Abstract
Our group proposes a new titanium smelting process via Bi–Ti alloy. This process is comprised of reduction of TiCl4 by Bi–Mg liquid alloy, separation of Ti from Bi by distillation, and Mg electrolysis. In this study, the Mg electrolysis with Bi–Mg liquid alloy cathode was investigated. We firstly measured the IR-corrected polarization curves on graphite and Bi–Mg liquid alloys by the current interruption method. The results indicated that the Bi–Mg alloy cathode can reduce the electricity consumption of the Mg electrolysis. In addition, from the relaxation curves on graphite and Bi–Mg alloys, the concentration overpotential on the Bi–Mg alloy is mainly due to mass transfer of Mg from the electrode/molten salt interface to the liquid alloy bulk. At current densities higher than 300 mA cm−2, Mg-rich solid phases such as Bi2Mg3 and/or pure solid Mg are assumed to be deposited on the Bi–Mg liquid alloy cathode. Finally, we estimated the electricity consumption of the Mg electrolysis in the new smelting process based on the measured overpotentials, assuming that Bi–Mg liquid alloy cathode is stirred sufficiently and a low current density, 275 mA cm−2, is applied. Under these conditions, the total electricity consumption of the Mg electrolysis in the new process will be lower than that in the Kroll process when the anode–cathode distance is smaller than 8 cm.
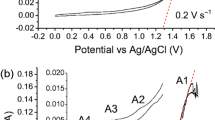
Graphic abstract
IR-corrected polarization curves of (a) Mg2+ reduction on graphite and Bi–Mg liquid alloys and (b) Cl2 evolution on graphite in MgCl2–NaCl–KCl at 550 °C were measured by the current interruption method, and electricity consumption of Mg electrolysis in the new Ti smelting process was estimated from these results.













Similar content being viewed by others
References
Kroll W (1940) The production of ductile titanium. J Electrochem Soc 78:35–47. https://doi.org/10.1149/1.3071290
Chen GZ, Fray DJ, Farthing TW (2000) Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 407:361–364. https://doi.org/10.1038/35030069
Ono K, Suzuki RO (2002) A new concept for producing Ti sponge: calciothermic reduction. JOM 54:59–61. https://doi.org/10.1007/BF02701078
Crowley G (2003) How to extract low-cost titanium. Adv Mater Process 161:25–27
Takeda O, Okabe TH (2006) High-speed titanium production by magnesiothermic reduction of titanium trichloride. Mater Trans 47:1145–1154. https://doi.org/10.2320/matertrans.47.1145
Okabe TH, Oda T, Mitsuda Y (2006) Titanium powder production by preform reduction process (PRP). J Alloys Compd 364:156–163. https://doi.org/10.1016/S0925-8388(03)00610-8
Zhang Y, Fang ZZ, Xia Y, Huang Z, Lefler H, Zhang T, Sun P, Free ML, Guo J (2016) A novel chemical pathway for energy efficient production of Ti metal from upgraded titanium slag. Chem Eng J 286:517–527. https://doi.org/10.1016/j.cej.2015.10.090
Maruyama S, Kado Y, Uda T (2013) Phase diagram investigations of the Bi–Ti system. J Phase Equilib Diffus 34:289–296. https://doi.org/10.1007/s11669-013-0243-0
Kado Y, Kishimoto A, Uda T (2015) New smelting process for titanium: magnesiothermic reduction of TiCl4 into liquid Bi and subsequent refining by vacuum distillation. Metall Mater Trans B 46:57–61. https://doi.org/10.1007/s11663-014-0164-2
Kishimoto A, Kado Y, Uda T (2016) Electrorefining of titanium from Bi–Ti alloys in molten chlorides for a new smelting process of titanium. J Appl Electrochem 46:987–993. https://doi.org/10.1007/s10800-016-0983-8
Kishimoto A, Kuramitsu A, Tsuchihashi K, Uda T (2016) Continuous production of Bi–Ti alloys by magnesiothermic reduction of TiCl4 for a new smelting process of Ti. J MMIJ 123:199–206. https://doi.org/10.2473/journalofmmij.132.199
Kishimoto A, Uda T (2018) Thermodynamics on the Bi–Fe–Ti system and the gibbs energy of Bi9Ti8. Metall Mater Trans B 49:2975–2985. https://doi.org/10.1007/s11663-018-1393-6
Tomonari T (2001) Chitan Kogyo to Sono Tenbo. The Japan Titanium Society, Japan
Furihata S, Akashi K, Kurosawa S (1981) The polarographic reduction wave of magnesium ion(II) in a molten LiCl–KCl eutectic mixture. Electrochim Acta 26:1107–1109. https://doi.org/10.1016/0013-4686(81)85085-2
Rao GM (1988) Electrochemical studies of magnesium ions in magnesium chloride containing chloride melt at 710 ± 10 °C. J Electroanal Chem 249:191–203. https://doi.org/10.1016/0022-0728(88)80359-0
Kisza A, Kaźmierczak J, Børresen B, Haarberg GM, Tunold R (1995) Kinetics and mechanism of the magnesium electrode reaction in molten magnesium chloride. J Appl Electrochem 25:940–946. https://doi.org/10.1007/BF00241588
Kisza A, Kaźmierczak J, Børresen B, Haarberg GM, Tunold R (1997) Kinetics and mechanism of the magnesium electrode reaction in molten MgCl2–NaCl binary mixtures. J Electrochem Soc 144:1646–1651. https://doi.org/10.1149/1.1837654
Børresen B, Haarberg GM, Tunold R (1997) Electrodeposition of magnesium from halide melts—charge transfer and diffusion kinetics. Electrochim Acta 42:1613–1622. https://doi.org/10.1016/S0013-4686(96)00322-2
Martínez AM, Børresen B, Haarberg GM, Castrillejo Y, Tunold R (2004) Electrodeposition of magnesium from CaCl2–NaCl–KCl–MgCl2 melts. J Electrochem Soc 151:C508–C513. https://doi.org/10.1149/1.1758814
Moser Z, Krohn C (1974) Thermodynamic properties of liquid magnesium–bismuth alloys. Metall Trans 5:979–985. https://doi.org/10.1007/BF02644308
Nayeb-Hashemi AA, Clark JB (1985) The Bi-Mg (bismuth–magnesium) system. Bull Alloys Phase Diag 6:528–533. https://doi.org/10.1007/BF02887150
Kosemura S, Ampo S, Fukasawa E, Hatta Y (2007) Production of titanium metal at Toho Titanium Co., Ltd. J MMIJ 123:693–697. https://doi.org/10.2473/journalofmmij.123.693
Güden M, Karakaya İ (1994) Electrolysis of MgCl2 with a top inserted anode and an Mg–Pb cathode. J Appl Electrochem 24:791–797. https://doi.org/10.1007/BF00578096
Demirci G, Karakaya İ (2007) Collection of magnesium in an Mg–Pb alloy cathode placed at the bottom of the cell in MgCl2 electrolysis. J Alloys Compd 439:237–242. https://doi.org/10.1016/j.jallcom.2006.08.064
Leung P, Heck SC, Amietszajew T, Mohamed MR, Conde MB, Dashwood RJ, Bhagat R (2015) Performance and polarization studies of the magnesium–antimony liquid metal battery with the use of in-situ reference electrode. RSC Adv 5:83096–83105. https://doi.org/10.1039/c5ra08606j
Newhouse JM, Sadoway DR (2017) Charge-transfer kinetics of alloying in Mg-Sb and Li-Bi liquid metal electrodes. J Electrochem Soc 164:A2665–A2669. https://doi.org/10.1149/2.1571712jes
Bard AJ (1976) Encyclopedia of electrochemistry of the elements, vol 10. Marcel Dekker Inc, New York
Komura A, Imanaga H, Watanabe N (1971) Chlorine evolution process in magnesium chloride–potassium chloride melt. Kogyo Kagaku Zassshi 74:867–871. https://doi.org/10.1246/nikkashi1898.74.5_867
The Electrochemical Society of Japan (2000) Denki Kagaku Binran, 5th edn. Maruzen Publishing Co., Ltd, Tokyo
Egan JJ, Bracker J (1974) Thermodynamic properties of some binary fused chloride mixtures obtained from e.m.f. measurements. J Chem Thermodyn 6:9–16. https://doi.org/10.1016/0021-9614(74)90201-8
Redlich O, Kister AT (1948) Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem 40:345–348. https://doi.org/10.1021/ie50458a036
Papatheodorou GN, Kleppa OJ (1967) Enthalpies of mixing in some binary alkaline-earth chlorides. J Chem Phys 47:2014–2020. https://doi.org/10.1063/1.1712231
Hersh LS, Kleppa OJ (1965) Enthalpies of mixing in some binary liquid halide mixtures. J Chem Phys 42:1309–1322. https://doi.org/10.1063/1.1696115
Pelton AD (2001) A general “geometric” thermodynamic model for multicomponent solutions. CALPHAD 25:319–328. https://doi.org/10.1016/S0364-5916(01)00052-9
Dinsdale AT (1991) SGTE data for pure elements. CALPHAD 15:317–425. https://doi.org/10.1016/0364-5916(91)90030-N
Perry GS, Fletcher H (1993) The magnesium chloride–potassium chloride phase diagram. J Phase Equilib 14:172–178. https://doi.org/10.1007/BF02667805
Chase NW Jr (1998) NIST-JANAF, thermochemical tables, 4th edn. ACS and AIP, Washington, D. C.
Acknowledgements
This work was supported by Advanced Low Carbon Technology Research and Development Program, Japan Science and Technology Agency, JST ALCA (Grant No. JPMJAL1006). Bi metal was supplied by Kamioka Mining & Smelting Co., Ltd.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
1.1 Calculation of the theoretical electricity consumption of Mg electrolysis
Data used for the thermodynamic calculation are given in Table 1. The activities of MgCl2 in Table 1 are calculated by a subregular solution model, in which the excess molar Gibbs energy of ternary molten salt, Gex, is represented as Eq. (8).
In Eq. (8), x1, x2, and x3 are the mole fractions of components 1, 2, and 3 in the ternary molten salts, respectively, and Ωij are interaction parameters of binary molten salts composed of i and j. The compositions of the molten salts are shown in Table 1. We defined the components 1 and 2 as MgCl2 and NaCl, respectively. The component 3 is defined as CaCl2 or KCl in the Kroll process or new process, respectively. The activity coefficient of MgCl2, γ1, can be calculated from the following equation.
In Eq. (9), R is the gas constant, T is the absolute temperature, P is the pressure. Then, interaction parameters of the binary salts, Ωij, are collected as below.
For MgCl2–NaCl, NaCl–CaCl2, and KCl–MgCl2 binary salts, data points of excess molar Gibbs energies for binary salts, \( G_{ij}^{\text{ex}} \), are reported in literature [30]. Then, Ωij are obtained by Eq. (10) and fitted by Eq. (11) which is a Redlich–Kister polynomial [31].
In Eqs. (10) and (11), yi and yj are mole fractions of i and j in the binary salts, and \( L_{n}^{ij} \) should be function of temperature. However, we assumed that \( L_{n}^{ij} \) are independent of temperature and adopted the data above 700 °C. Then, Ωij are function of the mole fractions. Figure 13 shows Ωij versus yi − yj and fitted curves by Eq. (11). The fitting parameters, \( {{L}}_{{n}}^{{ij}} \), are shown in Table 2. As for CaCl2–MgCl2 [32] and NaCl–KCl [33] molten salts, interaction parameters are reported as Eqs. (12) and (13), respectively.
Interaction parameters of MgCl2–NaCl, NaCl–CaCl2, and KCl–MgCl2 molten salts calculated from the reported data. Solid lines are fitted curves by Eq. (11)
Using these data, parameters \( L_{n}^{ij} \) are obtained as shown in Table 2.
Finally, each Ωij expressed as Eq. (11) is substituted into Eqs. (8) and (9) under the condition that yi − yj is equal to xi − xj, which is called a Muggianu-type approximation [34].
Rights and permissions
About this article
Cite this article
Kumamoto, K., Kishimoto, A. & Uda, T. Evaluation of overpotentials on graphite and liquid Bi–Mg electrodes by current interruption. J Appl Electrochem 49, 743–753 (2019). https://doi.org/10.1007/s10800-019-01320-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01320-3