb42759.x
Acknowledgments
This work was financially supported by Advanced Low Carbon Technology Research and Development Program (Japan Science and Technology Agency). Bi metal was supplied by Kamioka Mining & Smelting Co., Ltd. The authors appreciate their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishimoto, A., Kado, Y. & Uda, T. Electrorefining of titanium from Bi–Ti alloys in molten chlorides for a new smelting process of titanium. J Appl Electrochem 46, 987–993 (2016). https://doi.org/10.1007/s10800-016-0983-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0983-8