Abstract
Studies of stream ecosystem metabolism over decades are rare and focused on responses to a single factor, e.g., nutrient reduction or storms. Numerous studies document that light, temperature, allochthonous inputs, nutrients, and flow affect metabolism. We use measurements spanning ~ 40 years to examine the interplay of all these influences on metabolism in forested and meadow reaches of a rural stream in southeastern Pennsylvania, USA. Measurements made in 1971–1975 used benthic substrata transferred to chambers (Period 1, P1), and ones in 1997–2010 used open system methodology (P2). Metabolism was greater in the Meadow reach both periods. Gross primary productivity (GPP) was driven primarily by light and chlorophyll, and respiration (R) by temperature and inclusion of hyporheic metabolism. Annually, processes were nearly balanced (Forested reach) or dominated by autotrophy (Meadow reach) in P1. Heterotrophy predominated in both reaches in P2, fueled by litter inputs (Forested reach) and fine particulate organic matter from the agricultural watershed (Meadow reach). Storms reduced GPP, R, and chlorophyll in proportion to storm size, but had less influence than other environmental factors. Riparian-zone reforestation of the P1 Meadow reach resulted in incident light and GPP similar to that in the permanent Forested reach within ~ 20 years.
Similar content being viewed by others
Introduction
Odum’s seminal publication (1956) concerning primary productivity in flowing waters spawned numerous measurements of ecosystem metabolism (primary productivity and aerobic respiration) in streams and rivers. Some exemplary early studies identified key aspects of metabolism that have been substantiated in subsequent work, e.g., the dominance of respiration (R) over gross primary productivity (GPP; Hoskin, 1959) although not in all systems (Edwards & Owens, 1962), the stimulation of GPP and R by nutrients (wastewater treatment plant effluent; Flemer, 1970), and the importance of substratum stability to periphyton development and metabolism (Duffer & Dorris, 1966).
Fisher and Likens (1973) approached streams as open systems and Hynes (1975) elaborated the connection of a stream to its watershed through geology, hydrology, and detrital inputs. Extending this idea, the River Continuum Concept (Vannote et al., 1980) postulated a longitudinal transition for metabolism in temperate forested streams—from a dominance of respiration fueled by allochthonous inputs in shaded headwaters, to a predominance of primary productivity (at least seasonally) in mid-sized streams in response to greater light, and a return to respiration dominance in deep large rivers. Tests in systems in different biomes of the contiguous US (Minshall et al., 1983; Bott et al., 1985) including the 8th-order Salmon River, ID drainage (Minshall et al., 1992) confirmed predictions. McTammany et al. (2003) reported that GPP increased in downstream direction with an accompanying shift to autotrophy, but in some other studies the continuum remained heterotrophic throughout despite increasing GPP (e.g., Chessman, 1985; Meyer & Edwards, 1990). Other patterns occurred in systems of different character. For instance, the entire continuum could be predominately autotrophic in a grassland river depending on the flow regime through its effects on turbidity (Young & Huryn, 1996) or present an inverted form where open headwaters flow into mid-order reaches bounded by gallery forest (Wiley et al., 1990). Grimm and Fisher (1984) expanded consideration to the vertical dimension with a report that hyporheic metabolism contributed 50% of respiration in a desert stream and studies by Jones (1995), Pusch (1996), Fellows et al. (2001) and Battin et al. (2003), among others, enhanced the knowledge of this ecotone. Scatterplots in Hoellein et al. (2013) and Bernhardt et al. (2022) illustrate the predominance of heterotrophic metabolism in most streams.
A large-scale, multi-site investigation showed that light and nutrient (soluble reactive phosphorus) were the most important proximal controls on GPP, and the extent of the transient storage zone and soluble reactive phosphorus had greatest effect on R (Mulholland et al., 2001), while other studies highlighted the effects of flow (discussed below), organic matter content (Acuña et al., 2004) and temperature (Bott et al., 1985) on these processes. Although studies of the stream—watershed connection initially focused on allochthonous inputs and their utilization (sometimes quantifying their contribution to an energy budget, e.g., Fisher, 1977; Cummins et al., 1983) interest in more recent work has shifted to distal controls on instream metabolism through land use. Agricultural activities and urbanization in the watershed usually elevated GPP over that in streams in forested watersheds (Young & Huryn, 1999) or streams in both forested watersheds and watersheds recovering from agricultural land use (McTammany et al., 2007) or in reference streams in a study that encompassed multiple biomes (Bernot et al., 2010). Respiration in the impacted streams in these studies was either lower than in reference streams (Young & Huryn, 1999) or showed no difference between land use categories (McTammany et al., 2007; Bernot et al., 2010). Clapcott et al. (2010) reported that GPP and R both increased with the degree of vegetation removal in the watershed, treating results as responses to a disturbance gradient. Houser et al. (2005), however, reported that disturbance to upland soils and vegetation reduced only instream R and not GPP, which was controlled instead by riparian canopy shade.
A forested riparian zone reduces solar radiation reaching the stream, affecting available light and sometimes streamwater temperature, and is the major source of autumnal leaf litter. GPP was lower, and R was either lower or without difference, in reaches with a forested riparian zone compared to open reaches (Sweeney et al., 2004; Bott et al., 2006b). Alberts et al. (2016) showed that season interacted with riparian canopy cover to affect both GPP and R in streams in urban and reference watersheds. In studies of local and distal controls on metabolism, GPP and R decreased with riparian shade, which had greater effect on function than the most important watershed variable which was percentage of agricultural land (Bunn et al., 1999; Burrell et al., 2014).
Continuous monitoring of dissolved oxygen was used to study the daily, seasonal, and inter-annual variability of metabolism in small streams (Roberts et al., 2007; Beaulieu et al., 2013) and in larger systems (Izagirre et al., 2008; Dodds et al., 2013) in studies lasting 2 years. Longer-term studies are rare and usually have followed metabolic responses to a triggering factor, e.g., flood plain restoration (Roley et al., 2014, 5 years), reduction in wastewater effluent (Arroita et al., 2019, 20 years), or storms (Uehlinger, 2006, 15 years). Appling et al. (2018) based metabolism estimates for 365 US rivers on up to 9 years of data.
The impact of storms on periphyton and metabolism was first studied in Sycamore Creek, a desert stream subject to flash floods (Fisher et al., 1982). Continuous monitoring of dissolved O2 concentrations facilitated assessment of storm effects on metabolism in the River Necker (Uehlinger & Naegeli, 1998) and River Thur in Switzerland (Uehlinger 2000) and urban streams in the mid-Atlantic region of the US (Reisinger et al., 2017). Uehlinger’s (2006) analysis of 15 years of data for the Thur showed that storms reduced primary productivity by ~ 50% and respiration by ~ 20%, with recovery rates season dependent. Using data from 222 US rivers, Bernhardt et al. (2022) identified the flow regime and light as the primary regulators of river metabolism.
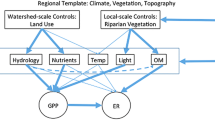
Here, we report studies of ecosystem metabolism in a forested and meadow reach of a stream draining a rural watershed in southeastern PA that span a period of 39 years. Our study stream is typical of many in the Eastern US Piedmont physiographic province. The naturally forested region was extensively deforested for timber, fuel, and agriculture during settlement. Some subsequent replacement occurred, and rural streams today traverse a landscape mosaic of meadows, pastures, cultivated fields, and second growth forests. Our objectives here are to: (1) present patterns of GPP and R in each reach, (2) compare early determinations of seasonal and annual rates of GPP and R with those obtained more recently, albeit with different methods, (3) interpret metabolism rates using concurrent measurements of light, temperature, chlorophyll, flow, and water chemistry together with data, e.g., organic matter inputs and hyporheic activity, from other studies of the site, (4) assess the impact of storms on metabolism, and (5) examine the response of metabolism to reforestation of a meadow riparian zone. Initial measures were made between 1971 and 1975 (Period 1, P1). Measures resumed in 1997 (Period 2, P2) and were expanded in 2005 to include the effect of riparian reforestation.
Materials and methods
Study sites
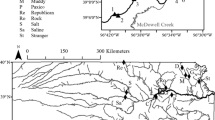
The 3rd-order study reaches were located on the east branch of White Clay Creek (Chester Co., PA), a tributary of the Christina River in the Delaware River watershed. The stream drains a study watershed of 7.2 km2. In 1973, predominant land uses were 60% horse pasture, 17% cultivated crop, and 18% woodlot (Vannote, unpublished data). In 1995, those values were 52%, 23%, and 21%, respectively (Newbold et al., 1997). Forested areas were dominated by tulip poplar (Liriodendron tulipifera L.), white oak (Quercus alba L.), and American beech (Fagus grandifolia Erhr.). Spicebush (Lindera benzoin (L.) Blume) was a common understory species. In 1975, the riparian zone of the drainage network upstream of the Stroud Water Research Center was 58% forested, 30% in meadow, and 12% semi-open (Vannote, unpublished data). By 1995, those values were 70%, 14%, and 16%, respectively (Newbold et al., 1997) and by 2005, nearly all the riparian zone was forested.
The reaches in P1 were each 150 m long, with the Meadow reach ~ 500 m downstream of the Forested reach (Fig. 1). The area surrounding the Forested reach changed little during the decades of interest except for the natural loss of a few large trees (Supplementary Fig. 1A–C). Native trees (as bare root seedlings) were planted in the riparian zone of the Meadow reach in 1989 and it is referred to as Reforested for P2. It is shown in its meadow (P1) and reforested (P2) condition in Supplementary Fig. 1D–F. In 1997, a permanent Meadow reach (150 m long) was established in an alluvial floodplain downstream of the Reforested reach (Fig. 1). This reach is referred to as Meadow 2 (Supplementary Fig. 1G, H). Tree canopy density measurements made in May 2005, July 2006, and June 2008 ranged from 74 to 84% at the Forested reach and from 60 to 83% at the Reforested reach. At the Meadow 2 reach, canopy density was ~ 48% (mean of 2005 and 2006 data) but decreased to 21% in 2008 (from tall grasses and a relatively high streambank) following the removal of scattered riparian trees.
Aerial photographs of the White Clay Creek study watershed in 1970 and 2005 with the stream highlighted in blue and study reaches in yellow. The stream flows from north to south. Coordinates for the downstream end of each reach are as follows: Forested (39.863222° N, − 75.78433° W), Reforested (39.85907° N, − 75.783641° W), and Meadow 2 (39.854803° N, − 75.784339° W). The Stroud Water Research Center is located streamside north of the road traversing the center of the photo
Shaded reaches were wider and shallower than the Meadow 2 reach. Measurements made during P2 yielded a mean width and depth of 4.77 m and 0.138 m, respectively, for the Forested reach, 4.05 m and 0.155 m for the Reforested reach, and 2.91 m and 0.191 m for the Meadow 2 reach. Stream slopes (m/1000 m) were 5.3, 6.4, and 3.8 in the Forested, Reforested, and Meadow 2 reaches, respectively. Mean percentages of streambed substrata characterized according to Hynes (1970) as soft (clay, silt, sand) and hard (pebble, cobble and boulder) were 34% and 66%, respectively, in the Forested reach, and 35% and 64%, in the Reforested reach, but were closer to equal (46% and 51%) in the Meadow 2 reach. Monthly mean baseflow ranged from 0.063 to 0.141 m3/s (annual mean 0.104 m3/s) during P1 and from 0.052 to 0.112 m3/s (annual mean 0.086 m3/s) during P2. Streamwater chemistry reflects the gneiss and schist bedrock, with moderate enrichment in nitrogen and phosphorus from agricultural activities (Supplementary Table 1).
Benthic algae were the dominant primary producers in all reaches. An abundant and diverse diatom (Bacillariophyta) flora occurred in the Forested reach, with a mean of 168 species in samples taken biweekly during P1 (Patrick, 1996). A bloom of Ulothrix zonata Kützing occurred each spring and other Chlorophyta, e.g., Stigeoclonium lubricum (Dillwyn) Kützing, Cladophora glomerata (L.) Kützing, Spirogyra sp.; Xanthophyta (Vaucheria sp.); and Cyanobacteria, e.g., Microcoleus vaginatus (Vaucher) Gomont, Schizothrix calcicola Gomont, occasionally developed a spotty visible growth. Filamentous Chlorophyta, Cyanobacteria, and Melosira sp. were common in both Meadow reaches.
Metabolism measurements
Metabolism was measured on from one to three days per week in the Forested reach between April 1971 and August 1974 and in the Meadow reach between July 1973 and November 1975 (P1). In 1997, measurements for 3–11 days began in the Forested and Meadow 2 reaches during warm and cold seasons. Concurrent measurements in the Reforested reach began in 2005 and concluded in all three reaches in January 2010 (P2).
During P1, metabolism was estimated from changes in dissolved O2 concentration measured in clear acrylic chambers (22 l; 68.5 cm long × 30.5 cm wide × 14 cm deep) equipped for water recirculation (Teel Model IP598 submersible pumps, Dayton Electric, Chicago, IL). Chambers were submerged in water jackets located on the streambank that were supplied continuously with stream water in order to keep chamber water near ambient temperature. Plastic trays (337 cm2, 5 cm deep, 60 per reach) were filled with streambed substrata and incubated in the streambed with surfaces contiguous for weeks to months prior to use in measurements. For measures, usually one (sometimes 2 or 3) tray(s) were transferred to a chamber. From one to three chambers were used per day. The chamber was sealed and dissolved O2 concentrations were measured using a Model 60 flow-through probe inserted in the recirculation line and Model 300 meter (Rexnord, Malvern, PA) and recorded on a strip chart recorder (Speed-o-Max M, Leeds and Northrop, North Wales, PA). Probes were calibrated before each run against Winkler dissolved O2 determinations. If O2 supersaturation was anticipated, the water jacket was covered with black plastic for 1 h to lower the dissolved O2 concentration. Rates of change for the preceding and succeeding hours were averaged and substituted for the hour the chamber was covered as data were processed. After sampling portions of the periphyton for chlorophyll as described below, trays with intact sediment were returned to the stream for continued incubation prior to use in another measurement. Chambers were scrubbed between use and recirculation lines were changed and boiled in water weekly.
For P2 open system measurements, dissolved O2 was monitored with either Model 600XL sondes (YSI, Inc., Yellow Springs, OH) coupled with a CR-500 data logger (Campbell Scientific, Logan, UT) in weatherproof housing (Rapid Creek Research, Boise, ID) or YSI Model 600XLM sondes with internal logging capability. After calibration of dissolved O2 probes in water saturated air and a quality control check, a sonde was positioned at the upstream and downstream end of each reach. Dissolved O2 and temperature were measured and logged every 15 min for up to 11 days during which reach-specific reaeration was determined once from propane injection (Marzolf et al. 1994, 1998; Young & Huryn 1998) with bromide as a conservative tracer (Bott et al., 2006b). In seven cases, reaeration coefficients were based on geomorphic and hydraulic variables (Owens et al., 1964; Tsivoglou & Neal, 1976). Quality control checks included incubation of sondes at a single location before and after each measurement series to obtain an offset value when applying the 2-station analysis procedure and, starting in 2000, daily checks of probe performance using an additional sonde and meter.
Complementary measurements
Flow data during P1 were obtained from a continuously recording gauging station located ~ 60 m downstream from the bottom of the Meadow reach. Flows for P2 were measured by bromide dilution concurrent with propane injections. Water temperature was monitored using a Taylor thermograph (P1) or thermistors on the YSI sondes (P2). During P1, total solar radiation was measured using a recording pyranometer (Model 8-48, Eppley, Newport, RI) in a field adjacent to the Meadow reach and a recording pyroheliometer (Model 5-3850, Belfort Instruments, Baltimore, MD) installed at the Forested reach in September 1973. During P2, above-water photosynthetically active radiation (PAR) was measured with a quantum sensor (Model 190, LI-COR, Lincoln, NB) mounted on a stake mid-stream at the top and bottom of each reach and logged to LI-COR Model 1400 data loggers. Total solar radiation data for P1 were converted to an approximation of PAR with empirically derived equations. For the Meadow, total solar radiation and PAR were measured concurrently using LI-COR sensors, Model 200-SA pyranometer, and Model 190 quantum, respectively, at an open site during summer. A regression of PAR data (mol quanta photons m−2 day−1) against total radiation (“Energy”) expressed as Mjoules m−2 day−1 yielded the equation: PAR = 1.933 Energy (R2 = 0.99, n = 14 days) which was applied to Meadow reach data. It is remarkably similar to one based on 2 years of data collected ~ 142 km south of our site (PAR = 2.04 Energy, R2 = 0.99; Fisher et al., 2003). Two regressions were used for the Forested reach. One, PAR = 1.30 Energy, R2 = 0.90, was applied to data collected between May 10 and October 31. It was based on data collected over 52 days in mid-summer with PAR sensors and an Eppley pyranometer moved sequentially to five locations under the forest canopy. A second regression, for use when selective removal of photosynthetically active wavelengths by leaves was not an issue, was based on data collected with LI-COR sensors in a greenhouse under neutral density screening. Data in the range of 1.5 to 9.2 MJoules m−2 day−1 (representative of values between November 1 and May 9 of P1) generated the equation: PAR = 1.83 Energy (R2 = 0.98, n = 9).
For P1 (beginning in September 1972), chlorophyll a was determined on from 4 to 10 periphyton samples (10.2 cm2 × 0.5 cm deep) that were scraped or cored from substrata at the end of each chamber measurement. Samples were extracted overnight in buffered acetone in the dark at ≤ 4°C and chlorophyll a was determined spectrophotometrically (Lorenzen, 1967). Reach averaged estimates were produced as detailed below for metabolism data. For P2, sample collection prior to 2000 followed Bott et al. (2006b). From 2000 on, samples of cover types amounting to ≥ 10% of those seen through a viewing bucket at 200 locations in the reach (10 lateral points on 20 transects) were collected and processed as described in Bott et al. (2006a) and then analyzed as in P1. Chlorophyll estimates per m2 for each cover type were weighted for the proportion of the reach with that cover type and summed. Stream width was measured at each transect and depth at each lateral point. The depths reported above were derived from one-dimensional transport with inflow and storage (OTIS) models (Runkel, 1998) of conservative tracer releases during reaeration measurements, and the physical measurements agreed with those values within 10% over all reaches. Streambed substrata characterization was done concurrent with cover type assessments. Tree canopy densities were determined as described in Bott et al. (2006a) at the time these other data were collected.
Water chemistry was monitored at approximately weekly intervals during each period although sampling dates did not always coincide with metabolism measurements. NH4+ was determined using the phenol –hypochlorite procedure (Solorzano, 1969) and the phenate procedure (EPA method 350.1) in P1 and P2, respectively; NO3− by chromotropic acid procedure (Am. Public Health Assoc. [APHA], 1971) in P1 and the cadmium reduction technique (EPA method 353.2) in P2; total alkalinity by methyl orange titration in P1 (APHA 1971) and Gran titration in P2 (EPA Method 310.1); and the following analyses during both periods: SiO2 (EPA method 370.1), SO42+ (EPA method 375.4), Cl− (EPA method 325.3), and PO43− (EPA method 365.1). EPA methods are found in US EPA (1993). Cations were determined by atomic absorption spectrometry during P1 and by EPA method 200.7 during P2. pH was measured with a meter. Missing water chemistry data were replaced with baseflow averages for the week in question obtained from other years within the appropriate period.
Data analyses
For P1, diel rate-of-change curves were created from hourly changes in dissolved O2 (Bott, 2006). The average nighttime respiration rate was extrapolated through the photoperiod and daily respiration (R24) computed. Photoperiod respiration was added to the net change in O2 during the photoperiod to generate gross primary productivity (GPP). Net daily metabolism (NDM = GPP – R24; alternatively, net ecosystem productivity) and the P/R ratio (GPP/R24) were determined. Data from days with more than one chamber measurement were averaged, first for riffle or pool habitat, and then over habitat, to generate the estimates of reach metabolism.
The premise for metabolism data analysis for P2 is that the net metabolic production of oxygen is the sum of the change in oxygen concentration and the loss to (or gain from) the atmosphere, i.e.,
where GPPt = gross primary productivity (g O2 m−2 h−1) at time t, Rt = respiration (g O2 m−2 h−1), Ct = dissolved oxygen concentration (g O2 m−3), Csat = saturation concentration of O2 at water temperature and atmospheric pressure of site (g O2 m−3), KL = gas transfer velocity (m h−1), z = depth (m)
Daily metabolism estimates were based on diel curves created from dissolved O2 change every 0.25 h, which were integrated over the respective daylight or nighttime hours using the 2-station analytical procedure (Bott 2006, after Owens, 1974) and deriving the metabolic parameters as described above. The single station approach using data from the downstream sonde was applied to one-third of the datasets because data quality precluded using the 2-station approach. A PAR value of 2 µmol quanta photons m−2 s−1 was used to differentiate night and day except when a higher value was needed to account for bright moonlight. Where groundwater inputs measurably augmented flow within the reach (Meadow 2, January and February), we corrected the GPP and R24, as described by McCutchan et al. (2002) and Hall & Tank (2005), using groundwater dissolved O2 measured in two nearby wells (8.60 mg l−1).
Data for days on which storms occurred during P2 (Feb. 23 & 24, 1998; July 15, 2006; Jan. 17, 2010) and post-storm days were deleted from the analyses (unless noted otherwise) because chlorophyll values (obtained pre-storm) and reaeration coefficients were inapplicable. Forested reach data for 2001 were deleted because of probe failure. Data were processed and analyzed using SAS (version 9.4) or JMP (Release 7; both from SAS Institute, Cary, NC) with the exception of coinertia analyses. Data were either log or arcsine square root transformed before use in statistical tests.
Differences in metabolism, chlorophyll, PAR, and temperature between the Forested and the respective Meadow reach and between seasons in a reach were tested using 2-way analysis of variance (ANOVA). Water chemistry data were averaged by week within period and tested for between-period difference in paired sample t-tests with data paired by week. Metabolism parameters, chlorophyll, PAR, and temperature were tested for differences between the Forested, Reforested, and Meadow 2 reaches using Dunnett’s tests with the Forested reach specified as the control and using only days with data for all reaches. Photosynthesis–irradiance curves were generated using data from May 2005, July 2006, and June 2008 in a hyperbolic tangent model (Jassby & Platt, 1976).
We collected three consecutive years of data for the Forested reach between April 1971 and April 1974 (P1) and we computed seasonally weighted annual metabolism for each of those years. For P2, a simple mean was inappropriate because the daily measurements were clustered in 12 runs (sonde deployments spanning 3–11 days), most of which were taken in late spring or summer, leaving autumn and winter under-represented. To remove the seasonal bias, we fit annual LOESS curves (SAS Proc LOESS) to the P1 data, using the resultant daily departure from the annual mean to calculate seasonally adjusted P2 daily values. The daily values were averaged to obtain an annual estimate for P2. The standard error for the annual mean estimate was calculated from the between-run mean squared error via ANOVA (SAS Proc GLM) of the adjusted daily values specifying “run” as a random effect (Neter et al., 1990, p. 656).
For storms that occurred during P1, we analyzed the impact on metabolism and biomass (chlorophyll) and the subsequent recovery. For both impact and recovery, post-storm values of the respective parameter were compared to the average of pre-storm values taken within 16 days of the storm and at least 21 days after a previous storm of similar or larger size. To assess impact, we averaged post-storm values over a period of 7 days unless truncated by a similar or larger storm. For each storm, we calculated resistance, R (sensu Grimm & Fisher 1989), as the ratio of post-storm to pre-storm averages. For clarity, we also express storm impact as a percent reduction, (1−R) × 100. We related resistance to storm size by fitting the regression coefficients a and b in the equation:
where Qmax is peak flow of the respective storm. Points for the regression were weighted to account for the number of pre-and post-storm values as follows: weight function = [npre + npost] −0.5| npre – npost|. The threshold for impact was the value of Qmax above which the predicted resistance was < 1. R was also calculated for two storms in the P2 period, using averaging intervals of 3–4 days pre-storm and post-storm days after streamflow returned to ≤ 17% of the mean of pre-storm data. For interpretive purposes, we convert R to percent reduction or 100 × (1−R).
Recovery from storms was determined using response ratios, RR (metabolism or chlorophyll on a given day, divided by pre-storm average; Reisinger et al., 2017), over a post-storm period of 25 days unless recovery was interrupted by a similar or larger storm. We estimated the rate of recovery, r, from the regression equation:
where RR(j) is the response ratio on day j after the storm peak and ln[RR(0)] is the estimated intercept. Time-to-recovery, i.e., for RR(j) to return to a value of 1, was then calculated as
We used log-transformed values in Eq. (3) to stabilize the variance. This transformation, however, implicitly assumes that recovery is exponential, i.e., increasing as recovery approaches completion. We therefore consider the transformation appropriate for estimating time-to-recovery (Tr), but not for describing the trajectory of recovery. For subsequent modeling (presented in the Discussion), we calculated a linearized recovery rate as r* = [1−RR(0)] ⋅ Tr−1, where r* represents daily recovery as a fraction of the fully recovered rate, or stock.
Recovery rates were analyzed separately for the warm season (May through October) and the cold season (November through March). If seasonal rates did not differ (extra-sums-of-squares principle; Draper & Smith, 1966), the seasons were combined. Recoveries during April were excluded because rapidly changing temperature and light complicated recovery trajectories. The analysis was carried out for storms with peak flow > 2 m3 s−1; smaller storms did not yield detectable recovery rates.
For comparison with the results of Uehlinger (2000), we estimated the average long-run, per-storm, resistance of all storms above a given threshold. For this, we used the 50-year continuous hydrograph for White Clay Creek, calculating R from Eq. (2) and using the peak flow for each excursion above the threshold.
We used the estimates of streambed shear stress to scale resistance measured in White Clay Creek to that reported by Uehlinger (2000) for the River Necker (Switzerland). Shear stress was calculated as τ = ρ g h s (Richards, 1982) where ρ is the density of water, g is the acceleration of gravity, h is stream depth, and s is the channel slope. For the River Necker, we estimated h for a given flow using a value of 0.04 for Manning’s roughness coefficient, appropriate for the river’s bed material (gravel and cobble) and sinuosity (1.4) (Arcement & Schneider, 1989).
Relationships between environmental factors and metabolism were synthesized using coinertia analysis with P1 and P2 data combined. Coinertia analyzes the common structure of two data tables, in our case, environmental variables and metabolic variables, by maximizing the covariance of the primary axes of principal component analyses of the individual datasets (Dolédec & Chessel, 1994; Dray et al., 2003). For P1, a data record represented either one day or the average of a group of days that metabolism and chemistry data (collected within 5–7 days of each other) were linked. For P2, a record was the group-averaged data for each measurement series, thus avoiding pseudoreplication from linking the same chemistry and chlorophyll data with multiple metabolism records. A period-specific monthly mean value was substituted for any missing chemical data. Storms were assigned to one of five thresholds: 0.3, 0.7, 1.2, 2.0, and 2.8 m3/s. These thresholds span a range of 3.5 to 33 times the mean annual baseflow of 0.086 m3/s. Days-since-storm of each threshold were counted to the central day for averaged group data. Cold and warm seasons were differentiated by a mean water temperature of 12°C. The number of groups of data and dates of collection were as follows: P1 Forested reach, 29 groups (12 Cold, 17 Warm), June 1973–July 1974; P1 Meadow reach, 39 groups (19 Cold, 20 Warm), July 1973–Nov. 1975; P2 Forested reach, 11 groups (4 Cold, 7 Warm), P2 Meadow 2 reach, 11 groups (3 Cold, 8 Warm), July 1997–Jan. 2010 for both reaches; and P2 Reforested reach, 5 groups (1 Cold, 4 Warm), May 2005–Jan. 2010. Coinertia was performed using the ade4 package in R (version 1.7-4).
The data used in coinertia analyses, with the addition of a code for Period (0, 1), were used in stepwise mixed model multiple linear regression (MLR) analyses of metabolism parameters and chlorophyll, with a probability of 0.15 for both entry and removal of terms. Akaike’s Information Criterion corrected for small sample size at each step, the statistical significance of included parameters, and model complexity guided model selection.
Results
Seasonal patterns of metabolism, chlorophyll, PAR, and temperature in the Forested and Meadow reaches
GPP in the Forested reach during spring was significantly (~ 2.6-fold) higher than during other seasons when canopy shade or low temperature restricted activity (Fig. 2A, Table 1). The high P1 values in January all occurred during unusually mild weather in 1973. Rates > 1.4 g O2 m−2 day−1 during autumn occurred in 1971 when weather was drier than in 1972. In 1971, there were 9 days with maximum flow ≥ 1.5 times baseflow between September 22 and December 21, whereas in 1972, there were 20 such days (Supplementary Fig. 2). Thus, GPP (in g O2 m−2 day−1) during autumn averaged 1.03 ± 0.59 (\(\overline{x}\) ± SD, n = 42) in 1971 but only 0.62 ± 0.32 (n = 27) in 1972. Fewer measurements in 1973 prevented discernment of a pattern. GPP during P2 was in the range of data for P1, and as for P1, mid-summer values were lower than those in mid-spring (Fig. 2A). The warm season mean exceeded the cold-season mean (Table 1). In the Meadow and Meadow 2 reaches, GPP tracked with solar radiation (Fig. 2B) and, even with the wide range in data, seasonal means differed as expected (Table 1). GPP in the Meadow reach was significantly greater than in the Forested reach in both periods.
Daily rates of Gross Primary Productivity (GPP, Panels A, B) Respiration (R24, Panels C, D), Net Daily Metabolism (NDM, Panels E, F), and P/R ratio (Panels G, H) in the Forested and Meadow reaches. Daily rates for P2 (red diamonds) are superimposed on P1 data (gray circles). Note the difference in scale for GPP. Years of data collection − P1: Forested reach, 1971–1974; Meadow reach, 1973–1975; and P2: Forested and Meadow reaches, 1997–2010
R24 values tended to be higher in P2 than in P1in both the Forested and respective meadow reach (more consistently so in the Forested reach) because hyporheic respiration was included in the open system P2 measurements (Fig. 2C, D). During P1, R24 in both reaches was significantly higher in spring and summer than in autumn and winter (Table 1). During P2, the difference between cold and warm seasons was significant in the Forested reach but not in the Meadow 2 reach, owing to a wide range of values in the warm season and high respiration in January. As for GPP, R24 was greater in the Meadow reach during each period. Regressions of R24 on GPP with P1 data had intercepts that were not significantly different from 0 and significant regression slopes for both reaches, indicating a coupling of respiration with GPP (Supplementary Table 2). Regressions of P2 data had intercepts that differed significantly from 0 and the slope for the Meadow 2 reach, while significant, was shallower, all indicative of a weaker association of R24 with GPP in P2 in both reaches.
In the Forested reach during P1, NDM (Fig. 2E) and P/R (Fig. 2G) indicated that respiration predominated under canopy shade (with lowest values in summer) while processes were balanced or dominated by GPP at other times (Table 1). GPP predominated in the Meadow reach during P1 (Fig. 2F, H). Seasonal changes in GPP and R24 led to significant differences in NDM there, but the changes were of similar magnitude so that P/R remained relatively constant (Table 1). Negative NDM and P/R < 1 at times when GPP usually prevailed are attributed to storm scour of periphyton growths. During P2 respiration predominated in both reaches with the exception of a few mid-spring measurements in the Forested reach and several warm season measures plus a few in February in the Meadow 2 reach. Thus, in P2, mean NDM values were more negative and mean P/R ratios were often no more than half of those for P1 at comparable times of year. Between-season differences for these parameters in P2 were non-significant, except for the P/R ratio for the Forested reach (Table 1). Meadow reach NDM in P1, and P/R during both periods, exceeded values for the Forested reach (Table 1).
Chlorophyll concentrations in the Forested reach during P1 peaked in late winter and spring prior to tree canopy development (Fig. 3A) and differed significantly from summer and autumn values (Table 1). P2 concentrations during the cold season were in the range of P1 values but higher values were measured in the warm season. In the Meadow reach during P1, a wide range of values occurred between March and November with lower values between December and February (Fig. 3B) and seasonal differences were few, given this variability (Table 1). Like the Forested reach, several P2 values exceeded P1 data. Chlorophyll concentrations were higher in the Meadow reach in both periods, but the difference was significant only for P1.
Chlorophyll a concentrations (Panels A, B), Photosynthetically Active Radiation (PAR, Panels C, D) and mean water temperature (Panels E, F) associated with daily metabolism measures in the Forested and Meadow reaches during P1 and P2. P1 and P2 data are presented as in Fig. 2. Note the difference in scale for PAR
During P1, average PAR measured concurrently with metabolism at the Forested reach was 90% of average Meadow PAR between Feb 15 and April 30 (prior to tree canopy closure) but only 25% between May 16 and July 31, similar to the average (26%) at that time in P2 (Fig. 3C). Seasonal means in P1 were highest in spring at the Forested reach and in spring and summer at the Meadow reach and showed expected seasonal differences in P2 (Table 2). PAR at the respective meadow reach (Fig. 3D) exceeded that at the Forested reach during each period (Table 2). Streamwater temperatures at the time of metabolism measures (Fig. 3E, F) did not differ between reaches in either period and differed seasonally as expected (Table 2).
Annual metabolism
Estimated annual GPP in the Forested reach was slightly (26%) greater in P2 than in P1 but annual R during P2 was 115% greater than that measured during P1 (Table 3). We had only two consecutive years of data for the Meadow reach during P1 and to generate a total for the second year we added winter data from the first year to original data for other three seasons of the second year. Annual GPP in the Meadow 2 reach was only 60% of that in the P1 Meadow reach but annual R increased by 32%. The small standard errors of these estimates for both periods indicate that values are robust and that between-year variability within period was low. Annual GPP and R in the Forested reach during P2 were 53% and 62%, respectively, of values for the Meadow 2 reach. P1 data suggested that GPP equaled or exceeded respiration in both reaches, but P2 open system measures documented that respiration was the predominant process in both reaches.
Effect of riparian reforestation on metabolism, chlorophyll, PAR and temperature
Groupings in Dunnett’s tests showed that during the warm season under a full tree canopy the Reforested and Forested reaches were alike with respect to GPP and PAR, but in the cold season, the Reforested reach grouped with the Meadow 2 reach (Table 4). R24 was greatest in the Meadow 2 reach both seasons. The Reforested reach grouped with it in the warm season and with the Forested reach only in the cold season. Given the high respiration in the Meadow 2 reach, NDM there was more negative than at the shaded reaches, which grouped together the cold season. Between-reach differences in NDM and the P/R ratio were non-significant in the warm season. However, while P/R ratios suggested roughly similar proportionality between GPP and R24 in each reach, the magnitude of values was such that NDM varied nearly sevenfold. Chlorophyll was greater in the Meadow 2 reach than in the shaded reaches in both seasons but because the number of estimates is low, statistical significance is lacking. Mean daily water temperatures during metabolism measurements did not differ among reaches in either season but the daily maximum water temperature in the Meadow 2 reach exceeded values for the shaded reaches in the warm season.
With one exception (Reforested reach, June 2008), photosynthesis–irradiance curves for the shaded reaches possessed an initial slope (α) that was 1.6–4.3-fold greater than for the Meadow 2 reach (Table 5). Normalization for chlorophyll resolved that exception and enhanced the magnitude of the differences between the shaded reaches and the Meadow 2 reach. Saturating intensities for the shaded reaches were always lower than those for the Meadow 2 reach. Despite such evidence of shade adaptation, maximum photosynthesis was the greatest in the Meadow 2 reach with one exception (Reforested reach, May 2005). That exception was a transient phenomenon, however, as evidenced by the data obtained in June and July.
Effect of storms on metabolism and chlorophyll
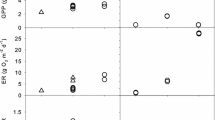
Storms reduced GPP, R24, and chlorophyll a when peak flows exceeded a threshold near or slightly less than 0.5 m3 s−1, which is 5.9 times the average annual baseflow of 0.086 m3 s−1 and has a return interval of 22 days (Fig. 4, Table 6). The reductions increased with storm size. At bankfull flow (4 m3 s−1; return interval 215 days), the estimated average reductions were 44% for GPP, 29% for R24, and 69% for chlorophyll a (Table 6). The storms in P2 were not included in the regressions but the reductions for GPP and R24 were in the range of those for P1 storms of similar magnitude (Fig. 4). Both the regression lines and a visual interpretation of the data suggest possible stimulation (resistance > 1) by small storms. As shown by the confidence intervals (Fig. 4), such stimulation is not statistically supported. However, for the P2 storms, we also saw some evidence for stimulation (Fig. 4).
Resistance for GPP, R24, and chlorophyll a regressed against storm peak discharge. Points for the regression were weighted to reflect the number of points used in each ratio as described in the text. There were no significant differences between the Forested and Meadow reaches. Data for the three reaches from two storms during P2 (red diamonds) are superimposed on P1 data
For GPP and R24, recovery from storms with peak flows > 2.0 m3 s−1 was faster in the warm season than in the cold season (P < 0.05; Fig. 5, Table 7). Although only the warm season recoveries were significant at P < 0.05, we nonetheless used the estimated cold-recovery slopes to calculate recovery times. GPP averaged 19 days to recovery in the warm season and 48 days in the cold season. For R24, the times to recovery were 16 days in the warm season and 63 days in the cold season. With fewer data for chlorophyll, we were unable to distinguish a seasonal effect (P > 0.05). Recovery time averaged over the entire year was 29 days.
Synthesis of factors affecting metabolism
The subset of data used to form groups for the coinertia and MLR analyses showed little bias relative to the full dataset. The relative percent difference between the means of grouped data and of all data for each parameter (by reach and period) differed by less than 13% except for NH4 (20%, 25% and 27% for Forested P1, P2, and Meadow P2, respectively), and NDM (28% for both the Forested P1 and Reforested reaches). The first two factors of the principal component analysis (PCA) of metabolic variables accounted for 60% and 36% of variance in the data, respectively (Fig. 6). All metabolic variables positioned negatively on axis 1, with GPP and R24 most strongly so. Metabolic variables aligned on the second axis with R24 most positive and metabolic balances (NDM, P/R) negative. The first two factors of the PCA of environmental parameters explained 36% and 14% of the variance in the data, respectively. PAR, temperature, and chlorophyll aligned negatively on axis 1, suggesting that it characterized season. Water chemical characteristics (Supplementary Table 1) were primarily associated with axis 2, with alkalinity and NO3 positive (higher in P2) and PO4 and NH4 negative (higher in P1), suggesting that it characterized Period. NH4 aligned approximately opposite of chlorophyll, which is suggestive of its role as an algal nutrient, but SiO2, NO3, and PO4 appeared independent of chlorophyll. Days-since-storm of all thresholds aligned positively on axis 2 in proximity (indicative of their inter-correlation, with highest r values between next ranked storm sizes).
Results of coinertia analysis showing the percent variability associated with axis 1 and 2 of separate principal component analyses (PCAs) of environmental and metabolic variables and the co-structure of the two data tables associated with the first two axes of the coinertia analysis. Loadings of environmental and metabolic variables on axes 1 and 2 from the PCAs are shown as triangles and circles, respectively. Vectors for the metabolic variables are not shown to preserve clarity. The centroids (± 1 SE) of scores for each reach by season are shown in Panel A, and for each reach by Period in Panel B. GPP gross primary productivity, R24 daily Respiration, NDM net daily metabolism, P/R GPP/R24, Temp temperature, Chla Chlorophyll a, Alk total alkalinity, DSS days-since-storm of indicated threshold
Coinertia analysis had a RV (R value, a coefficient indicating overall similarity between two data tables) of 0.29 and a Monte Carlo P value of 0.01, which suggests modest but significant similarity between the metabolic and environmental data matrices. The first two axes of the coinertia analysis described 57% and 42% of co-structure in the data, respectively. GPP was most strongly related to PAR but also to chlorophyll and temperature. R24 was related primarily to temperature and chlorophyll and oriented ~ 180° from NH4 (perhaps because some O2 uptake was related to nitrification). Days-since-storm of any size had less impact on metabolic variables than these other environmental variables.
Scores computed for each reach by season and by period are shown in Fig. 6A and B, respectively. Centroids of scores for environmental and metabolic variables were close for both cold and warm seasons for the Forested and Meadow reaches (indicating that data matrices were reasonably well-correlated) and slightly further apart for the Reforested reach, presumably because of fewer observations for that reach (Fig. 6A). Meadow reach scores were located to the left of scores for each shaded reach for each season, consistent with the greater PAR, GPP and chlorophyll there. Season scores for each reach were oriented in agreement with the temperature trajectory.
Reach-period score separation was primarily driven by respiration, NDM, P/R and water chemistry (Fig. 6B). Centroids of scores for environmental and metabolic variables were close for the Forested reach in both periods and the Meadow reach in P1, and only slightly further apart for the Meadow 2 and the Reforested reaches. P2 scores were located toward the top of Axis 2 and aligned with alkalinity, NO3 and respiration (higher in P2), whereas P1 scores aligned with NH4, PO4, NDM and P/R (higher in P1). Again, Meadow reach scores were located to the left of shaded reach scores. The error bars associated with centroids for environmental variables of the Forested and Reforested reaches overlapped more than those for metabolic variables for those reaches. The distinct separation of Meadow and shaded reaches indicates the decisive role played by light in regulation of metabolism.
MLR analyses revealed that the relative importance (partial regression coefficient, β) of PAR differed at each reach. PAR was the most important environmental variable in all but one of the GPP, NDM, and P/R models for the shaded reaches but the least important variable in the GPP and NDM models for the Meadow reach and absent from its P/R model (Supplementary Table 3). Temperature was the most important variable in the models for R24 at all reaches. Although PAR was the most important environmental variable in the model for chlorophyll in the Forested reach, Days-since-storm > 2.8 m3 s−1 (our highest threshold) was most important followed by temperature and a suite of chemical factors in the Meadow reach and the model for the Reforested reach was non-significant. PAR and temperature were significantly correlated at the Meadow reach (r = 0.583, P < 0.001), but not at the Forested (r = 0.038) and Reforested (r = 0.035) reaches. Period was absent from the GPP model for the Forested reach and from the R24 and chlorophyll models for the Meadow reach.
Discussion
Factors affecting metabolic patterns and algal biomass
Seasonal patterns and coinertia analysis documented that PAR was a principal driver of GPP and the major factor separating reaches, consistent with other studies in temperate (e.g., Bott et al., 1985; Mulholland et al., 2001; Bernhardt et al, 2022) and subtropical (Bunn et al., 1999; Mosisch et al., 2001) streams. The seasonal pattern of GPP in the Forested reach, with a spring maximum and low rates in summer under full canopy shade, has been seen in other studies (e.g., Hill & Dimick 2002; Roberts et al., 2007). Nutrients had minor influence on this pattern. Mean water column concentrations of NH4, NO3, and PO4 during June–August of P1 were 93%, 92%, and ~ 140%, respectively, of concentrations during March–May. MLR models showed that PAR had greater influence on GPP at the shaded reaches, presumably because PAR reached saturation intensity at the Meadow reach on most days. Period code did not enter the GPP model for the Forested reach because the surrounding area was relatively unchanged during this research (Supplementary Fig. 1), resulting in reasonably similar GPP. The inclusion of Period code in the MLR model for GPP at the Meadow reach reflects effects from both reach geomorphology and measurement technique. The Meadow 2 reach was meandering (Fig. 1) and more deeply incised (Supplementary Fig. 1) than the P1 Meadow reach. Thus, shade from the streambanks and bank vegetation affected periphyton development and P2 metabolism measurements. In contrast, water jackets cast little shade on chambers during P1 metabolism measurements made on the streambank.
The differences in P-I curve parameters between the shaded reaches and Meadow 2 reach could have resulted from differences in predominant algal taxa, higher biomass-specific concentrations of photosynthesis enhancing pigments in algae in the shaded reaches (Richardson et al., 1983), or both. Others have shown that physiological pigment adjustments, while beneficial, could not overcome the effect of reduced light encountered seasonally (Laviale et al., 2009) or resulting from riparian shade (Hill et al., 1995) and we too found that GPP in the Meadow reach exceeded that in the shaded reaches.
The higher chlorophyll concentrations reported for P2 are attributed primarily to the change in sampling protocol although lower streamflow resulting in less periphyton disturbance during P2 may have been a contributing factor. An estimate of chlorophyll from samples of predominant cover types during P2 was the appropriate complement to open system metabolism measurements but fewer samples in P2 prevented verification of the patterns observed during P1.
Coinertia and MLR analyses indicated that temperature was the major driver of respiration, given its large seasonal variation, but measurement method also affected respiration results. The trays used in chamber measurements during P1 restricted water exchange with underlying sediments whereas open system measures fully captured hyporheic activity. Thus, P1 annual respiration estimates for the Forested and Meadow reach were 47% and 76% of P2 values for those reaches, and Period was included in the MLR for the Forested reach. Battin et al. (2003), using mini-piezometers, estimated that hyporheic respiration in our Forested reach was equivalent to ~ 1 g O2 m−2 day−1, which is 38% of our P2 estimate for the whole stream and thus in rough agreement with the difference between our P1 and P2 estimates. Similarly, Fellows et al. (2001), using a combination of chamber and open system measurements, documented that the hyporheos contributed from 40 to 93% of whole system respiration in four New Mexico streams.
Respiration was also influenced by chlorophyll, but to a lesser extent. Hall and Beaulieu (2013) estimated that respiration by autotrophs and inseparably attached heterotrophs was 44% of GPP. Using 44%, respiration attributed to autotrophs during P2 for the Forested and Meadow 2 reach averaged 28% and 33% of total respiration, respectively, (from Table 3) and 30% for the Reforested reach (mean of cold and warm seasons from Table 6). Thus, ~ 70% of total respiration in all reaches reflects the decomposition of algal or allochthonous detritus, both particulate and dissolved, by heterotrophic organisms.
In P1, R24 was coupled significantly with GPP and the respiratory demand could be met by GPP in both reaches. The annual Meadow production exceeded Meadow respiration, generating a net production (NDM) of 787 g O2 m−2 year−1, and a P/R ratio of 1.66 (Table 3). Similarly, annual Forest production exceeded Forest respiration, generating a net production (NDM) of 31 g O2 m−2 year−1, and a P/R ratio of 1.07. In P2, however, when we used open system measurements that included hyporheic respiration, the metabolic picture was quite different. Annual Meadow respiration exceeded Meadow production, and net metabolism (NDM) in the Meadow was -404 g O2 m−2 year−1, for a P/R ratio of 0.74 (Table 3). Likewise in the Forest respiration exceeded production, for a net metabolism (NDM) of -363 g O2 m−2 year−1, and a P/R ratio of 0.63. In both reaches, the inclusion of hyporheic respiration flipped the annual net production from positive to negative and, correspondingly, the P/R ratio from > 1 to < 1.
The P2 annual NDM in Forested reach of -363 g O2 m−2 year−1 (Table 3), translated to carbon using a respiratory quotient of 0.90 (rounding up 0.85 from Wetzel, 2001, p. 189), results in a consumption of 123 g C m−2 year−1. The Forest receives annual litterfall of 156 g C m−2 year−1 (50% of AFDM), approximately balancing the net consumption. Finding net heterotrophy in the Meadow 2 reach is not unusual as there are several reports of heterotrophy in open reaches, particularly in rural and agricultural watersheds (Young & Huryn, 1999; Mulholland et al., 2001; Griffiths et al., 2013; Roley et al., 2014) and we found this condition in 5 of 12 other nearby streams (Bott et al., 2006b). However, identifying the source of the supporting carbon has been largely unresolved. The annual NDM in Meadow 2 of − 404 g O2 m−2 year−1 (Table 3) equates to a carbon consumption of 136 g C m−2 year−1. Grasses and herbs from the surrounding floodplain delivered either in overland flow or from slumping streambanks undoubtedly contribute, but only in relatively small amounts. Uptake of labile DOC has been shown to be appreciable in White Clay Creek (Kaplan & Bott, 1985; Kaplan et al., 2008), but the relatively low concentrations of labile DOC in ground- and soil–water sources (McLaughlin & Kaplan, 2013) imply that most of the labile DOC originates from instream primary production or allochthonous particles. We suspect that the major source of carbon that supports the heterotrophy is supplied as fine particulate organic matter (FPOM) delivered from the upstream, largely agricultural, landscape. The annual transport of FPOM in White Clay Creek is 56,000 kg AFDM or 28,000 kg C year−1, assuming 50% C, 97% of which is delivered during storms (Newbold et al., 1997). At 136 g C m−2 year−1, 1 km of meadow reach (3 m wide) would respire 409 kg C year−1, ~ 1.5% of the transported FPOM. That is, there is sufficient carbon delivered to support the meadow heterotrophy, provided that it is entrapped and mineralized at sufficient rates. A recent survey showed that the Meadow 2 reach had a habitat-weighted average C content in the sediments of 800 g C m−2 based on the stratified sampling of major bed forms, while that of the Forested reach was 330 g C m−2 (Oviedo-Vargas, personal communication). It seems likely that this stock of sedimentary carbon is maintained and renewed as storms deliver carbon and rework the sediments. The stock of 800 g C m−2 would support the average NDM of − 136 g C m−2 year−1at a turnover time of 5.9 years, which is comparable to the turnover time of 6.4 years for organic matter in sediments of woodland streams (Hedin, 1990). That the turnover time is shorter than typical of in situ soil carbon is consistent with recent recognition that soil carbon becomes more bioavailable and degrades more rapidly upon disturbance and transport to streams and rivers (e.g., Cole & Caraco, 2001; Mayorga et al., 2005; McCallister & Georgio, 2012; Zhao et al., 2021). That NDM was more negative in the meadow than in the upstream forest we attribute to the meadow’s greater storage of sedimentary carbon (800 vs. 330 g C m−2), which in turn reflects a more incised and less stable meadow channel. Along with the greater C content in Meadow 2 sediments, we found a greater percentage of fine substrata in the Meadow 2 reach, which would provide greater surface area for microbial attachment and activity (Marxsen, 2001; Mori et al., 2017).
Concentrations of several water chemical constituents differed between periods (Supplementary Table 1) and contributed to reach separation in coinertia analyses. Lower PO43− and NH4+ concentrations during P2 presumably reflect watershed-scale land preservation resulting from the implementation of conservation easements, best management practices and reforestation. Higher NO3 concentrations in P2 are possibly the legacy of fertilizer application rates that increased during the 1980s and subsequently mixed into old groundwater (Van Meter et al., 2018). The higher P2 concentrations of Ca2+ and Na+ may reflect increased use of road salt, although a corresponding increase in Cl− was not statistically significant. Higher P2 concentrations may also be related to lower streamflow resulting in less dilution of groundwater sources. Overall, coinertia and MLR analyses indicated that nutrients had less impact on metabolic parameters than PAR or temperature.
Storms had negative impacts on GPP, R24, and chlorophyll a, and impacts increased with storm size. The reductions in GPP and R24 that we observed, even for the largest storms, were relatively modest when compared to other reports. We first note that our estimates of reductions (Table 6) were based on post-storm measurements that averaged 3.9 days after peak flow, and thus were attenuated by recovery that occurred during this time. Based on the recovery rates (Table 7), our estimates of resistance, R, were approximately 10% higher (less impactful) than had they been measured immediately after the storm. For example, where we observed a reduction of 56% (R = 0.44), the immediate post-storm reduction actually may have been 60% (R = 0.40). Our storm flows ranged up to ~ 10 m3 s−1, for which the estimated average reductions were 56% for GPP, 39% for R24, and 81% for chlorophyll a. In contrast, Reisinger et al. (2017) reported a median reduction of 88% for GPP and 79% for ecosystem respiration (ER) among 11 floods in urbanized watersheds. Other studies in urban or suburban watersheds have observed at least some reductions ranging from 60 to 99% (Beaulieu et al., 2013; Larsen & Harvey, 2017; Qasem et al., 2019). Urbanized streams are typically “flashy”—subject to sudden, large increases in flow emanating from impervious surfaces (Walsh et al., 2005). Grimm and Fisher (1989) reported similarly high reductions in chlorophyll (> 90%) in a desert stream, which also was characterized as flashy. There have been few other studies of storm effects in streams that are not affected by high impervious surface area. In a woodland stream, Roberts et al. (2007) reported that in one of two large storms studied, GPP was reduced by 90%; however, in the other, an autumn storm, GPP actually increased because leaf litter was cleared from the streambed.
Perhaps the study most comparable to ours is that of Uehlinger (2000) who reported effects of storms on GPP and ER in two Swiss rivers that were similar to the White Clay watershed in land cover and slope, although much larger in drainage area. In the River Necker, the smaller of the two, Uehlinger reported that storms exceeding 28 m3 s−1 reduced GPP by 53% and ER by 24%, on average. We used bed shear stress to scale flows from the Necker to the much smaller flows of White Clay Creek. We estimated that 28 m3 s−1 in the River Necker produced a bed shear of 53 Newtons m−2 and that the same bed shear would occur in White Clay Creek at 2.8 m3 s−1. Based on our estimates of resistance (Table 6), the average reductions from all storms > 2.8 m3 s−1 in the 50-year record are 45% for GPP and 30% for R24. Thus, it appears that at comparable bed shears, White Clay Creek and the River Necker exhibit comparable resistance, with White Clay being possibly more resistant to GPP reductions and the Necker more resistant to R24 reductions.
Our results suggested a threshold flow below which storms might stimulate metabolism, as has been observed by others. Qasem et al. (2019) observed frequent stimulation of ER and less for GPP after relatively small storms. Acuña et al. (2004), Roberts et al. (2007), and Larsen & Harvey (2017) all observed stimulation attributable to the clearing of overlying leaf litter or the delivery of fine particulate organic matter.
We estimated recovery of metabolism in 2–3 weeks in the warm months and 7–9 weeks in the cold season. Our warm season recovery times were longer than those reported by Reisinger et al. (2017) (~ 1–2 weeks), Qasem et al. (2019) (< 1 week) and Roberts et al. (2007) (< 1 week). However, our recovery times were similar to those of Uehlinger (2000) who reported recovery of P/R ratios within three weeks, but longer in winter months. Our result showing that GPP in the autumn of 1972 was only 61% of that in 1971 also indicates that frequent storms, not necessarily severe, will reduce algal productivity by curtailing full recovery. We saw no seasonal effect on the recovery time of chlorophyll a recovery, but attribute this to the relatively limited sample size. The annually averaged recovery time of four weeks was between our warm- and cold-season times for metabolism and longer than reported for chlorophyll a by Grimm and Fisher (1989) (~ 2 weeks).
The importance of storms as a regulator of ecosystem metabolism depends not only on the response to specific storm, but also on the flow regime—the distribution in magnitude and timing of peak flows. Following Cronin et al. (2007), we used the White Clay Creek’s 50-year (1969–2018) hydrograph to simulate a daily record in which metabolism is set back in response to each flow peak, begins to recover, but is again set back by the next peak regardless of whether full recovery has occurred. More formally, daily values of resistance, R, were calculated from Eq. (2) using the regression coefficients of Table 6 and maximum daily flow. The algorithm tracked daily values of the variable, f, which represented GPP, R24, or chlorophyll stock, as a fraction of the value (1.0) it would have in the absence of storm influence. To reflect storm setback, f (initialized at f = 1) was multiplied by R on each day (or “storm day”) for which R < 1, but only once and by the minimum value of R when storm days were consecutive. On all other days (R ≥ 1), f increased by the respective linearized recovery rate (Table 7) until the next storm day or until full recovery (f = 1) was attained. The resulting 50-year averages, fave, expressed as percent reduction attributable to storms, or [100*(1−f)], were 22, 19 and 25% for GPP, R24, and chlorophyll a, respectively.
By comparing measures of annual metabolism among 222 streams and rivers in the conterminous U. S., Bernhardt et al. (2022) found that both GPP and ER (equivalent to our R24) were ~ 60% lower in those with highly variable flow regimes (lowest quartile) than in those with the most stable flow regimes (uppermost quartile). We infer from these results that, for a stream or river of average, or median, flow variability, storms might be expected to reduce metabolism by ~ 30%. Using the metric employed by Bernhardt et al. (L-moment daily discharge), we find that White Clay Creek’s flow regime is very near the median among the rivers that they analyzed. Thus, our estimate for White Clay Creek of a ~ 20% reduction by storms is less than suggested by the analysis of Bernhardt et al. Given that many factors (including uncertainties in data analysis) may account for this difference, we find it noteworthy that two very different approaches (ours, a mechanistic synthesis of measurements from a single stream; theirs, a statistical comparison among many streams and rivers) are in broad agreement.
Our long-run reductions suggest that in streams such as White Clay Creek which are unaffected by urbanization, storms do have an important, but not necessarily dominant, influence on metabolism. Coinertia and MLR analyses bore this out, documenting that the other factors associated with reach metabolism discussed above (PAR, temperature, chlorophyll) were more important to metabolic function than storms. More work on the complex interactions between storms, other environmental parameters, periphyton growth form and community composition, and ecosystem metabolism is needed.
Influence of riparian zone management on metabolism and algae
In Fig. 7, P1 and P2 annual GPP values are placed in the context of published data (Bott et al., 1985) for the Forested reach, upstream 1st- and 2nd-order reaches, and a nearby 5th-order reach, all also forested, obtained with chamber incubations in situ during 1976. GPP gradually increased along this forested continuum to a maximum in the 5th-order reach where channel widening forced separation of the tree canopy and light reached the streambed through a relatively shallow water column. However, GPP in the 3rd-order Meadow and Meadow 2 reaches was ~ 3 and 1.6 times greater, respectively, than in the 5th-order reach despite the sixfold greater width (17.4 m) of the latter, which underscores the disruption caused by riparian deforestation to the trajectory of GPP expected for a continuum of reaches in natural condition in this region.
Annual estimates of GPP in the Forested (F) and Meadow (M) reaches made during P1 compared to measurements made in 1976 in forested 1st- through 3rd-order reaches in White Clay Creek and 5th-order Buck and Doe Run (data from Bott et al., 1985) and P2 measurements in the Forested, Reforested (R), and Meadow 2 (M2) reaches. Values are from Table 3 for the Forested and Meadow reaches and based on Table 4 for the Reforested reach. Information in the x-axis labels is as follows: Stream order- Reach type- Period (if from this study) and Year(s) of measurement
We do not have details concerning the rate of change in GPP in the Reforested reach, but we can state that the change in metabolic status we report occurred within 16–21 years of planting. It is noteworthy that riparian trees planted along agriculturally impacted streams in southeast Australia restored net ecosystem productivity to rates typical of forested streams within 20 years (Giling et al., 2013). In a study of thirty 1st-order mid-Atlantic Piedmont streams the recovery of other, non-metabolic, parameters occurred in a roughly similar time frame (Orzetti et al., 2010).
While riparian shade reduces areal estimates of GPP, the greater width of a shaded stream helps to offset those lower values. Productivity in the Forested reach during P2 was 53% of Meadow 2 productivity when expressed per unit area, but 86% of Meadow 2 productivity when expressed per unit length of stream. Another consideration is that the food quality of algae in a shaded reach can be higher, providing a benefit for the stream food web. Diatoms, predominant in our shaded reaches, are rich in polyunsaturated fatty acids (PUFAs), compounds that nearly all benthic invertebrates and fish must acquire from their food sources (Torres-Ruiz et al., 2007). Field studies have shown that the concentration of, e.g., eicosapentaenoic acid, a PUFA indicator of high food quality found in diatoms, was greater in periphyton from shaded reaches (Cashman et al., 2013; Guo et al., 2016). Other benefits of riparian shade have been documented for streams in rural landscapes (Sweeney et al., 2004; McTammany et al., 2007; Burrell et al., 2014) and in suburban and urban areas (Alberts et al., 2016).
A review of literature concerning riparian forest buffers led Sweeney and Newbold (2014) to recommend a buffer width of ≥ 30 m to protect the physical, biological, and chemical characteristics of streams draining ≤ 5th -order watersheds. This approach to stream restoration is grounded in knowledge of stream–watershed interactions and stream ecosystem function. Furthermore, it is self-sustaining and easier to accomplish than costly and invasive engineering projects involving channel reconfiguration.
Conclusions
Returning to our objectives, we have presented values for daily, seasonal, and annual metabolic rates in forested and meadow reaches in a stream typical of many in the region. GPP and R were greater in the meadow reach in both periods of measurement. Open system measurements documented the predominance of R in all study reaches. We have evaluated the factors influencing metabolism and supported our interpretations quantitatively in an ecosystem context. The respiration demand in the Forested reach was met by litter inputs, and in the Meadow 2 reach by FPOM delivered from the upstream landscape. Storms affected metabolism but their influence was not as strong as that of PAR and chlorophyll on GPP, or temperature on respiration. This may be because our rural study stream, which was designated an Exceptional Value stream by the State of Pennsylvania in 1984, has a less flashy hydrograph than desert streams or ones impacted by development in urban-suburban areas. Although GPP was unnaturally high in meadow reaches, we have documented that reforestation of the riparian zone of one of them has restored metabolism there close to that measured in a reach located in mature forest within two decades.
Data availability
The dataset used in this study is available from the Environmental Data Initiative (EDI) as https://doi.org/10.6073/pasta/bd4f4a90fe4942c10aedd978f81d3b09
Change history
23 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10750-023-05403-3
References
Acuña, V., A. Giorgi, I. Muñoz, U. Uehlinger & S. Sabater, 2004. Flow extremes and benthic organic matter shape the metabolism of a headwater Mediterranean stream. Freshwater Biology 49: 960–971.
Alberts, J. M., J. J. Beaulieu & I. Buffam, 2016. Watershed land use and seasonal variation constrain the influence of riparian canopy cover on stream ecosystem metabolism. Ecosystems 20: 553–567.
American Public Health Association [APHA], 1971. Standard methods for the examination of water and wastewater. American Public Health Association, New York.
Appling, A. P., J. S. Read, L. A. Winslow, M. Arroita, E. S. Bernhardt, N. A. Griffiths, R. O. Hall Jr., J. W. Harvey, J. B. Heffernan, E. H. Stanley, E. G. Stets & C. B. Yackulic, 2018. Data Descriptor: the metabolic regimes of 356 rivers in the United States. U. S. Geological Survey https://doi.org/10.5066/F70864KX.
Arcement, G. J., Jr. & V. R. Schneider, 1989. Guide for selecting Manning's roughness coefficients for natural channels and flood plains. USGS Water-supply Paper 2339.
Arroita, M., A. Elongi & R. O. Hall Jr., 2019. Twenty years of daily metabolism show riverine recovery following sewage abatement. Limnology & Oceanography 64: 577–592.
Battin, T. J., L. A. Kaplan, J. D. Newbold & S. P. Hendricks, 2003. A mixing model analysis of stream solute dynamics and the contribution of a hyporheic zone to ecosystem function. Freshwater Biology 48: 995–1014.
Beaulieu, J. J., C. P. Arango, D. A. Balz & W. D. Shuster, 2013. Continuous monitoring reveals multiple controls on ecosystem metabolism in a suburban stream. Freshwater Biology 58: 918–937.
Bernhardt, E. S., P. Savoy, M. J. Vlah, A. P. Appling, L. E. Koenig, R. O. Hall Jr., M. Arroita, J. R. Blaszczak, A. M. Carter, M. Cohen, J. W. Harvey, J. B. Heffernan, A. M. Helton, J. D. Hosen, L. Kirk, W. H. McDowell, E. H. Stanley, C. B. Yackulic & N. B. Grimm, 2022. Light and flow regimes regulate the metabolism of rivers. Proceedings of the National Academy of Sciences USA 119: e2121976119. https://doi.org/10.1073/pnas.2121976119.
Bernot, M. J., D. J. Sobota, R. O. Hall Jr., P. J. Mulholland, W. K. Dodds, J. R. Webster, J. L. Tank, L. R. Ashkenas, L. W. Cooper, C. N. Dahm, S. V. Gregory, N. B. Grimm, S. K. Hamilton, S. L. Johnson, W. H. McDowell, J. L. Meyer, B. Peterson, G. C. Poole, H. M. Valett, C. Arango, J. J. Beaulieu, A. J. Burgin, C. Crenshaw, A. M. Helton, L. Johnson, J. Merriam, B. R. Niederlehner, J. M. O’Brien, J. D. Potter, R. W. Sheibley, S. M. Thomas & K. Wilson, 2010. Inter-regional comparison of land-use effects on stream metabolism. Freshwater Biology 55: 1874–1890.
Bott, T. L., 2006. Primary productivity and community respiration. In Hauer, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology 2nd ed. Elsevier, Amsterdam: 663–690.
Bott, T. L., J. T. Brock, C. S. Dunn, R. J. Naiman, R. W. Ovink & R. C. Petersen, 1985. Benthic community metabolism in four temperate stream systems: an inter-biome comparison and evaluation of the river continuum concept. Hydrobiologia 123: 3–45.
Bott, T. L., D. S. Montgomery, J. D. Newbold, D. B. Arscott, C. L. Dow, A. K. Aufdenkampe, J. K. Jackson & L. A. Kaplan, 2006a. Ecosystem metabolism in streams of the Catskill Mountains (Delaware and Hudson River watersheds) and Lower Hudson Valley. Journal of the North American Benthological Society 25: 1018–1044.
Bott, T. L., J. D. Newbold & D. B. Arscott, 2006b. Ecosystem metabolism in Piedmont streams: reach geomorphology modulates the influence of riparian vegetation. Ecosystems 9: 398–421.
Bunn, S. E., P. M. Davies & T. D. Mosisch, 1999. Ecosystem measures of river health and their response to riparian and catchment degradation. Freshwater Biology 41: 333–345.
Burrell, T. K., J. M. O’Brien, S. E. Graham, K. S. Simon, J. S. Harding & A. R. McIntosh, 2014. Riparian shading mitigates stream eutrophication in agricultural catchments. Freshwater Science 33: 73–84.
Cashman, M. J., J. D. Wehr & K. Truhn, 2013. Elevated light and nutrients alter the nutritional quality of stream periphyton. Freshwater Biology 58: 1447–1457.
Chessman, B. C., 1985. Estimates of ecosystem metabolism in the LaTrobe River, Victoria. Australian Journal of Marine and Freshwater Research 36: 873–880.
Clapcott, J. E., R. G. Young, E. O. Goodwin & J. R. Leathwick, 2010. Exploring the response of functional indicators of stream health to land-use gradients. Freshwater Biology 55: 2181–2199.
Cole, J. J. & N. F. Caraco, 2001. Carbon in catchments: connecting terrestrial carbon losses with aquatic metabolism. Marine and Freshwater Research 51: 101–110.
Cronin, G., J. H. McCutchan Jr., J. Pitlick & W. M. Lewis Jr., 2007. Use of shields stress to reconstruct and forecast changes in river metabolism. Freshwater Biology 52: 1587–1601.
Cummins, K. W., J. F. Sedell, F. J. Swanson, G. W. Minshall, S. G. Fisher, C. E. Cushing, R. C. Petersen & R. L. Vannote, 1983. Organic matter budgets for stream ecosystems: problems in their evaluation. In Barnes, J. R. & G. W. Minshall (eds), Stream Ecology: Application and Testing of General Ecological Theory. Plenum, New York: 299–353.
Dodds, W. K., A. M. Veach, C. M. Ruffing, D. M. Larson, J. L. Fischer & K. H. Costigan, 2013. Abiotic controls and temporal variability of river metabolism: multiyear analyses of Mississippi and Chattahoochee River data. Freshwater Science 32: 1073–1087.
Dolédec, S. & D. Chessel, 1994. Co-inertia analysis: an alternative method for studying species-environment relationships. Freshwater Biology 31: 277–294.
Draper, N. R. & H. Smith, 1966. Applied regression analysis. Wiley, New York.
Dray, S., D. Chessel & J. Thioulouse, 2003. Co-inertia analysis and the linking of ecological data tables. Ecology 84: 3078–3089.
Duffer, W. R. & T. C. Dorris, 1966. Primary productivity in a southern Great Plains stream. Limnology and Oceanography 11: 143–151.
Edwards, R. W. & M. Owens, 1962. The effects of plants on river conditions. IV. The oxygen balance of a chalk stream. Journal of Ecology 50: 207–220.
Fellows, C. S., H. M. Valett & C. N. Dahm, 2001. Whole-stream metabolism in two montane streams: contribution of the hyporheic zone. Limnology and Oceanography 46: 523–531.
Fisher, S. G., 1977. Organic matter processing by a stream-segment ecosystem: Fort River, Massachusetts, U.S.A. Internationale Revue Der Gesamten Hydrobiologie 62: 701–727.
Fisher, S. G. & G. E. Likens, 1973. Energy flow in Bear Brook, New Hampshire: an integrative approach to stream ecosystem metabolism. Ecological Monographs 43: 421–439.
Fisher, S. G., L. J. Gray, N. B. Grimm & D. E. Busch, 1982. Temporal succession in a desert stream ecosystem following flash flooding. Ecological Monographs 52: 93–110.
Fisher, T. R., A. B. Gustafson, G. M. Radcliffe, K. L. Sundberg & J. C. Stevenson, 2003. A long-term record of photosynthetically available radiation (PAR) and total solar energy at 38.6°N, 78.2° W. Estuaries 26: 1450–1460.
Flemer, D. A., 1970. Primary productivity of the North Branch of the Raritan River, New Jersey. Hydrobiologia 35: 273–296.
Giling, D. P., M. R. Grace, R. MacNally & R. M. Thompson, 2013. The influence of native replanting on stream ecosystem metabolism in a degraded landscape: can a little vegetation go a long way? Freshwater Biology 58: 2601–2613.
Griffiths, N. A., J. L. Tank, T. V. Royer, S. S. Roley, E. J. Rosi-Marshall, M. R. Whiles, J. J. Beaulieu & L. T. Johnson, 2013. Agricultural land use alters the seasonality and magnitude of stream metabolism. Limnology and Oceanography 58: 1513–1529.
Grimm, N. B. & S. G. Fisher, 1984. Exchange between interstitial and surface water: implications for stream metabolism and nutrient cycling. Hydrobiologia 111: 219–228.
Grimm, N. B. & S. G. Fisher, 1989. Stability of periphyton and macroinvertebrates to disturbance by flash floods in a desert stream. Journal of the North American Benthological Society 8: 293–307.
Guo, F., M. J. Kainz, F. Sheldon & S. E. Bunn, 2016. Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 181: 449–462.
Hall, R. O., Jr. & J. L. Tank, 2005. Correcting whole-stream estimates of metabolism for groundwater inputs. Limnology and Oceanography Methods 3: 222–229.
Hall, R. O., Jr. & J. J. Beaulieu, 2013. Estimating autotrophic respiration in streams using daily metabolism data. Freshwater Science 32: 507–516.
Hedin, L. O., 1990. Factors controlling sediment community respiration in woodland stream ecosystems. Oikos 57: 94–105.
Hill, W. R. & S. M. Dimick, 2002. Effects of riparian leaf dynamics on periphyton photosynthesis and light utilisation efficiency. Freshwater Biology 47: 1245–1256.
Hill, W. R., M. G. Ryon & E. M. Schilling, 1995. Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76: 1297–1309.
Hoellein, T. J., D. A. Bruesewitz & D. C. Richardson, 2013. Revisiting Odum (1956): a synthesis of aquatic ecosystem metabolism. Limnology and Oceanography 58: 2089–2100.
Hoskin, C. M., 1959. Studies of oxygen metabolism of streams of North Carolina. Publications of the Institute of Marine Science of Texas 6: 186–192.
Houser, J. N., P. J. Mulholland & K. O. Maloney, 2005. Catchment disturbance and stream metabolism: patterns in ecosystem respiration and gross primary production along a gradient of upland soil and vegetation disturbance. Journal of the North American Benthological Society 24: 538–552.
Hynes, H. B. N., 1970. The Ecology of Running Waters. University of Toronto Press, Toronto.
Hynes, H. B. N., 1975. The stream and its valley. Verhandlungen Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 19: 1–15.
Izagirre, O., U. Agirre, M. Bermejo, J. Pozo & A. Elosegi, 2008. Environmental controls of whole-stream metabolism identified from continuous monitoring of Basque streams. Journal of the North American Benthological Society 27: 252–268.
Jassby, A. D. & T. Platt, 1976. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnology and Oceanography 21: 540–547.
Jones, J. B., Jr., 1995. Factors controlling hyporheic respiration in a desert stream. Freshwater Biology 34: 91–99.
Kaplan, L. A. & T. L. Bott, 1985. Acclimation of stream-bed heterotrophic microflora: metabolic responses to dissolved organic matter. Freshwater Biology 15: 479–492.
Kaplan, L. A., T. N. Wiegner, J. D. Newbold, P. H. Ostrom & H. Gandhi, 2008. Untangling the complex issue of dissolved organic carbon uptake: a stable isotope approach. Freshwater Biology 53: 855–864.
Larsen, L. G. & J. W. Harvey, 2017. Disrupted carbon cycling in restored and unrestored urban streams: critical timescales and control. Limnology and Oceanography 62: S160–S182.
Laviale, M., J. Prygiel, Y. Lemoine, A. Courseaux & A. Créach, 2009. Stream periphyton photoacclimation response in field conditions: effect of community development and seasonal changes. Journal of Phycology 45: 1072–1082.
Lorenzen, C. J., 1967. Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnology and Oceanography 12: 343–346.
Marxsen, J., 2001. Bacterial production in different streambed habitats of an upland stream: sandy versus coarse gravelly sediments. Archiv Für Hydrobiologie 152: 543–565.
Marzolf, E. R., P. J. Mulholland & A. J. Steinman, 1994. Improvements to the diurnal upstream-downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Canadian Journal of Fisheries and Aquatic Sciences 51: 1591–1599.
Marzolf, E. R., P. J. Mulholland & A. J. Steinman, 1998. Reply: improvements to the diurnal upstream-downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Canadian Journal of Fisheries and Aquatic Sciences 55: 1786–1787.
Mayorga, E., A. K. Aufdenkampe, C. A. Masiello, A. V. Krusche, J. I. Hedges, P. D. Quay, J. E. Richey & T. A. Brown, 2005. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature 436: 538–541.
McCallister, S. L. & P. A. Del Giorgio, 2012. Evidence for the respiration of ancient terrestrial organic C in northern temperate lakes and streams. Proceedings of the National Academy of Sciences, USA 109: 16963–16968.
McCutchan, J. H., Jr., J. F. Saunders III., W. M. Lewis Jr. & M. G. Hayden, 2002. Effects of groundwater flux on open-channel estimates of stream metabolism. Limnology and Oceanography 47: 321–324.
McLaughlin, C. & L. A. Kaplan, 2013. Biological lability of dissolved organic carbon in stream water and contributing terrestrial sources. Freshwater Science 32: 1219–1230.
McTammany, M. E., J. R. Webster, E. F. Benfield & M. A. Neatrour, 2003. Longitudinal patterns of metabolism in a southern Appalachian river. Journal of the North American Benthological Society 22: 359–370.
McTammany, M. E., E. F. Benfield & J. R. Webster, 2007. Recovery of stream ecosystem metabolism from historical agriculture. Journal of the North American Benthological Society 26: 532–545.
Meyer, J. L. & R. T. Edwards, 1990. Ecosystem metabolism and turnover of organic carbon along a blackwater river continuum. Ecology 71: 668–677.
Minshall, G. W., R. C. Petersen, K. W. Cummins, T. L. Bott, J. R. Sedell, C. E. Cushing & R. L. Vannote, 1983. Interbiome comparison of stream ecosystem dynamics. Ecological Monographs 53: 1–25.
Minshall, G. W., R. C. Petersen, T. L. Bott, C. E. Cushing, K. W. Cummins, R. L. Vannote & J. R. Sedell, 1992. Stream ecosystem dynamics of the Salmon River, Idaho: an 8th-order system. Journal of the North American Benthological Society 11: 111–137.
Mori, N., B. Debeljak, K. Kocman & R. Simčič, 2017. Testing the influence of sediment granulometry on heterotrophic respiration with a new laboratory flow-through system. Journal of Soils and Sediments 17: 1939–1947.
Mosisch, T. D., S. E. Bunn & P. M. Davies, 2001. The relative importance of shading and nutrients on algal production in subtropical streams. Freshwater Biology 46: 1269–1278.
Mulholland, P. J., C. S. Fellows, J. L. Tank, N. B. Grimm, J. R. Webster, S. K. Hamilton, E. Martí, L. Ashkenas, W. B. Bowden, W. K. Dodds, W. H. McDowell, M. J. Paul & B. J. Peterson, 2001. Inter-biome comparison of factors controlling stream metabolism. Freshwater Biology 46: 1503–1517.
Neter, J., W. Wasserman & M. H. Kutner, 1990. Applied Linear Statistical Models, 3rd ed. Irwin, Homewood.
Newbold, J. D., T. L. Bott, L. A. Kaplan, B. W. Sweeney & R. L. Vannote, 1997. Organic matter dynamics in White Clay Creek, Pennsylvania, USA. Journal of the North American Benthological Society 16: 46–50.
Odum, H. T., 1956. Primary production in flowing waters. Limnology and Oceanography 1: 102–117.
Orzetti, L. L., R. C. Jones & R. F. Murphy, 2010. Stream condition in Piedmont streams with restored riparian buffers in the Chesapeake Bay watershed. Journal of the American Water Resources Association 46: 473–485.
Owens, M., 1974. Measurements on non-isolated natural communities in running waters. In Vollenweider, R. A. (ed), A Manual on Methods for Measuring Primary Production in Aquatic Environments. IPB Handbook 12 2nd ed. Blackwell, Oxford: 111–119.
Owens, M., R. W. Edwards & J. W. Gibbs, 1964. Some reaeration studies in streams. International Journal of Air and Water Pollution 8: 469–486.
Patrick, R., 1996. Chapter 5. Headwaters—Mid Atlantic Piedmont Streams. In: Rivers of the United States, The eastern and southeastern states, vol. III. Wiley, New York: 169–236.
Pusch, M., 1996. The metabolism of organic matter in the hyporheic zone of a mountain stream, and its spatial distribution. Hydrobiologia 323: 107–118.
Qasem, K., S. Vitousek, B. O’Connor & T. Hoellein, 2019. The effect of floods on ecosystem metabolism in suburban streams. Freshwater Science 38: 412–424.
Reisinger, A. J., E. J. Rosi, H. A. Bechtold, T. R. Doody, S. S. Kaushal & P. M. Groffman, 2017. Recovery and resilience of urban stream metabolism following Superstorm Sandy and other floods. Ecosphere 8: e01776. https://doi.org/10.1002/ecs2.1776.
Richards, K., 1982. Rivers: Form and Process in Alluvial Channels. Methuen & Company, New York.
Richardson, K., J. Beardall & J. A. Raven, 1983. Adaptation of unicellular algae to irradiance: an analysis of strategies. New Phytologist 93: 157–191.
Roberts, B. J., P. J. Mulholland & W. R. Hill, 2007. Multiple scales of temporal variability in ecosystem metabolism rates: results from 2 years of continuous monitoring in a forested headwater stream. Ecosystems 10: 588–606.
Roley, S. S., J. L. Tank, N. A. Griffiths, R. O. Hall Jr. & R. T. Davis, 2014. The influence of floodplain restoration on whole-stream metabolism in an agricultural stream: insights from a 5-year continuous data set. Freshwater Science 33: 1043–1059.
Runkel, R. L, 1998. One dimensional transport with inflow and storage (OTIS): a solute transport model for streams and rivers. US Geological Survey Water Resources Investigation Report 98–4018.
Solorzano, L., 1969. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceanography 14: 799–801.
Sweeney, B. W. & J. D. Newbold, 2014. Streamside forest buffer width needed to protect stream water quality, habitat and organisms: a literature review. Journal of the American Water Resources Association 50: 560–584.
Sweeney, B. W., T. L. Bott, J. K. Jackson, L. A. Kaplan, J. D. Newbold, L. J. Standley, W. C. Hession & R. J. Horwitz, 2004. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proceeding of the National Academy of Sciences, USA 101: 14132–14137.
Torres-Ruiz, M., J. D. Wehr & A. A. Perrone, 2007. Trophic relations in a stream food web: importance of fatty acids for macroinvertebrate consumers. Journal of the North American Benthological Society 26: 509–522.
Tsivoglou, H. C. & L. A. Neal, 1976. Tracer measurement of reaeration: III. Predicting the reaeration capacity of inland streams. Journal of the Water Pollution Control Federation 489: 2669–2689.
U.S. Environmental Protection Agency [US EPA], 1993. Determination of inorganic substances in environmental samples. EPA-600/R-93-100.
Uehlinger, U., 2000. Resistance and resilience of ecosystem metabolism in a flood-prone river system. Freshwater Biology 45: 319–332.
Uehlinger, U., 2006. Annual cycle and inter-annual variability of gross primary production and ecosystem respiration in a floodprone river during a 15-year period. Freshwater Biology 51: 938–950.
Uehlinger, U. & M. W. Naegeli, 1998. Ecosystem metabolism, disturbance, and stability in a prealpine gravel bed river. Journal of the North American Benthological Society 17: 165–178.
Van Meter, K. J., P. Van Cappellen & N. B. Basu, 2018. Legacy nitrogen may prevent achievement of water quality goals in the Gulf of Mexico. Science 360: 427–430.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Walsh, C. J., A. H. Roy, J. W. Feminella, P. D. Cottinghim, P. M. Groffman & R. P. Morgan II., 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24: 706–723.
Wetzel, R. G., 2001. Limnology: Lake and River Ecosystems, 3rd ed. Academic Press, San Diego.
Wiley, M. J., L. L. Osbourne & R. W. Larimore, 1990. Longitudinal structure of an agricultural prairie river system and its relationship to current stream ecosystem theory. Canadian Journal of Fisheries and Aquatic Sciences 47: 373–384.
Young, R. G. & A. D. Huryn, 1996. Interannual variation in discharge controls ecosystem metabolism along a grassland river continuum. Canadian Journal of Fisheries and Aquatic Sciences 53: 2199–2211.
Young, R. G. & A. D. Huryn, 1998. Comment: improvements to the diurnal upstream-downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Canadian Journal of Fisheries and Aquatic Sciences 55: 1784–1785.
Young, R. G. & A. D. Huryn, 1999. Effects of land use on stream metabolism and organic matter turnover. Ecological Applications 9: 1359–1376.
Zhao, M., L. Jacobs, S. Boullion & G. Govers, 2021. Rapid soil organic carbon decomposition in river systems: effects of the aquatic microbial community and hydrodynamical disturbance. Biogeosciences 18: 1511–1523.
Acknowledgements
We thank Roger Baumgartner, Chris Cain, Chad Colburn, Aaron deLong, Michael Hartshorne, Brian Hughes, Michael Humphreys, David Montgomery, Nancy Parsons, Jean Pierson, Floyd Ritter, Rajiv Shah, William Shaw, Robert Smith, David VanHorn, Burley Vannote, and Bruce Wallace for field and laboratory assistance; Melanie Arnold, Bruce Boydell, Heather Brooks, Charles Dow, Cindy Dunn, Christa Evans, Robert Fox, John Hendrickson, Michelle Stafford, Tim Smith, and Erica Zwilling for data analytical support, and James Spotilla (Drexel University) for the loan of an Eppley radiation sensor.
Funding
Funding for this research was provided by the Rockefeller Foundation, the Francis Boyer Trust, National Science Foundation/Environmental Protection Agency Water and Watersheds Grant DEB-9613588, National Science Foundation Grants DEB-0424681 and DEB-9726986, and Pennswood 2 Endowment of the Stroud Water Research Center.
Author information
Authors and Affiliations
Contributions
TLB was responsible for the overall direction of the field studies, data analysis, data interpretation, and preparation of the manuscript. JDN modeled the conservative tracer releases, conceptualized and performed the analysis of storm impacts and recovery, and contributed to data interpretation and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
The authors have followed accepted principles of ethical and professional conduct in the performance of this research.
Additional information
Handling editor: Stefano Amalfitano
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bott, T.L., Newbold, J.D. A multi-year analysis of factors affecting ecosystem metabolism in forested and meadow reaches of a Piedmont stream. Hydrobiologia 850, 4243–4269 (2023). https://doi.org/10.1007/s10750-023-05296-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05296-2