Abstract
Biological early warning systems (BEWSs) monitor the behaviour or physiology of living organisms as an indirect mechanism to sense local environmental changes, and have become a widely established tool for monitoring water pollution. Complementary to conventional chemical and physical techniques, their strength lies in the ability to continuously monitor water quality, providing direct and rapid warning of toxic discharges. Bivalve molluscs (mussels and clams) are ideal sensing organisms for BEWSs, owing to their: high water filtration capacity; sensitivity to numerous, diverse pollutants; considerable longevity; high abundance; and wide range of physical responses to environmental change. Two behavioural metrics of bivalves have dominated in ecotoxicological studies, and consequently spawned development into BEWSs: valve movements, typically measured using electromagnetic techniques; and bivalve cardiac activity, typically measured using infrared photoplethysmography. However, other, less studied bivalve behaviours may exhibit pollutant impacts, including shell growth and dissolution, premature egg/larval release, horizontal movement, and burial. Such metrics may, with further research, provide bases for future BEWSs, some through current valve movement monitoring technology, others potentially through acoustic telemetry. Future bivalve-based BEWSs may become more informative and sensitive through the greater nuance that lies in integration of different metrics simultaneously and/or multi-species monitoring. Future directions may seek techniques that reduce disturbance to bivalves and enable observation of a more natural mode of behaviour, through using untethered, or entirely unequipped, specimens. With rigorous controls for individual- and population-level variation, bivalves have great power as biosensors and, bolstered by the scope for future advancements, this field has the potential to make significant contributions to future water quality management.
Similar content being viewed by others
Introduction
Aquatic environments frequently experience chemical pollution, the origin of which may be a point source (e.g. governmental or industrial wastewater) or nonpoint source (e.g. airborne deposition, or runoff from urban or agricultural regions) (Connon et al., 2012). It is essential to protect drinking water sources from such chemical contamination, the discharge of which can often be sporadic and unpredictable (White et al., 2002). Situations of accidental or deliberate contamination must be detected rapidly and a speedy response coordinated, owing to the potential for serious impacts on human health (Storey et al., 2011). Consequently, early warning monitoring has been developed, permitting rapid identification of adverse water quality changes.
Most countries employ physical and chemical sensors in the real-time monitoring of aquatic environments. However, physical and chemical sensors are expensive to maintain and reveal only local, short-term water quality changes (Bae & Park, 2014). Moreover, many chemical spill events occur sporadically and contain unpredicted pollutants, and so often go undetected (Mikol et al., 2007). Chemical monitoring of aquatic environments is further hindered by the wide diversity of chemical classes that may be present, ranging from polar and nonpolar organic molecules, to metals and ionic species (Mikol et al., 2007). Additionally, chemical analytical techniques may not capture the toxic effects of chemical mixtures which may be additive, synergistic or antagonistic (Costa et al., 2011, 2012).
The complexity of quantifying the plethora of chemical pollutants continuously, in real-time, including both emerging pollutants and transformation products, combined with the inability to measure toxicity directly, has led to the employment of biomonitors in aquatic environments (White et al., 2002; Connon et al., 2012). Biological early warning systems (BEWSs) have been built to monitor the responses of organisms, based on behaviour or physiology, allowing continuous sensing of a wide range of pollution (Bae & Park, 2014). Alongside the test organism, BEWSs have two further elements: an automated detection system, and an alarm system (Gerhardt et al., 2006). Hence, altered responses, resulting from pollution, can be rapidly detected and the alarm raised, allowing appropriate action to be taken.
The organisms deployed in BEWSs for aquatic pollution span various trophic levels and range from vertebrates (e.g. fish) to macroinvertebrates (e.g. molluscs, crustaceans, and insects) and microbes (e.g. bacteria and algae) (Gerhardt et al., 2006; Bae & Park, 2014). BEWSs using animals typically employ behavioural parameters, since these are amongst the fastest and most sensitive indicators of pollutants, and their monitoring is usually non-invasive and relatively inexpensive (Gerhardt et al., 2006; Gerhardt, 2007; Bae & Park, 2014). Bivalve molluscs possess a unique and desirable assortment of characteristics, making them suitable for use in a BEWS, including their high abundance and wide distribution, predominantly sessile nature, ease of handling, and high bioaccumulation ability (Markert et al., 2003). Bivalves have been employed as the sensing organisms in BEWSs, from widespread routine monitoring of surface waters (e.g. Borcherding, 2006) to integration within drinking water facilities (McGuire, 2019; Micu, 2020), and extensive research has revealed different metrics by which bivalve behaviour may be assessed to infer stress resulting from contaminants. With increased understanding of bivalve ecology and the emergence of smaller, cheaper and more sensitive technologies, there are opportunities for significant advancements in the development of bivalve-based biosensors. Furthermore, bivalve-based BEWSs may help improve bivalve conservation practices; since the survival of many bivalve species is imperilled by our poor understanding of their ecology, and of anthropogenic influences thereon (Bogan, 1993), bivalve biosensors can improve our knowledge and highlight damaging activities. Additionally, assessment of the health of bivalves in their environment with such BEWSs may allow them to act as sentinels for other organisms (Aldridge et al., 2007; Chowdhury et al., 2016). Lastly, there is also clear potential benefit to the bivalve aquaculture industry; such biosensors can help inform farming decisions, improving animal welfare and also the sustainability and profitability of farming practices (Andrewartha et al., 2015).
Various informative reviews exist on the topic of BEWSs and general on-line biomonitoring approaches (e.g. Gerhardt et al., 2006; Mikol et al., 2007; Bae & Park, 2014). These can provide a useful overview to the area, with explanations of the principles, examples and case studies of ways different organisms have been employed in monitoring, and approaches to processing data for such systems. However, since bivalves do not form the key focus, many of the finer details on the employment of bivalves are vague or missing. This review will primarily focus on the different metrics by which bivalve behaviour has been, or may yet be, evaluated, in response to external factors, chiefly pollutants. Various technologies that have been deployed for their measurement will be explored and compared, with particular focus on those compatible with field use and automated sensing (key for BEWSs). Relevant case studies will be provided, as well as suggestions of potential areas for further research and development.
Methods to characterise bivalve behaviour
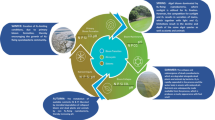
Bivalves largely fall into two groups when being selected for use in biosensors: sessile and mobile species. In their natural habitat, sessile bivalves cement themselves to hard substrates using byssus threads and include the freshwater zebra and quagga mussels (Dreissena spp.), the brackish water false mussels (Mytilopsis spp.), and the marine mussels, such as Mytilus spp. and Perna spp. Mobile bivalves are non-cementing and locomote through sediments using an enlarged, muscular foot; they include the freshwater unionid mussels such as Unio spp. and Anodonta spp., the brackish clams such as Rangia spp., and a wide number of marine taxa. A diverse array of metrics exist by which to measure bivalve behaviour, all with potential for development into BEWS assemblies (Fig. 1). Of these, the two best studied are valve movements and cardiac activity, metrics applicable in both sessile and mobile species. This section provides an explanation and evaluation of both metrics, and of the techniques employed to measure them.
Valve movements
Background
Valve movements (Fig. 1a) are a well-established metric of bivalve behaviour, which has received considerable attention in the literature. Interest began over 100 years ago (Marceau, 1909), expanding significantly over the past thirty to forty years. Starting with laboratory experimentation and evolving to field-based studies, commercial BEWSs now exist, employing valve movements to indicate unfavourable water conditions. Mussel monitors first appeared on the River Rhine in 1992 and various valve monitors exist worldwide (White et al., 2002); for a review on the principles of valve movement use, set in the context of an established proprietary commercial BEWS, see Kramer & Foekema (2001). Through dose-dependent (e.g. Liu et al., 2016) and time-dependent (e.g. Tran et al., 2003) studies of bivalve responses, sophisticated modelling can allow prediction of changes resulting from contaminants. Systems have even been proposed to quantitatively estimate contaminant concentration from observed valve movement changes (Jou & Liao, 2006; Chen et al., 2012).
Using valve movements to monitor contaminants relies on the bivalve’s ability, upon detecting a perturbation (e.g. toxic chemical stressor), to alter its behaviour; this may involve counteracting the change, or else, to lower or isolate as far as possible its exposure to ambient water. Most species maintain open valves during submersion, permitting feeding and respiration. However, prolonged closure (especially coordinated closure among multiple individuals) often indicates an environmental perturbation, due either to natural changes in conditions, or pollutant(s) (Kramer et al., 1989; Kramer & Foekema, 2001; Barile et al., 2016). Responses to pollutants depend on the local physical conditions, sensitivity of the individual concerned, and nature of the stressor itself (toxicity/concentration). Potential responses to toxicants include: closing of valves for a fixed period of time (avoidance behaviour) (e.g. Ait Fdil et al., 2006; Giari et al., 2017); flapping (increasing pumping), i.e. rapid cycles of abduction (valve-opening) and adduction (valve-closing) (e.g. Basti et al., 2009; Bamber & Westerlund, 2016); narrowing valve gape (lowering/ceasing pumping) (Ait Fdil et al., 2006; Giari et al., 2017); wide gaping, indicative of death (Kramer & Foekema, 2001; Andrade et al., 2016).
In analysing valve movement behaviour, various approaches and endpoints can be considered. Valve position is typically expressed as percentage of maximum opening (e.g. Comeau & Babarro, 2014; Clements et al., 2018), angle in degrees (e.g. Robson et al., 2007; Powell et al., 2017), or in units of distance (across valve gape) (e.g. Liu et al., 2016; Basti et al., 2009). Behaviour is profiled at a defined rate (dependent on technique capabilities or desired frequency) and various statistics extracted, used to assess traits of normal behaviour and effects of perturbations. Typical attributes measured include: time spent in open/closed positions (e.g. Chambon et al., 2007; Hartmann et al., 2016); average valve openness over a set time interval (e.g. García-March et al., 2008; Liu et al., 2016); frequency of valve movements (e.g. Liu et al., 2016; Hartmann et al., 2016); speed of movements (e.g. Robson et al., 2007; Durrieu et al., 2016); distance travelled (reflecting the amount of valve movement) (e.g. Redmond et al., 2017; Bamber & Westerlund, 2016); cases of excessive opening (gaping, suggestive of death) (Kramer & Foekema, 2001; Andrade et al., 2016). Different pollutants, or concentrations thereof, can impact different attributes to varying degrees; therefore, the most sensitive (and informative) varies with pollutant type or concentration (e.g. Liu et al., 2016; Giari et al., 2017; Redmond et al., 2017), rendering analysis of a range of attributes advantageous.
Established BEWSs typically measure and average attributes over set time intervals, e.g. five (Borcherding, 2006) or fifteen minutes (Kramer & Foekema, 2001), comparing with previously collected data. Alternatively (especially for large, high-frequency datasets), behaviour of individuals may be modelled, allowing more sophisticated behavioural analysis; various techniques have proved effective (Jou & Liao, 2006; Schmitt et al., 2011; Sow et al., 2011; Azaïs et al., 2014). Historical data allows a “learning period”, in which baseline thresholds of behaviour (in the monitored environment) are defined, allowing divergence to be identified in newly collected data (Kramer & Foekema, 2001). Comparison may employ whole group averages (e.g. Borcherding, 2006), or separate individual averages (e.g. Kramer et al., 1989; Sow et al., 2011), with the latter often considered more informative (e.g. Redmond et al., 2017). When newly collected data exceeds thresholds of normal behaviour, alarms can alert of a potential perturbation (e.g. Kramer et al., 1989); triggering typically stems from significant deviation from normal behaviour by a pre-determined proportion of individuals.
Assessment of valve movement attributes may also be used in toxicity testing; attributes are assessed in sets of exposed individuals and compared with controls, allowing measurement of toxicant reference levels for different species (e.g. Curtis et al., 2000; Ait Fdil et al., 2006). Sensitivity naturally depends on the species involved (e.g. Liu et al., 2016), whilst a range of factors (that define the conditions of the exposure experiments) can affect the recorded toxicity, including: physical conditions like temperature (Borcherding, 2006); types of attribute examined (e.g. Redmond et al., 2017); number of specimens tested (e.g. Borcherding, 1992); and length of exposure time (e.g. Liao et al., 2009). Reference levels indicate animal sensitivity and, thereby, have utility in BEWS coordination. However, owing to their specificity, they are of limited applicability beyond the controlled settings they were generated under; the natural environment (where BEWSs typically operate) contains diverse mixtures whose compound effects can vary markedly from those of individual contaminants (Costa et al., 2011, 2012).
Advantages and disadvantages of measuring valve movements
Certain stressors can induce specific valve movement patterns, potentially enabling pollutant identity to be inferred. For example, mercury induces a distinctly different pattern of behaviour to other trace metals in the Asian Clam, Corbicula fluminea (O.F. Müller, 1774) (Tran et al., 2007). However, quantitative estimation, or identification of pollutants, is beyond the scope of most valve movement monitors in most settings. Consequently, BEWSs developed using this metric benefit from deployment with chemical and physical monitoring strategies. For instance, a sudden alteration in valve movements of zebra mussels [Dreissena polymorpha (Pallas, 1771)] was detected using a commercial BEWS on the River Ruhr at Fröndenberg (North Rhine-Westphalia, Germany) in 1994, triggering an alarm; a water sample was taken, later analysis of which revealed a chemical pollutant which acted as a ‘fingerprint’, enabling identification of the company responsible for its discharge (Borcherding, 2006).
Despite their utility, valve movements exhibit responses to various factors that may be independent of exposure to pollutants, including changes to respiration, feeding, metabolic activity, excretion, locomotion, and reproductive activity (Nagai et al., 2006; Robson et al., 2009; Coquereau et al., 2016; Clements et al., 2018). Therefore, valve movements can be highly variable within an individual. Furthermore, owing to natural variation of such factors between separate individuals, and (moreso) in separate species, valve movements diverge between individuals (Kramer & Foekema, 2001) and species (e.g. Kramer et al., 1989; Liu et al., 2016). Valve movements also alter under various ambient factors like temperature, season, food availability, and physical vibrations (Kramer & Foekema, 2001; Borcherding, 2006; Clements et al., 2018). Furthermore, valve movements exhibit rhythms (i.e. coordinated patterns of opening/closing), regulated by external physical factors like light (e.g. Garcia-March et al., 2016; Comeau et al., 2018). For instance, circadian rhythms exist in raft-cultivated mussels (Mytilus galloprovincialis Lamarck, 1819) in Spain, where maximum valve opening occurs at night, and minimum by day (Comeau et al., 2018). Rhythms can be highly uniform. To minimise their potential to trigger alarms falsely, rhythms should be: accounted for, e.g. by modelling rhythmic patterns to allow dynamic changes in baseline reference data (e.g. Sow et al., 2011); or avoided, by control of the physical factors governing them.
However variation within and between individuals is accounted for, rigorous testing of behaviours under a variety of conditions is key for BEWS development. Laboratory environments allow strict control of ambient conditions under which pollutant effects may be gauged, but can affect natural rhythms, impairing understanding of typical behaviour (Wilson et al., 2005; Garcia-March et al., 2016); further, thorough characterisation in the field is therefore advisable. Measurement of physical conditions, e.g. temperature, pH, light levels, or turbidity, is often necessary in BEWSs, to allow dynamic changes to thresholds, improving alarm accuracy (e.g. Borcherding, 2006). Furthermore, isolation of subjects from predators is wise, not only to protect cohorts and their attached equipment, which predators may dislodge (e.g. García-March et al., 2008), but also because predators may impact behaviour; bivalves are sensitive to cues of predators, such as damage to conspecifics, which causes individuals to alter valve gape behaviour so as to lower their chances of detection (Robson et al., 2007; Dzierżyńska-Białończyk et al., 2019). One last consideration is the fact that, once bivalves close upon pollutant exposure, they can remain closed long after favourable conditions return, limiting their utility in the interim (Kramer & Foekema, 2001).
Technologies
A raft of different techniques exist for measuring valve movements. Some are mechanical in nature, typically inferring or measuring physical displacement of a thread or wire attached to the free valve of a fixed specimen (e.g. Englund et al., 1994; Markich et al., 2000). However, mechanical techniques have largely fallen out of use, being less robust and more cumbersome than more recent approaches, and better suited to laboratory use. Alternatively, video and image analysis techniques may be employed; videos or photographs of bivalves may be analysed by eye or using image analysis software, often using a known visual cue (e.g. a shell tag for tracking, or an adjacent reference object of known dimensions) to gauge openness of valves (Newell et al. 2001; Sénéchal et al. 2008; Speiser & Johnsen, 2008; Coquereau et al., 2016; Dzierżyńska-Białończyk et al., 2019). Video and image analysis techniques also typically lack field compatibility, as well as the necessary automated detection of typical BEWSs. However, numerous alternative approaches exist (Fig. 2), capable of remote, automated valve movement detection. Many have proved appropriate for field use, and certain techniques have been developed into BEWSs.
Cumulative number of published papers using valve movement sensing technology, compatible with BEWS use, between 1960 and 2019. Note the order in the legend reflects the order of the plots. Data were collated using a systematic review from a Web of Science literature search, on 22/11/19, with advanced search term: TS = (bivalve* OR mussel* OR clam* OR scallop* OR oyster*) AND TS = (sensor* OR biosensor* OR sense* OR sensing OR detect* OR detector* OR detection OR activity OR movement*) AND TS = (valve* OR gape* OR gaping). Each publication was read to identify whether one or more technologies were used
Reed switch
Reed switches are simple electrical switches that respond to magnetic fields. These devices have been applied to valve movement monitoring, principally in a well-established commercial BEWS, the Dreissena-Monitor (Envicontrol, Germany); this system employs 84 zebra mussels, Dreissena polymorpha, divided equally between two channels (Borcherding, 2006), fixed onto supports, and equipped with a magnet on the free valve. With a reed switch positioned opposite free valves, opening (or closing) of valves acts to close (or open) the reed switch, allowing a simple binary readout (open/closed) for all mussels. This is robust, reliable and effective for water quality monitoring (e.g. Borcherding, 2006). Magnetic relay sensors may be used analogously to provide binary information (Irlandi & Mehlich, 1996). However, binary measurement conceals intricacies of valve movement which most modern techniques measure as a continuous variable; even graded bands of openness, gauged using multiple reed switches (Garcia‐March et al., 2008), may obscure behavioural nuances. A computer records average percentages of open mussels and number of valve movements for the Dreissena-monitor. Using such large cohorts helps nullify individual peculiarities, and is a particular strength of this technique. Furthermore, although most alternative electromagnetic induction techniques require a continuous draw of electricity (García-March et al., 2008), reed switch energy consumption is low, making it ideal for more remote BEWS deployments, such as general surface water monitoring, where mains power (power grid electricity) may be unavailable.
Dreissena-monitor alarms are set, based upon dynamic limits, which are informed by toxicity testing. This may be carried out using the same device. Instead of feeding live water through both channels, with each acting as validation for the other (during continuous water monitoring), a toxicant may be applied to one (test) channel, the other channel acting as a control; differences in opening/closing behaviour between the channels helps determine toxicant reference levels (Borcherding, 2006).
Electromagnetic induction
Electromagnetic induction forms the key principle of many valve movement techniques (Véró & Salánki, 1969; Kádár et al., 2001; White et al., 2002), including another commercial BEWS, the Musselmonitor® (Delta Consult B.V., The Netherlands), and the high-frequency non-invasive (HFNI) valvometry technique (“Molluscan Eye”, n.d.). Systems employ two small coils, either one on a support the bivalve is fixed to and the other on the free valve (for the Musselmonitor®), or one to each valve of freely moving specimens (in the HFNI set-up). A high-frequency oscillating current is passed through one coil (transmitter), generating a magnetic field, which induces a current in the second coil (receiver) inversely proportional to the distance between coils (Kramer et al., 1989; Curtis et al., 2000; Chambon et al., 2007; Barile et al., 2016; Giari et al., 2017). The range of operation is set such that the generated signal is (essentially) linearly related to the distance.
The Musselmonitor® can be deployed either as an in situ or flow-through system, with eight mussels. A learning period informs later evaluation, performed on behaviours of individuals. Such evaluation and measurement settings can be optimised for the conditions of use, allowing definition of sensible threshold criteria and appropriate alarm generation (Kramer & Foekema, 2001; Giari et al., 2017). This system is effective, the measurements robust, and the statistics performed simple (involving averages of attributes within individuals over set time intervals). However, this simplicity may average out some behavioural intricacies. The device must connect directly to a PC to allow data visualisation, although remote data-logging is possible through a networking module. Furthermore, the in situ version can operate in the field without mains power, using an internal, rechargeable battery (Kramer & Foekema, 2001). Therefore, the Musselmonitor® is suitable for a broad range of BEWS settings, from remote deployments of the in situ version, to controlled deployments of the flow-through version, e.g. in drinking water facility monitoring, with connected computer(s).
The HFNI valvometry system, employing 16 bivalves, possesses two electronic cards: The first sits in the water and measures valve activity; the second remains outside the water, drives the first card, and manages the transfer of the data to a central workstation, where it is processed (Schwartzmann et al., 2011; Sow et al., 2011). This system seeks to enable free movement, with flexible wires and no fixing; this is advantageous, as fixing may alter valve movement patterns (Kramer & Foekema, 2001), and is generally most suitable for sessile, byssus-forming bivalves. The HFNI technique is therefore suitable for application with both sessile and mobile species. Although each valve is equipped, sensors are lightweight, and appear to effect no difference on valve movement behaviours (Tran et al., 2003), though this naturally depends on the size and species of bivalve used. The HFNI system has many further advantages, including its ability to employ low power consumption in field-based units and broadcast data to a central workstation for processing. Furthermore, the sensors have in-built anti-fouling and are maintenance-free (Andrade et al., 2016). Data are collected at a high frequency and modelled, potentially allowing discernment of intricacies that slower data collection rates and simpler data processing protocols may overlook. The HFNI system is an effective technology for BEWS monitoring, having been widely demonstrated in marine monitoring. The principles of the system are robust and, since remote use is not a problem, such a system could be analogously applied in various other BEWS settings, from monitoring of surface waters to drinking water facilities.
Hall sensor
Hall sensors have been widely employed in laboratory and field settings, and may form effective BEWSs. The Hall effect sensor is a device whose output voltage alters, depending on the external magnetic field. Through attachment of a Hall sensor to one valve, and a small rare earth magnet to the opposite valve (as is typical), the distance between valves can be measured continuously (Wilson et al., 2005; Nagai et al., 2006; Robson et al., 2007, 2009; Andrewartha et al., 2015; Hartmann et al., 2016; Liu et al., 2016; Clements et al., 2018; Comeau et al., 2018). Magnetoresistive sensors also act as transducers of magnetic field strength, and can be used analogously (Garcia-March et al., 2016).
Hall sensor approaches have many strengths: employing off-the-shelf components, minimal loads are typically applied to valves (lighter than Musselmonitor® coils, marginally heavier than HFNI coils), high voltages (typical in coil-based methods) are not required, and power consumption can be low (Wilson et al., 2005; Nagai et al., 2006; Comeau et al., 2018). Since valves are not usually fixed, the method is suitable for sessile and mobile bivalve species. Hall sensors also permit very high sampling rates; this may be important, since too low a sampling rate can underestimate adduction and abduction events, causing potential information loss or biases in results (Robson et al., 2009). The Musselmonitor® and HFNI systems (which employ sequential data collection to prevent interference of separate coil pairs) may exhibit such information loss through slower sampling rates. However, though not routinely a problem, Hall sensor systems may experience interference if multiple equipped animals are kept in close proximity. Furthermore, Hall sensor output is not linearly related to the distance, so rigorous and repeated calibration is recommended (Wilson et al., 2005). Overall, Hall sensors provide a promising BEWS technique, and should be easily adaptable into real-world BEWS applications in a broad range of settings.
Optical techniques
Attachment of a light-emitting fibre and light-receiving (and measuring) fibre, one to each valve, allows valve openness to be measured through received light intensity (Frank et al., 2007). Alternatively, by focussing a laser beam on the valve edge of a specimen and measuring the reflected light to gauge distance (a process called laser triangulation), valve movements may also be recorded through received light intensity (Redmond et al., 2017).
Both techniques have been considered for field usage. The fibre optic technique allows isolation of the sensor from the electronics, eliminating any potential for electronic noise (possible with electromagnetic techniques). Fixing is key for both approaches, although laser triangulation may be less intrusive, since no components are fitted onto valves. However, optical techniques may lack the robustness of electromagnetic approaches, since they require good visibility, which is frequently lacking in turbid systems, or where bivalves naturally burrow (Wilson et al., 2005; Robson et al., 2009). Remote deployments, such as general surface water or offshore monitoring, may thereby be incompatible. However, more controlled BEWS settings, such as drinking water monitoring facilities, may be compatible, where: lighting may be artificially provided, when necessary; water to be monitored may have undergone filtration to remove sediment; and mains power provision would negate energy consumption (of sensors/associated computing) concerns. As such, optical techniques do not yet offer a credible technology for ubiquitous BEWS deployment.
Alternative technologies
Various alternative approaches may be appropriate in BEWSs, though utility is speculative due to lack of widespread use or immaturity of technology. For instance, inductive proximity sensors have allowed laboratory measurement of valve movements. Generating an electromagnetic field using a high frequency oscillator, metal objects entering the sensor’s field cause distortion, altering the amplitude of the oscillator and changing the output voltage (Allen et al., 1996; Miller et al., 1999). By fitting a metal disc to one valve of a fixed specimen, and placing a proximity sensor opposite, distance changes caused by valve movements are read in voltage changes (Allen et al., 1996; Miller et al., 1999; Ayad et al., 2011). Accelerometers may also allow monitoring of valve movements; as instruments capable of measuring acceleration in three axes, attachment of an accelerometer to one valve of a fixed specimen can allow valve displacements to be calculated (Coquereau et al., 2016). Lastly, experiments with passive acoustics, whereby hydrophones measure sounds produced by different types of valve movements (such as adduction or swimming), has indicated that such a method may allow identification of valve movements in multiple, unattached animals (Di Iorio et al., 2012; Coquereau et al., 2016).
Cardiac activity
Background
Cardiac activity (Fig. 1b) has been widely studied in a range of different bivalves, under laboratory and field conditions, and under various abiotic and biotic factors. It provides information on health, stress, and physiology; since this is impacted by environmental conditions, cardiac activity may be employed as an indicator of alterations in ambient factors (Sarà & de Pirro, 2011; Xing et al., 2016, 2019; Bakhmet et al., 2019). As an integrated organismal response to contamination, it is an effective metric for water quality biomonitoring in bivalves (Kholodkevich et al., 2017). However, baseline cardiac activity is naturally influenced by factors such as respiration (e.g. Gurr et al., 2018) or feeding (e.g. Widdows, 1973), as well as activity like adduction (e.g. Brand, 1972); consequently these must be considered, although cardiac activity can remain constant under large fluctuations in such factors (Thompson & Bayne, 1972).
Employing cardiac activity for monitoring pollutants relies on the bivalve’s ability to sense and respond to a perturbation in the ambient environment, such as a toxic contaminant. The response may involve increasing metabolic activity, to counteract the change, or to lower activity and avoid exposure. From cardiac traces, changes are often apparent in the heart rate; heart rate can increase or decrease in response to changes in local water conditions, depending on the pollutant (toxicity/concentration), and the animal’s sensitivity. Alterations in rates may sometimes be attributed to direct action of chemical contaminants on the bivalve. For instance, copper may directly impact nervous stimulation of the myocytes themselves (Nicholson, 2003). However, increases in rate are generally seen as the bivalve heart compensating for stressful situations, increasing their metabolism for excreting toxicants, whilst decreases are often linked to valve closure (a typical isolation response) to avoid stress (Nicholson, 1999b; Sarà & de Pirro, 2011). Indeed, valve closure and heart rate are closely linked; lowering of heart rate usually occurs during valve closure (e.g. Trueman & Lowe, 1971), and cessation of the heartbeat (cardiac arrest) is common, often forming a part of normal, periodic behaviour (e.g. Bakhmet et al., 2005). Upon reopening, heart rate typically increases immediately beforehand, temporarily overshooting baseline rate before restoration of normal rate, likely to allow removal of accumulated anaerobic respiration products (generated during oxygen deprivation associated with valve closure) (e.g. Trueman & Lowe, 1971). Heartbeats can also adopt different patterns in response to pollutants, such as “burst activity” (consisting of series of beats separated by pauses), or entirely irregular patterns (Grace & Gainey, 1987). Such patterns are discernible through closer analysis of the inter-pulse duration. Strength of contractions is given by the amplitude on cardiac traces, whilst the rate-amplitude product may help gauge oxygen consumption. The former is greatest under optimal growth conditions (and decreased by stressors), whilst the latter can inform of stress to the heart (increasing when bivalves counteract stressors through increasing metabolic activity) (e.g. Xing et al., 2019).
In assessing cardiac behaviour, various attributes may be extracted and analysed, ideal as BEWS foundations: heart rate monitoring is typical, as means at set intervals; monitoring of the inter-pulse duration is beneficial, since further information lies in the patterns of heartbeats (Bakhmet et al., 2009) and mean heart rates can hide such intricacies of behavioural response (Depledge et al., 1996); analysis of the amplitude and rate-amplitude product may provide a further insight into pollutant impacts on cardiac activity. Analogously to valve movements, cardiac activity attributes can also be assessed in pollutant exposures to generate toxicant reference levels for different species (e.g. Davenport & Manley, 1978). Measured toxicity naturally depends on specific experimental parameters, like time (e.g. Grace & Gainey, 1987) or attribute types (e.g. Curtis et al., 2000); its utility is therefore limited, though it may guide deployment or interpretation of BEWSs. Very little has been written on developing bivalve cardiac activity into real-world BEWSs. However, its integration within aquaculture monitoring (Andrewartha et al., 2015) and a commercial BEWS, the Biota Guard marine monitoring system (Biota Guard AS, Norway) (Dragsund et al., 2013), indicates there is value in measuring cardiac activity in BEWSs; small short- or long-term behavioural alterations can inform of local environmental perturbations (Dragsund et al., 2013).
Advantages and disadvantages of measuring cardiac activity
Cardiac activity is a culmination of various physiological factors which respond to perturbations (e.g. chemical stressors). Dose- or time-dependent responses of heart rate to different pollutants can be studied (e.g. Curtis et al., 2000). For instance, copper is an essential trace element for bivalves, though toxic in excess. Using the blue mussel (Mytilus edulis Linnaeus, 1758) as a paradigm of bivalve behaviours, copper typically induces bradycardia (reduced heart rate), as well as valve closure and greater heart rate variability (Davenport, 1977; Davenport & Manley, 1978; Grace & Gainey, 1987; Curtis et al., 2000). However, others have reported tachycardia (increased heart rate) as a response (Brown et al., 2004; Bakhmet et al., 2012). These reported differences may be attributed to the varying concentrations of copper applied. Higher concentrations may cause immediate toxic effects, leading to isolation (valve closure) and bradycardia. Lower concentrations may lead to compensatory measures, inducing tachycardia.
Although closely linked, cardiac patterns and valve activity may not always respond simultaneously. Heart rate alone may alter upon chemical application, unaccompanied by altered valve gape (Nicholson, 2003). Therefore, heart rate changes may not always be attributable to valve position (Grace & Gainey, 1987; Curtis et al., 2000), and this metric can reveal independent information on bivalve responses. Furthermore, cardiac activity reveals specimen health through their adaptive capability, since changes in cardiac activity provide an integrated measure of physiological fitness (Turja et al., 2014; Kholodkevich et al., 2017). For instance, when groups of blue mussels (Mytilus trossulus Gould, 1850) from an unpolluted site underwent temporary transplantation to separate sites, either polluted or unpolluted, subsequent toxicity testing revealed differences; those temporarily exposed to polluted sites took longer to recover typical heart rates than those exposed to the unpolluted sites, owing to their impacted health (e.g. Turja et al., 2014).
Unlike many physiological effects, cardiac activity monitoring is simple and robust, easily applied in the field for remote, real-time monitoring of environmental water quality. As the bivalve heart has no endothelial lining, and is directly immersed in the haemolymph, cardiac activity is rapidly affected by toxic substances, enabling rapid detection (Nicholson, 1999a). Furthermore, although heart rate alone may seem a basic index, informative heartbeat patterns can occur in cardiac traces, revealed through simultaneous monitoring of inter-pulse duration. Most techniques for measuring cardiac activity are affected by valve and organ (e.g. foot) movements, causing artefacts in traces, e.g. peaks, slow waves, or baseline drift. These can make heart rate difficult to determine and often renders amplitude unusable (Seo et al., 2016). Although amplitude (or rate-amplitude product) is occasionally used to measure cardiac activity (e.g. Bakhmet & Zdorovenov, 2010; Xing et al., 2019), it is typical that only qualitative information about the strength of contractions (from amplitude) is available (Trueman et al., 1973). Furthermore, cardiac activity traces do not always exhibit the same pattern; single or double (or occasionally more) peaks can occur in the waveforms, making elucidation of cardiac attributes more complex (e.g. Seo et al., 2016).
The high variability in cardiac activity presents additional challenges for monitoring, with variation occurring not only between different species of bivalve (e.g. Marshall & McQuaid, 1993; Bakhmet & Khalaman, 2006; Xing et al., 2016) but also within a species (e.g. Bakhmet et al., 2005; da Cândido & Romero, 2006; Sarà & de Pirro, 2011). Heart rate can regularly decrease to a temporary arrest, without coordination between animals (Bakhmet & Khalaman, 2006), and cardiac activity can alter, depending on the size and weight (Nicholson, 2002; Xing et al., 2016, 2019) or age of individuals (Xing et al., 2016, 2019), and under physiological stresses like spawning (Xing et al., 2016). Furthermore, rhythms (coordinated cardiac behavioural patterns) exist in many species, some set by ambient physical factors such as light or tide, others controlled by endogenous biochemical or physiological factors, including reproduction (e.g. Bakhmet & Khalaman, 2006; Bakhmet et al., 2019; Xing et al., 2019). Indeed, heart rate is altered by various physical factors, such as temperature and salinity (e.g. DeFur & Mangum, 1979; Braby & Somero, 2006; Xing et al, 2016). However, rhythms can be altered by laboratory experimentation (Bakhmet & Zdorovenov, 2010) and not all individuals may exhibit the same pattern. Therefore, extensive characterisation of behaviour is key before BEWS employment can be considered, under both laboratory, and field conditions. Encapsulation of individuals within BEWSs is also propitious, not only to protect cohorts (and monitoring equipment) from predator attacks, but also because their presence can impact cardiac activity; the scent of a predator can elevate bivalve heart rate (Rovero et al., 1999).
Bivalve cardiac activity provides a good basis for BEWSs, as exemplified by the commercial system, the Biota Guard marine monitoring system (Dragsund et al., 2013). After thorough characterisation of behaviours, a suitable monitoring frequency should be established and individual ranges of typical behaviour should emerge, deviations from which should allow sensible alarm triggering. Physical monitoring alongside such a BEWS would be advantageous; since various physical factors impact behaviour, appropriate dynamic adjustment of alarm thresholds should improve sensitivity. By employing an appropriate sample size, sudden alterations (concurrent between multiple individuals) could alert of environmental perturbations.
Technologies
Various technologies have been implemented for measurement of bivalve cardiac activity. The earliest methods were incompatible with BEWS requirements, involving visual inspection of the heart (or proxy of cardiac activity) after shell dissection, to manually count beats (Segal, 1956; Pickens, 1965; Scott & Major, 1972). Visual observation after dissection, or direct microscopic examination (through thin spat shells) remain viable laboratory techniques (Pandolfo et al., 2009; Parker et al., 2015; Liu & Wang, 2016). Similarly, detailed cardiac imaging through ultrasound (Haefner et al., 1996) or magnetic resonance imaging (MRI) (Lannig et al., 2008; Seo et al., 2016; Sukhotin et al., 2017), or measurement of haemolymph flow through Laser Doppler Perfusion Monitoring (Lannig et al., 2008), provide further means of extracting cardiac attributes; but, as expensive, laboratory techniques, these are unsuitable for BEWS constructs. Fortunately, established techniques exist for non-destructive and field-compatible measurement of cardiac activity (Fig. 3).
Cumulative number of published papers using cardiac activity sensing technology, compatible with BEWS use, between 1960 and 2019. Note the order in the legend reflects the order of the plots. Data were collated using a systematic review from a Web of Science literature search, on 22/11/19, with advanced search term: TS = (bivalve* OR mussel* OR clam* OR scallop* OR oyster*) AND TS = (heart* OR cardiac) AND TS = (rate* OR activity). Each publication was read to identify whether one or more technologies were used
Impedance pneumography
Impedance pneumography was, for a long time, the preferred method. As a technique allowing measurement of pulsatile changes in volume, it is ideal for measuring the contractions of cardiac activity. Useful in the laboratory, and even long-term (at least several weeks) field deployments (Trueman et al., 1973), implementation often involves the drilling of two holes through the bivalve shell (one per valve), each side of the pericardial cavity. Electrodes are inserted, connected by flexible wires to an impedance pneumograph; through applying an oscillating current between electrodes, the latter converts changes in impedance (from varying heart volume) to a voltage signal, often recorded on a pen recorder (Trueman, 1967; Trueman & Lowe, 1971; Trueman et al., 1973; DeFur & Mangum, 1979; Stickle & Sabourin, 1979; Grace & Gainey, 1987; Marshall & McQuaid, 1993; Stenton-Dozey & Brown, 1994; Nicholson, 1999a, 1999b, 2002, 2003; Braby & Somero, 2006; da Cândido & Romero, 2006).
Flexible wires allow uninhibited movement, and the equipment is comparatively lightweight, weighing no more than supposedly non-impacting infrared photoplethysmographic technology (Kholodkevich et al., 2017); this may lower impacts on behaviour, though it naturally depends on the size/species concerned. However, the approach is particularly invasive; drilling increases stress in specimens, causing higher mortality (under pollutant exposure) (Stenton-Dozey & Brown, 1994), or lower growth (Trueman & Lowe, 1971), than unequipped specimens, as well as potential artefacts (Bakhmet et al., 2009). Since alternative, less invasive approaches exist, it is unlikely to be used more widely for BEWS strategies.
Infrared photoplethysmography
Evolving more recently than impedance pneumography, infrared photoplethysmography has become the current preferred technique. Used both in the laboratory and long-term (several months) field deployments (Kholodkevich et al., 2017), commercial BEWSs also employ this technique. The approach typically involves attachment of a reflective optocoupler onto a bivalve shell over the cardiac region, containing an infrared light-emitting diode and a phototransistor, aligned parallel. The former emits infrared light; reflecting off the cardiac region, received light intensity is then measured (as current) by the phototransistor. Periodic changes of the heart shape and size, associated with cardiac activity, impart a pattern on this light, allowing extraction of cardiac attributes. The unit connects by flexible wires to other components, typically amplifying and filtering the current, and feeding this to an oscilloscope or computer (Depledge & Anderson, 1990; Depledge et al., 1996; Fedotov et al., 2000; Bakhmet et al., 2005, 2009, 2012, 2019; Bakhmet & Khalaman, 2006; Bakhmet & Zdorovenov, 2010; Sarà & de Pirro, 2011; Dragsund et al., 2013; Turja et al., 2014; Andrewartha et al., 2015; Seo et al., 2016; Xing et al., 2016, 2019; Kholodkevich et al., 2017; Zhang et al., 2019). Two optical fibres can alternatively be attached to shells, and used to emit and collect the infrared light of an analogous emitter/receiver unit, allowing it to remain detached from specimens (Fedotov et al., 2000; Turja et al., 2014; Kholodkevich et al., 2017).
Infrared photoplethysmographic approaches generally allow unimpeded movement, with flexible wires. The technique is reputed to be non-invasive (Seo et al., 2016; Xing et al., 2016), with the heavier fibre optic set-up demonstrated not to impact typical behaviours (Kholodkevich et al., 2017), though this would depend on size/species. However, manual shell thinning or drilling can be necessary for thick-shelled specimens (e.g. Andrewartha et al., 2015), necessitating some degree of invasiveness. Nevertheless, power use can be low and the technique is robust, rapid, efficient, and economical (Seo et al., 2016; Xing et al., 2016). The ease of this technique, combined with its lower invasiveness, has likely made it the preferred method, and fostered its integration in real-world BEWS monitoring (Dragsund et al., 2013; Andrewartha et al., 2015). This technique offers the best means for ubiquitous cardiac activity bivalve-based BEWS applications.
Alternative techniques
Edge-detection software can allow heart rate to be detected in videos of juveniles, by comparing pixel colour at a reference point (stationary during the cardiac cycle) with that at a point of interest (moving during the cardiac cycle) (Domnik et al., 2016). This allows non-invasive remote detection of heart rate. However, video microscopy relies on ideal positioning, transparency of shells (atypical beyond juveniles) and good visibility of specimens. Therefore, the technique is unlikely to enter widespread BEWS use. Further optical means may also be exploited to measure cardiac activity, allowing light to be imprinted with rhythmic changes associated with the cardiac cycle, and subsequently measured to extract cardiac attributes. For instance, a lamp may be mounted to one valve, and a photocell (a device whose output voltage alters with light intensity) to the opposite (Dietz & Tomkins, 1980). Compatible with ambient light, and exploiting off-the-shelf components, this technique may have promise, though viability in a field setting (and BEWS potential) is unproved. Alternatively, valves can remain unequipped; a laser beam can instead be directed at the cardiac region (by low-power laser diode), with reflected light collected by a fibre optic bundle, and measured by photodiode (Ritto et al., 2003, 2005). Enabling good resolution, the technique is simple and employs off-the-shelf components. However, precise positioning of specimens is important and the surrounding medium naturally affects readings, so this technique is likely to be of use only in more controlled BEWS settings.
Alternative metrics and future directions
Beyond valve movements and cardiac activity, alternative behavioural metrics (Fig. 1c–f) have received comparatively little attention, particularly in BEWS development. Moreover, monitoring a combination of behaviours (and attributes within them), or a combination of species simultaneously, may generate more nuanced information, producing more informative and sensitive systems. These areas for potential improvement of systems will be explored in this section. Emerging technologies, offering potential improvements and step-changes to both existing and alternative measures of behaviour will also be highlighted. Furthermore, few monitoring designs properly account for variation in pollutant responses within a species, potentially causing faulty adjustment of system sensitivity to levels that are too high (causing false alarms) or too low (causing missed pollution discharges). Suggestions will be provided of how to tackle this difficulty, to improve current bivalve-based BEWS approaches.
Bivalve growth
Growth of bivalves (Fig. 1c) is impacted by their health and metabolism, altering under: stress, such as from handling (Trueman & Lowe, 1971; Andrewartha et al., 2015); ambient physical factors, like temperature (Zhang et al., 2019); or exposure to chemical stressors (Manly & George, 1977; Muncaster et al., 1990; Bonel et al., 2013; Nobles & Zhang, 2015; Mincy & McKinney, 2019). Changes in growth are often studied manually through weight or length measurements, as well as other forms of physical or laboratory examination, such as sclerochronology (Steinhardt et al., 2016), involving investigation of shell growth rings (Lundquist et al., 2019), or scope for growth (e.g. Zhang et al., 2019), involving measurement of the energy available for growth through various physiological responses. For remote field monitoring, whole animal wet weight is an unsuitable index of growth, owing to the impracticality of remote continuous weight measurement, and the potential uncertainty of measurements, stemming from the varying quantities of water bivalves may hold within their mantles. Therefore, growth is more easily gauged through the shell. Shell growth may possibly be observed using the current valve movement monitoring technology at low frequency (Wilson et al., 2005); the technique measures the distance between the two valves, which should increase as a bivalve grows, with growth originating in the mantle cavity. By this means, (daily) incremental increase in minimum (or maximum) opening distance may be observed as the growth rate (Schwartzmann et al., 2011). Some work has commenced in this area, although greater utility may be realised with further application.
Growth rate changes are slow changes; this metric would not allow very rapid pollution detection, but may be useful for more general water quality monitoring during long-term environmental change, at the scale of months or years. Lowest growth will likely be seen under suboptimal water quality. However, it should be noted that growth may not always lower with increases in contaminants; certain contaminants, e.g. lead and cadmium, positively correlate with growth in the freshwater mussel Anodonta anatina (Linnaeus, 1758) (Manly & George, 1977), and metals can affect the chemical composition of shell material, causing deformation and blistering (Spann et al., 2010). Furthermore, growth can be preferentially directed, sometimes in favour of the soft tissue rather than the shell (Gagné et al., 2001), such that effects may not always be observable, and slow-growing species may offer little information in the limited windows over which specimens may be sensibly applied in BEWSs (Newton et al., 2001). However, growth measurements should generally reflect pollutant impacts.
Growth measurement may be especially useful in recording toxin-related shell dissolution events. Valve closure (in response to a contaminant) is a typical avoidance behaviour, and the resulting limited oxygen availability leads to anaerobic respiration, which results in the formation of acidic species. These are neutralised through the dissolution of calcium carbonate from shells, leading to shell thinning. This effect has been observed in response to various contaminants, as well as unfavourable physical conditions (Crenshaw & Neff, 1969; Akberali et al., 1977; Akberali & Black, 1980; Alyakrinskaya, 2005; Nagarajan et al., 2006). Dissolution occurs on the inner shell surface, and seems to occur primarily from newly deposited calcium, with the least soluble components (e.g. nacreous layer) least likely to experience dissolution (Akberali, 1980; Alyakrinskaya, 2005). Although dissolution is likely more pronounced further from shell edges, it is possible that erosion may also operate at shell perimeters (since these may also act as a buffer for acidic species in the extrapallial fluid), leading to reduced separation of valves (and closer valve sensors). Whether thinning brings valves closer or not, thinning would likely be (indirectly) detectable through the consequent lowered/non-existent growth, leading to lowered/non-existent incremental growth readings from valve movement sensors.
Release of eggs and glochidia
Spawning behaviour often exhibits a pattern on valve movements (Andrade et al., 2016). However, in certain freshwater Unio spp., premature release of eggs and immature glochidia larvae (Fig. 1d) can occur under periods of stress (e.g. reduced oxygen availability, or high temperatures), likely as a mechanism to remove the physiological burden of brooding (which reduces functional gill surface area) (Aldridge & McIvor, 2003; Gascho Landis et al., 2015). This may be a more general stress response, likely susceptible to various environmental pollutants; valve closure (avoidance behaviour) lowers oxygen availability and would likely, depending on the period of closure, elicit gill evacuation to lessen hypoxic stress. The process can also respond directly to certain pollutants. For instance, serotonin and its receptor ligands are important in egg maturation and spawning, and various selective serotonin reuptake inhibitors (used as human antidepressents), e.g. fluoxetine, may be found in sewage effluent and enter surface waters; these can disrupt regulated water uptake and induce spawning (Fong et al., 1996; Fong, 1998; Fong & Molnar, 2008; Cunha & Machado, 2011; Lazzara et al., 2012). Therefore, premature release could inform of pollutant presence, though this metric requires further research to establish expected and abnormal periods of release. Gill evacuation in Unio spp. involves a rapid, repeated shunting of the valves (Aldridge & McIvor, 2003) likely discernible through valve movement sensors.
Horizontal movement and burial
Physical activity is another behavioural biomarker which could present an effective tool by which to measure water quality, since it integrates an organism’s response to contamination (Kholodkevich et al., 2017). Many bivalves move laterally (Fig. 1e), and vertically (Fig. 1f), both motions typically entailing valve movements. Naturally, species vary; some are completely sessile, whilst others are more mobile, and sensitivity to ambient conditions can affect the necessity of, or inclination towards, movement (e.g. Amyot & Downing, 1997; Peck et al., 2004; Gough et al., 2012; Mitchell et al., 2018). For instance, under drought conditions, species with lower desiccation tolerance track the receding water line more keenly than those less susceptible to desiccation (Gough et al., 2012). Similarly to other behaviours, horizontal movement and burial are likely impacted by chemical stressors, which affect health and impair activity. For instance, cigarette butt leachate, containing a spectrum of toxic compounds, significantly negatively impacts bivalve burial performance when administered at environmentally-relevant doses (Montalvão et al., 2019). However, activity may not only decrease in response to stressors; prolonged exposure to the antidepressant, fluoxetine, increases lateral and vertical motion (Hazelton et al., 2014). Movement can also provide a means of avoiding stressors, such as desiccation or unfavourable temperatures (Amyot & Downing, 1997; Gough et al., 2012; Mitchell et al., 2018). Therefore, although significantly more research is needed to gauge the utility of this metric, horizontal movement and burial may reflect impacts of perturbations in the environment.
The monitoring of horizontal movement and burial is challenging. This metric is likely most informative when movement is unimpeded. Techniques involving manual measurement of positions (Amyot & Downing, 1997; Mitchell et al., 2018) or manual searching with transmitters and receivers (Gough et al., 2012), would be incompatible with a BEWS. Video or image analysis can provide a tool by which bivalve positions may be tracked (e.g. Zapitis et al., 2021). If automated, perhaps through sophisticated machine learning techniques, this may provide a basis for tracking animal positions in a BEWS. However, the use of such systems would likely be limited to more controlled settings, since the good visibility necessary is unachievable in many field settings.
Alternatively, various biotelemetry solutions, which have been employed with fish, might analogously be employed in bivalves. For instance, acoustic telemetry may be possible; bivalve shells may be equipped with a transmitter (including requisite battery power), that emits low frequency sound signals, and these may be detected by hydrophones. Using multiple hydrophones as receivers, the 3D position may potentially be determined in real-time for equipped bivalves, as for fish (Karp, 2014). Accelerometers can be used alongside such systems to help describe fine-scale movements (e.g. Meese & Lowe, 2020). However, acoustic telemetry applications typically use aquatic life whose movements are of far greater magnitude, and over a far wider geographical range, than those in question (Leclercq et al., 2018; Naughton et al., 2021; Nebiolo & Meyer, 2021). Bivalve motions lie on the scale of < 1 to 10 s of centimetres per day (Amyot & Downing, 1997; Gough et al., 2012; Mitchell et al., 2018), and since positional accuracy of acoustic telemetry typically falls above this scale (e.g. Meese & Lowe, 2020), and rarely at the sub-metre scale (e.g. Leclercq et al., 2018), it is possible this method may provide positions on too crude a scale to be informative. Furthermore, a further potential challenge is the attenuation of signals that burial would impose in such telemetry set-ups; with a change in medium (sediments to water), reflection of signals at the interface of different media could impair signal strength, as could sediment in itself. There are other potential drawbacks, e.g. the ability of subjects to migrate away from sites of interest, and vulnerability to predation (owing to lack of encapsulation within any housing device). However, assuming these are not such weighty issues, and that tailoring of the approach for the smaller area ranges and positional accuracy requirements of bivalves were possible, such a technique could be compatible with wider BEWS deployment.
Combining measures
Bivalves are sensitive to myriad aquatic pollutants, as may be determined through measurement of the various behavioural metrics identified here. Through combination of different metrics, more nuanced information on bivalve behavioural responses may be gained, potentially allowing ‘fingerprints’ of pollutants to be discerned. For instance, measurement of the valve movement sensitivity to copper revealed the detection limit of the freshwater bivalve, Dreissena polymorpha, to be 0.03–0.1 mg/L (Borcherding, 2006), or possibly as low as 0.01 mg/L (“Detectie limieten - Mosselmonitor”, n.d.). In the marine bivalve, Mytilus edulis, this limit may be as low as 0.005 mg/L (“Detectie limieten - Mosselmonitor”, n.d.). The World Health Organisation limit for safe copper levels in drinking water is 2 mg/L (Herschy, 2012), demonstrating the great power bivalves possess as biosensors for water quality. A notable finding from copper exposure experimentation was that the resulting drop in median valve position was a less sensitive indicator than the drop in median heart rate, and the increase in heart rate variability exhibited even greater sensitivity (Curtis et al., 2000). This suggests that finer details in behavioural changes (e.g. heart rate variability) can be particularly useful for detecting pollutants at their lowest concentrations, whilst alterations on an increasingly larger scale (e.g. heart rate or valve position) are more useful in detecting higher pollutant concentrations. This is consistent with findings comparing measured valve movement attributes; when a stressor was applied to a bivalve (dispersed crude oil to M. edulis), mean valve gape was a useful behavioural endpoint at higher pollutant concentrations, whilst distance travelled (a measure of the sum of valve movement in a fixed time interval) proved most sensitive at the lowest concentrations (Redmond et al., 2017). This does not suggest lower value in the less intricate assessments of behaviour; indeed, such differential effects should provide information on the severity (concentration/toxicity) of a pollution episode.
As the examples above highlight, different metrics, and different attributes therein, provide different types of information on bivalve responses to pollutants, each displaying its own sensitivity to different pollutants. Therefore, integration of different metrics/attributes within a BEWS allows enhanced understanding of bivalve responses, increasing the information on pollutants that may be present, and improving system sensitivity. Little has been published on the subject of integrating measures; some work has been undertaken in simultaneous measurement of valve movements and cardiac activity (e.g. Curtis et al., 2000; Andrewartha et al., 2015), and a commercial BEWS, the Biota Guard marine monitoring system, was developed utilising both metrics (Dragsund et al., 2013). Integration with further metrics, e.g. burial or horizontal movement, may reveal further intricacies of behaviour that could improve bivalve-based BEWS practices. Indeed, by combining a range of behavioural measurements, it might be possible to identify toxin-specific ‘fingerprints’, which could prove especially powerful in providing a rapid indication on the nature of a pollution event.
Before integrating technologies, it is important to ensure techniques continue to robustly measure desired metrics, without sacrificing accuracy. For instance, although Hall sensing strategies (to measure valve movements) may potentially measure heart rate simultaneously (at high frequency) (Wilson et al., 2005), and although various cardiac monitoring techniques, e.g. impedance pneumography, can provide indications of valve movements (e.g. Trueman, 1967), unless techniques provide high quality information, simultaneous equipment of bivalves with each separate metric’s technology is best. However, since behaviour can be affected by attachment of devices, experimentation should ensure that techniques are non-intrusive, i.e. that behaviour is not impacted.
Multi-species monitoring
Commercial BEWSs may not always provide the desired sensitivity. For instance, when freshwater Chinese giant mussels [Sinanodonta woodiana (I. Lea, 1834)] were exposed to a variety of chemical pollutants (ranging from chromium to sodium dodecyl sulphate and crude oil) in the Musselmonitor®, very little alarm triggering occurred, even at contamination levels that greatly exceeded those permissible in drinking water (Giari et al., 2017). These tests indicated that, with this species, under these pollutants, the system would have very limited (likely no) useful applications.
Although the unavailing outcome observed in this example may be due to ineffectual setting of alarm thresholds, it is likely that S. woodiana lacks the desired sensitivity to the pollutants tested. Sensitivities naturally vary between species; certain pollutants affect different species to different degrees. For instance, although S. woodiana caused no alarm triggering with phenol up to 100 mg/L during testing, another freshwater species, Dreissena polymorpha, was found (using the Musselmonitor®) to have a detection limit of 14.0 mg/L (“Detectie limieten - Mosselmonitor”, n.d.). Therefore, since high sensitivity to a wide spectrum of pollutants is desirable, it should be beneficial to employ more than one species. Multiple species, deployed simultaneously, may provide better all-round sensing capacity. This should improve the biosensing ability of BEWSs employing bivalves.
Emerging technologies
The most popular techniques for measuring valve movements and cardiac activity are reasonably mature. However, in the constantly evolving world of electronics, on which BEWSs depend, it is easy to envisage many ways that bivalve-based BEWSs could improve in light of such innovation. For instance, smaller and lighter electronic components would lower the likelihood that typical behaviours are encumbered, and render equipment of bivalves for simultaneous measurement of multiple metrics more feasible. The miniaturisation and increased affordability of components should facilitate equipment of larger samples of bivalves; larger cohorts would improve the quality of information gathered, and render multi-species monitoring more practicable, further enhancing systems. As the quantity of information gathered increases, advances in computational processing and statistical techniques should aid interpretation of data and improve understanding of bivalve responses, improving BEWS sensitivity and informativeness. Furthermore, as batteries become more efficient, remote operation should improve.
One direction that future BEWSs may take is to develop strategies that involve equipping bivalves without the requirement of tethering. Some work has been done creating so-called backpacks for bivalves, containing all the electronic components necessary for measuring behavioural metrics and transmitting collected data, as well as a battery (e.g. Taylor et al., 2013). Untethering allows observation of a more natural mode of life, particularly desirable where mobile species of bivalve are employed. Naturally, equipment weight is significant. Further refinement is necessary, to lower intrusiveness and improve data transmission. However, with electrical innovation, this approach may become far more widespread in future BEWSs. Such a technique would also complement measurement of metrics like horizontal movement and burial.
An alternative approach in future bivalve-based BEWSs is to monitor bivalves without impacting their behaviour or mode of life at all, by removing the necessity for equipping animals. One technology that fulfils this non-intrusive approach is passive acoustics; pioneering work has shown different forms of valve movement produce distinct sounds (Di Iorio et al., 2012; Coquereau et al., 2016), themselves distinct from sounds produced by other benthic organisms, which can be measured by hydrophones over a large area (Di Iorio et al., 2012). The method is applicable to mobile and sessile species, for large cohorts and long-term monitoring. However, the application of this technique in bivalves is little developed; the magnitude of sounds generated is variable, and not all types of movement sounds can always be detected (Di Iorio et al., 2012). Therefore, considerably more research is needed before this technique could be developed for BEWS use.
Accounting for variation at the individual and population level
There are many potential sources of variability in behaviours within bivalves. For instance, cardiac activity can be affected by size and weight of individuals, as well as by age and physiological stresses, like spawning (Xing et al., 2016). Also, whilst behaviours like valve movement can contain various rhythms, which are often highly regulated between different individuals, some individuals can exhibit divergent behaviour (Garcia-March et al., 2016; Comeau et al., 2018). Additionally, responses of bivalves to pollutants depend upon historical exposure; tolerance can develop in a population by selection of certain resistant genotypes, leading to different responses between populations. Furthermore, many bivalves possess inducible “plastic” responses, allowing pollutant tolerance to be modified. For instance, metallothionein, a protein that detoxifies heavy metals, may be upregulated, altering subsequent responses. Such phenotypic alterations have been observed in valve movements and cardiac activity (Bakhmet et al., 2009; Barile et al., 2016; Giari et al., 2017).
There are practical ways to cope with different sources of variation in bivalve behaviours. For instance, desensitisation of cohorts, caused by phenotypic alterations stemming from repeated exposures, renders routine replacement of individuals advisable for maintenance of system sensitivity (Kramer & Foekema, 2001). Consideration of historical exposure is also important when selecting populations to employ, and affords a means of providing high sensitivity (through assembling cohorts from populations with limited pollutant exposure).
The effects of variation between individuals within a bivalve population can be lowered by employing appropriate sample sizes. BEWS cohort size is rarely discussed. However, it is a very significant factor which can severely impact the reliability and sensitivity of systems. Different commercial BEWSs employ different numbers of individuals; the Musselmonitor® employs just eight, whilst the Dreissena-Monitor employs 84, and the Biota Guard marine monitoring system, 32. Differences in choices of sample size are not always apparent. It may be that relatively small samples are appropriate; when an individual’s own behaviour is used as a control, the effect of natural variability lessens (Kramer & Foekema, 2001). Variability effects may be further diminished by highly intensive characterisation of individuals’ behaviours using high frequency data collection, although this necessitates large data sets, and may involve more complex statistical and computational approaches (Jou & Liao, 2006; Schmitt et al., 2011; Sow et al., 2011; Azaïs et al., 2014). However, toxicity testing using pentachlorophenol (a pesticide) revealed detection limits were significantly lower for the Dreissena-Monitor than the Musselmonitor®, despite the considerably simpler (binary) readout of valve gape possible under the former (Borcherding, 1992). The greater sensitivity may be attributed to the markedly larger sample sizes employed by the Dreissena-Monitor, highlighting the importance of ensuring sample sizes are sufficiently large to accommodate the innate variability of individuals. This recommends thorough testing of innate specimen variability, to ensure that the number of specimens used allows reliable results to be generated, which are independent of the number of individuals employed. For instance, prior testing with the Dreissena-Monitor indicated a cohort of at least 24 individuals was necessary (Borcherding, 1992). A potential means of establishing appropriate population sizes for a species (i.e. number of replicates) is to determine the number of specimens required to give a coefficient of variation (ratio of standard error to arithmetic mean) of 0.2 at the most, which is considered a reasonable error in most benthic samples (Elliott, 1971; Aldridge et al., 2007).
Other than simply controlling certain parameters, e.g. size/age/sex of individuals, another means of reducing variation between individuals used in BEWSs is to employ specimens from a known clonal lineage. This technique is employed in biomonitoring strategies involving the water flea, Daphnia magna Straus, 1820 (Shaw et al., 2008), and is a viable option for bivalves; for instance, the Asian clam, Corbicula fluminea, possesses one genotype across Europe, making it possible to control for genotype. However, it is important to remember that this is an invasive species, and use of such species should be discouraged, owing to the threat invasives pose to native flora and fauna. Various BEWSs do use invasive species, such as the Dreissena-Monitor (Dreissena polymorpha is invasive in Europe), but such species should only be used when there is no risk of escape (both directly and through reproduction) or when the species is already established. Furthermore, there is emerging evidence that translocation of native bivalves risks introduction of harmful pathogens into the recipient ecosystem (Brian & Aldridge, 2019; Brian et al., 2021). The species deployed in BEWSs should ideally always be local and native (Kramer & Foekema, 2001).
Conclusions
As predominantly sessile, ubiquitous, and easy-to-handle organisms, sensitive to a broad spectrum of water pollutants, bivalves offer an effective tool as biosensors of water quality. Extensive research has proven their value in bioindication and biomonitoring strategies, promoting interest in their employment as BEWS sensing organisms. Different behaviours may be used as metrics in such BEWSs; focus has been on valve movements and cardiac activity, which have their advantages and disadvantages (as summarised in Table 1). A wide variety of technologies have been developed to monitor these metrics, including some commercial BEWSs. However, this narrow focus has ignored other aspects of bivalve behaviour, e.g. burial and egg/glochidia release, which research suggests may exhibit sensitivity to perturbations, like pollutants. These alternative metrics may, through advancements, offer novel glimpses into bivalve ecology and provide other bases for BEWS development, potentially using alternative technologies (Table 2).
The various metrics may independently provide extensive information, necessary for sensitive BEWSs. However, combination of these metrics, and different attributes within them, has the potential to deliver greater understanding of responses, providing more intricate details and likely improving system sensitivity. In addition, simultaneous deployment of multiple bivalve species, each with different sensitivities, should help provide better all-round biosensing abilities in bivalve-based BEWSs. The continuing evolution of the world of electronics, generating smaller and cheaper components, increases the feasibility of multiple metric measurement and larger, multi-species cohorts in BEWSs. As the quantity of information generated increases, advancements in computing and statistical approaches should aid data processing and more sophisticated alarm triggering. Future directions may entail use of untethered, or entirely unequipped, animals. Provided that sources of variation, both at the individual and population level are considered and controlled for as far as practical, BEWSs employing bivalves have great power. The scope for new developments in the area of bivalve-based BEWSs is significant, and this field has the potential to provide important social, economic and environmental benefits, from improving future management of water quality to improved aquaculture practices and conservation of aquatic ecosystems.
References
Ait Fdil, M., A. Mouabad, A. Outzourhit, A. Benhra, A. Maarouf, & J. C. Pihan, 2006. Valve movement response of the mussel mytilus galloprovincialis to metals (Cu, Hg, Cd and Zn) and phosphate industry effluents from Moroccan Atlantic coast. Ecotoxicology (London, England) Ecotoxicology 15: 477–486
Akberali, H. B., 1980. 45Calcium uptake and dissolution in the shell of Scrobicularia plana (da Costa). Journal of Experimental Marine Biology and Ecology Elsevier 43: 1–9.
Akberali, H. B. & J. E. Black, 1980. Behavioural responses of the bivalve Scrobicularia plana (da Costa) subjected to short-term copper (Cu II) concentrations. Marine Environmental Research 4: 97–107.
Akberali, H. B., K. R. M. Marriott & E. R. Trueman, 1977. Calcium utilisation during anaerobiosis induced by osmotic shock in a bivalve mollusc [24]. Nature 266: 852–853.
Aldridge, D. C. & A. L. McIvor, 2003. Gill evacuation and release of glochidia by Unio pictorum and Unio tumidus (Bivalvia: Unionidae) under thermal and hypoxic stress. Journal of Molluscan Studies 69: 55–59.
Aldridge, D. C., T. M. Fayle & N. Jackson, 2007. Freshwater mussel abundance predicts biodiversity in UK lowland rivers. Aquatic Conservation: Marine and Freshwater Ecosystems 17: 554–564.
Allen, H. J., W. T. Waller, M. F. Acevedo, E. L. Morgan, K. L. Dickson & J. H. Kennedy, 1996. A minimally invasive technique to monitor valve-movement behavior in bivalves. Environmental Technology (united Kingdom) 17: 501–507.
Alyakrinskaya, I. O., 2005. Functional significance and weight properties of the shell in some mollusks. Biology Bulletin 2005 32:4 Springer 32: 397–418, https://doi.org/10.1007/s10525-005-0118-y.
Amyot, J. P. & J. A. Downing, 1997. Seasonal variation in vertical and horizontal movement of the freshwater bivalve Elliptio complanata (Mollusca: Unionidae). Freshwater Biology 37: 345–354.
Andrade, H., J. C. Massabuau, S. Cochrane, P. Ciret, D. Tran, M. Sow & L. Camus, 2016. High frequency non-invasive (HFNI) bio-sensors as a potential tool for marine monitoring and assessments. Frontiers in Marine Science 3: 1–10.
Andrewartha, S. J., N. G. Elliot, J. W. McCulloch, & P. B. Frappell, 2015. Aquaculture sentinels: smart-farming with biosensor equipped stock. Journal of Aquaculture Research & Development 07
Ayad, M. A., M. A. Fdil & A. Mouabad, 2011. Effects of cypermethrin (pyrethroid insecticide) on the valve activity behavior, byssal thread formation, and survival in air of the marine mussel Mytilus galloprovincialis. Archives of Environmental Contamination and Toxicology 60: 462–470.
Azaïs, R., R. Coudret & G. Durrieu, 2014. A hidden renewal model for monitoring aquatic systems biosensors. Environmetrics 25: 189–199.
Bae, M. J. & Y. S. Park, 2014. Biological early warning system based on the responses of aquatic organisms to disturbances: a review. Science of the Total Environment 466–467: 635–649. https://doi.org/10.1016/j.scitotenv.2013.07.075.
Bakhmet, I. N. & V. V. Khalaman, 2006. Heart rate variation patterns in some representatives of Bivalvia. Biology Bulletin 33: 276–280.
Bakhmet, I. N. & P. E. Zdorovenov, 2010. Variability in cardiac activity of the bivalves Mytilus edulis and Modiolus modiolus. Russian Journal of Marine Biology 36: 223–225.
Bakhmet, I. N., V. J. Berger & V. V. Khalaman, 2005. The effect of salinity change on the heart rate of Mytilus edulis specimens from different ecological zones. Journal of Experimental Marine Biology and Ecology 318: 121–126.
Bakhmet, I. N., N. N. Fokina, Z. A. Nefedova & N. N. Nemova, 2009. Physiological-biochemical properties of blue mussel Mytilus edulis adaptation to oil contamination. Environmental Monitoring and Assessment 155: 581–591.
Bakhmet, I. N., A. J. Komendantov & A. O. Smurov, 2012. Effect of salinity change on cardiac activity in Hiatella arctica and Modiolus modiolus, in the White Sea. Polar Biology 35: 143–148.
Bakhmet, I. N., A. Sazhin, N. Maximovich & D. Ekimov, 2019. In situ long-term monitoring of cardiac activity of two bivalve species from the White Sea, the blue mussel Mytilus edulis and horse mussel Modiolus modiolus. Journal of the Marine Biological Association of the United Kingdom 99: 833–840.
Bamber, S. D., & S. Westerlund, 2016. Behavioral responses of Arctica islandica (Bivalvia: Arcticidae) to simulated leakages of carbon dioxide from sub-sea geological storage. Aquatic Toxicology. 180: 295–305.
Barile, N. B., M. Scopa, S. Recchi & E. Nerone, 2016. Biomonitoring of coastal marine waters subject to anthropogenic use: development and application of the biosensor Mosselmonitor®. Ovidius University Annals of Chemistry 27: 81–86.
Basti, L., K. Nagai, Y. Shimasaki, Y. Oshima, T. Honjo & S. Segawa, 2009. Effects of the toxic dinoflagellate Heterocapsa circularisquama on the valve movement behaviour of the Manila clam Ruditapes philippinarum. Aquaculture 291: 41–47.
Bogan, A. E., 1993. Freshwater bivalve extinctions (mollusca: Unionoida): a search for causes. Integrative and Comparative Biology 33: 599–609.
Bonel, N., L. C. Solari & J. Lorda, 2013. Differences in density, shell allometry and growth between two populations of limnoperna fortunei (mytilidae) from the río de la plata basin, Argentina. Malacologia 56: 43–58.
Borcherding, J., 1992. Another early warning system for the detection of toxic discharges in the aquatic environment based on valve movements of the freshwater mussel Dreissena polymorpha. The Zebra Mussel Dreissena Polymorpha - Ecology, Biological Monitoring and First Applications in the Water Quality Management 4: 127–146.
Borcherding, J., 2006. Ten years of practical experience with the Dreissena-Monitor, a biological early warning system for continuous water quality monitoring. Hydrobiologia 556: 417–426.
Braby, C. E. & G. N. Somero, 2006. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). Journal of Experimental Biology 209: 2554–2566.
Brand, A. R., 1972. The mechanism of blood circulation in Anodonta anatina (L.) (Bivalvia, Unionidae). Journal of Experimental Biology 56: 361–379.
Brian, J. I., & D. C. Aldridge, 2019. Endosymbionts: an overlooked threat in the conservation of freshwater mussels? Biological Conservation. 155–165.
Brian, J. I., I. S. Ollard, & D. C. Aldridge, 2021. Don’t move a mussel? Parasite and disease risk in conservation action. Conservation Letters 14: e12799. https://doi.org/10.1111/conl.12799.
Brown, R. J., T. S. Galloway, D. Lowe, M. A. Browne, A. Dissanayake, M. B. Jones & M. H. Depledge, 2004. Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquatic Toxicology 66: 267–278.
Chambon, C., A. Legeay, G. Durrieu, P. Gonzalez, P. Ciret & J. C. Massabuau, 2007. Influence of the parasite worm Polydora sp. on the behaviour of the oyster Crassostrea gigas: A study of the respiratory impact and associated oxidative stress. Marine Biology 152: 329–338.
Chen, W. Y., L. J. Jou, S. H. Chen & C. M. Liao, 2012. A real-time biomonitoring system to detect arsenic toxicity by valve movement in freshwater clam Corbicula fluminea. Ecotoxicology 21: 1177–1187.
Chowdhury, G. W., A. Zieritz & D. C. Aldridge, 2016. Ecosystem engineering by mussels supports biodiversity and water clarity in a heavily polluted lake in Dhaka, Bangladesh. Freshwater Science 35: 188–199. https://doi.org/10.1086/684169.
Clements, J. C., L. A. Comeau, C. E. Carver, É. Mayrand, S. Plante & A. L. Mallet, 2018. Short-term exposure to elevated pCO2 does not affect the valve gaping response of adult eastern oysters, Crassostrea virginica, to acute heat shock under an ad libitum feeding regime. Journal of Experimental Marine Biology and Ecology 506: 9–17.
Comeau, L. A. & J. M. F. Babarro, 2014. Narrow valve gaping in the invasive mussel Limnoperna securis: implications for competition with the indigenous mussel Mytilus galloprovincialis in NW Spain. Aquaculture International 22: 1215–1227. https://doi.org/10.1007/s10499-013-9742-6.
Comeau, L. A., J. M. F. Babarro, A. Longa & X. A. Padin, 2018. Valve-gaping behavior of raft-cultivated mussels in the Ría de Arousa, Spain. Aquaculture Reports 9: 68–73. https://doi.org/10.1016/j.aqrep.2017.12.005.
Connon, R. E., J. Geist & I. Werner, 2012. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors (switzerland) 12: 12741–12771.
Coquereau, L., A. Jolivet, H. Hégaret & L. Chauvaud, 2016. Short-term behavioural responses of the great scallop pecten maximus exposed to the toxic alga alexandrium minutum measured by accelerometry and passive acoustics. PLoS ONE 11: 1–15.
Costa, R., D. C. Aldridge & G. D. Moggridge, 2011. Preparation and evaluation of biocide-loaded particles to control the biofouling zebra mussel, Dreissena polymorpha. Chemical Engineering Research and Design Institution of Chemical Engineers 89: 2322–2329. https://doi.org/10.1016/j.cherd.2011.02.027.
Costa, R., D. C. Aldridge & G. D. Moggridge, 2012. Multicomponent molluscicide mixtures for zebra mussel control. Journal of Great Lakes Research 38: 317–325.
Crenshaw, M. A. & J. M. Neff, 1969. Decalcification at the mantle-shell interface in molluscs. Integrative and Comparative Biology 9: 881–885.
Cunha, E. M. & J. Machado, 2011. Parturition in Anodonta cygnea induced by selective serotonin reuptake inhibitors (SSRIs). Canadian Journal of Zoology 79: 95–100. https://doi.org/10.1139/z00-187.
Curtis, T. M., R. Williamson & M. H. Depledge, 2000. Simultaneous, long-term monitoring of valve and cardiac activity in the blue mussel Mytilus edulis exposed to copper. Marine Biology 136: 837–846.
da Cândido, L. T., & S. M. B. Romero, 2006. Heart rate and burrowing behavior in the mussel Anodontites trapesialis (Bivalvia: Mycetopodidae) from lotic and lentic sites. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology 145: 131–136.
Davenport, J., 1977. A study of the effects of copper applied continuously and discontinuously to specimens of Mytilus edulis (L.) exposed to steady and fluctuating salinity levels. Journal of the Marine Biological Association of the United Kingdom University of Cambridge Centre of International Studies 57: 63–74.
Davenport, J. & A. Manley, 1978. Sea-water copper concentrations by the mussel Mytilus edulis. Journal of Marine Biology University of Cambridge 58: 843–850.
DeFur, P. L., & C. P. Mangum, 1979. The effects of environmental variables on the heart rates of invertebrates. Comparative Biochemistry and Physiology -- Part A: Physiology 62: 283–294.
Depledge, M. H., & B. B. Anderson, 1990. A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology 96A: 473–477
Depledge, M. H., A. K. Lundebye, T. Curtis, A. Aagaard & B. B. Andersen, 1996. Automated interpulse-duration assessment (AIDA): a new technique for detecting disturbances in cardiac activity in selected macroinvertebrates. Marine Biology 126: 313–319.
Detectie limieten - Mosselmonitor. , (n.d.)., https://aquadect.nl/detectie-limieten/.
Di Iorio, L., C. Gervaise, V. Jaud, A. A. Robson & L. Chauvaud, 2012. Hydrophone detects cracking sounds: non-intrusive monitoring of bivalve movement. Journal of Experimental Marine Biology and Ecology. 432–433: 9–16. https://doi.org/10.1016/j.jembe.2012.07.010.
Dietz, T. H., & R. U. Tomkins, 1980. The effect of temperature on heart rate of the freshwater mussel, Ligumia subrostrata. Comparative Biochemistry and Physiology -- Part A: Physiology 67: 269–271.
Domnik, N. J., E. T. Polymeropoulos, N. G. Elliott, P. B. Frappell & J. T. Fisher, 2016. Automated non-invasive video-microscopy of oyster spat heart rate during acute temperature change: impact of acclimation temperature. Frontiers in Physiology 7: 1–12.
Dragsund, I., M. Kompen, E. Holmslet, E. Sønneland & O. Christie, 2013. The Biota Guard marine oil leak monitoring system—novel sampling application of bivalve PAT biosensors. TOS Forum 2013: 15.
Durrieu, G., Q. K. Pham, A. S. Foltête, V. Maxime, I. Grama, V. Le Tilly, H. Duval, J. M. Tricot, C. Ben Naceur, & O. Sire, 2016. Dynamic extreme values modeling and monitoring by means of sea shores water quality biomarkers and valvometry. Environmental monitoring and assessment. Environmental Monitoring and Assessment 188. https://pubmed.ncbi.nlm.nih.gov/27286974/.
Dzierżyńska-Białończyk, A., Ł Jermacz, J. Zielska & J. Kobak, 2019. What scares a mussel? Changes in valve movement pattern as an immediate response of a byssate bivalve to biotic factors. Hydrobiologia 841: 65–77.
Elliott, J. M., 1971. Some Methods for the Statistical Analysis of Samples of Benthic Invertebrates, Freshwater Biological Association, Ambleside:
Englund, V. P. M., M. P. Heino & G. Melas, 1994. Field method for monitoring valve movements of Bivalved molluscs. Water Research 28: 2219–2221.
Fedotov, V. P., S. V. Kholodkevich & A. G. Strochilo, 2000. Study of contractile activity of the crayfish heart with the aid of a new non-invasive technique. Journal of Evolutionary Biochemistry and Physiology 36: 288–293.
Fong, P. P., 1998. Zebra mussel spawning is induced in low concentrations of putative serotonin reuptake inhibitors. Biological Bulletin Marine Biological Laboratory 194: 143–149.
Fong, P. P. & N. Molnar, 2008. Norfluoxetine induces spawning and parturition in estuarine and freshwater bivalves. Bulletin of Environmental Contamination and Toxicology 81: 535–538. https://doi.org/10.1007/s00128-008-9558-7.
Fong, P. P., R. Deguchi & K. Kyozuka, 1996. Serotonergic ligands induce spawning but not oocyte maturation in the bivalve Mactra chinensis from Central Japan. The Biological Bulletin 191: 27–32.
Frank, D. M., J. F. Hamilton, J. Evan Ward & S. E. Shumway, 2007. A fiber optic sensor for high resolution measurement and continuous monitoring of valve gape in Bivalve molluscs. Journal of Shellfish Research 26: 575–580.
Gagné, F., C. Blaise, M. Salazar, S. Salazar & P. D. Hansen, 2001. Evaluation of estrogenic effects of municipal effluents to the freshwater mussel Elliptio complanata. Comparative Biochemistry and Physiology - C Toxicology and Pharmacology 128: 213–225.
García-March, J. R., M. Á. Sanchís Solsona & A. M. García-Carrascosa, 2008. Shell gaping behaviour of Pinna nobilis L., 1758: circadian and circalunar rhythms revealed by in situ monitoring. Marine Biology 153: 689–698.
Garcia-March, J. R., S. Jiménez, M. A. Sanchis, S. Monleon, J. Lees, D. Surge & J. Tena-Medialdea, 2016. In situ biomonitoring shows seasonal patterns and environmentally mediated gaping activity in the bivalve, Pinna nobilis. Marine Biology Springer, Berlin Heidelberg 163: 1–12.
Gascho Landis, A. M., T. L. Mosley, W. R. Haag & J. A. Stoeckel, 2015. Effects of temperature and photoperiod on lure display and glochidial release in a freshwater mussel. Freshwater Science 31: 775–786. https://doi.org/10.1899/11-082.1.
Gerhardt, A., 2007. Aquatic behavioral ecotoxicology—prospects and limitations. Human and Ecological Risk Assessment 13: 481–491.
Gerhardt, A., M. K. Ingram, I. J. Kang & S. Ulitzur, 2006. In situ on-line toxicity biomonitoring in water: recent developments. Environmental Toxicology and Chemistry 25: 2263–2271.
Giari, L., F. Vincenzi, E. A. Fano, I. Graldi, F. Gelli & G. Castaldelli, 2017. Sensitivity to selected contaminants in a biological early warning system using Anodonta woodiana (Mollusca). Water SA 43: 200–208.
Gough, H. M., A. M. Gascho Landis & J. A. Stoeckel, 2012. Behaviour and physiology are linked in the responses of freshwater mussels to drought. Freshwater Biology 57: 2356–2366.
Grace, A. L. & L. F. Gainey, 1987. The effects of copper on the heart rate and filtration rate of Mytilus edulis. Marine Pollution Bulletin 18: 87–91.
Gurr, S. J., J. Goleski, F. P. Lima, R. Seabra, C. J. Gobler & N. Volkenborn, 2018. Cardiac responses of the bay scallop Argopecten irradians to diel-cycling hypoxia. Journal of Experimental Marine Biology and Ecology. 500: 18–29.
Haefner, P. A., B. Sheppard, J. Barto, E. McNeil & V. Cappellino, 1996. Application of ultrasound technology to molluscan physiology: noninvasive monitoring of cardiac rate in the blue mussel, Mytilus edulis Linnaeus, 1758. Journal of Shellfish Research 15: 685–687.
Hartmann, J. T., S. Beggel, K. Auerswald, B. C. Stoeckle & J. Geist, 2016. Establishing mussel behavior as a biomarker in ecotoxicology. Aquatic Toxicology. 170: 279–288.
Hazelton, P. D., B. Du, S. P. Haddad, A. K. Fritts, C. K. Chambliss, B. W. Brooks & R. B. Bringolf, 2014. Chronic fluoxetine exposure alters movement and burrowing in adult freshwater mussels. Aquatic Toxicology Elsevier 151: 27–35.
Herschy, R. W., 2012. Water quality for drinking: WHO guidelines. Encyclopedia of Earth Sciences Series 876–883.
Irlandi, E. A. & M. E. Mehlich, 1996. The effect of tissue cropping and disturbance by browsing fishes on growth of two species of suspension-feeding bivalves. Journal of Experimental Marine Biology and Ecology 197: 279–293.
Jou, L. J. & C. M. Liao, 2006. A dynamic artificial clam (Corbicula fluminea) allows parsimony on-line measurement of waterborne metals. Environmental Pollution 144: 172–183.
Kádár, E., J. Salánki, R. Jugdaohsingh, J. J. Powell, C. R. McCrohan & K. N. White, 2001. Avoidance responses to aluminium in the freshwater bivalve Anodonta cygnea. Aquatic Toxicology 55: 137–148.
Karp, C., 2014. Summary of freshwater fisheries telemetry methods. 24.
Kholodkevich, S. V., T. V. Kuznetsova, A. N. Sharov, A. S. Kurakin, U. Lips, N. Kolesova & K. K. Lehtonen, 2017. Applicability of a bioelectronic cardiac monitoring system for the detection of biological effects of pollution in bioindicator species in the Gulf of Finland. Journal of Marine Systems 171: 151–158. https://doi.org/10.1016/j.jmarsys.2016.12.005.
Kramer, K. J. M. & E. M. Foekema, 2001. The “Musselmonitor®” as biological early warning system. In Butterworth, F. M., A. Gunatilaka & M. E. Gonsebatt (eds), Biomonitors and Biomarkers as Indicators of Environmental Change. Springer, Boston.
Kramer, K. J. M., H. A. Jenner, & & D. De Zwart, 1989. The valve movement response of mussels: a tool in biological monitoring. Hydrobiologia.
Lannig, G., A. S. Cherkasov, H. O. Pörtner, C. Bock & I. M. Sokolova, 2008. Cadmium-dependent oxygen limitation affects temperature tolerance in eastern oysters (Crassostrea virginica Gmelin). American Journal of Physiology - Regulatory Integrative and Comparative Physiology 294: 1338–1346.
Lazzara, R., M. Blázquez, C. Porte & C. Barata, 2012. Low environmental levels of fluoxetine induce spawning and changes in endogenous estradiol levels in the zebra mussel Dreissena polymorpha. Aquatic Toxicology 106–107: 123–130.
Leclercq, E., B. Zerafa, A. J. Brooker, A. Davie & H. Migaud, 2018. Application of passive-acoustic telemetry to explore the behaviour of ballan wrasse (Labrus bergylta) and lumpfish (Cyclopterus lumpus) in commercial Scottish salmon sea-pens. Aquaculture 495: 1–12.
Liao, C. M., S. F. Jau, C. M. Lin, L. J. Jou, C. W. Liu, V. H. C. Liao & F. J. Chang, 2009. Valve movement response of the freshwater clam Corbicula fluminea following exposure to waterborne arsenic. Ecotoxicology 18: 567–576. https://doi.org/10.1007/s10646-009-0314-5.
Liu, X. & W. X. Wang, 2016. Physiological and cellular responses of oysters (Crassostrea hongkongensis) in a multimetal-contaminated estuary. Environmental Toxicology and Chemistry 35: 2577–2586.
Liu, H., X. Chen, I. J. Kang, Y. Shimasaki, Y. Oshima & J. Yang, 2016. The valve movement response of three freshwater mussels Corbicula fluminea Müller 1774, Hyriopsis cumingii Lea 1852, and Anodonta woodiana Lea 1834 exposed to copper. Hydrobiologia 770: 1–13.
Lundquist, S. P., T. A. Worthington & D. C. Aldridge, 2019. Freshwater mussels as a tool for reconstructing climate history. Ecological Indicators 101: 11–21.
Manly, R., & W. O. George, 1977. The occurrence of some heavy metals in populations of the freshwater mussel Anodonta anatina (L.) from the river thames. Environmental Pollution (1970) 14: 139–154.
Marceau, F., 1909. Recherches sur la morphologie, et l’histologie, et la physiologie compares des muscles adducteurs des mollusques acphales. Archives De Zoologie Expérimentale Et Générale 2: 295–469.
Markert, B. A., A. M. Breure & H. G. Zechmeister (eds), 2003. Bioindicators & Biomonitors: Principles, concepts and applications. Elsevier, Oxford.
Markich, S. J., P. L. Brown, R. A. Jeffree & R. P. Lim, 2000. Valve movement responses of Velesunio angasi (Bivalvia: Hyriidae) to manganese and uranium: an exception to the free ion activity model. Aquatic Toxicology 51: 155–175.
Marshall, D. J. & C. D. McQuaid, 1993. Differential physiological and behavioural responses of the intertidal mussels, Choromytilus meridionalis (Kr.) and Perna perna L., to exposure to hypoxia and air: a basis for spatial separation. Journal of Experimental Marine Biology and Ecology 171: 225–237.
McGuire, M., 2019. Check out these mussels: Minneapolis using mollusks to monitor water quality – WCCO. CBS Minnesota. https://minnesota.cbslocal.com/2019/05/14/mussels-helping-monitor-water-quality-in-minneapolis/.
Meese, E. N. & C. G. Lowe, 2020. Active acoustic telemetry tracking and tri-axial accelerometers reveal fine-scale movement strategies of a non-obligate ram ventilator. Movement Ecology 8: 1–17. https://doi.org/10.1186/s40462-020-0191-3.
Micu, A., 2020. In Poznan, Poland, eight clams get to decide if people in the city get water or not. ZME Science. https://www.zmescience.com/science/poznan-mussel-water-plants-892524/.
Mikol, Y. B., W. R. Richardson, W. H. Van Der Schalie, T. R. Shedd & M. W. Widder, 2007. An Online real-time biomonitor for contaminant surveillance in water supplies. Journal/american Water Works Association 99: 107–115.
Miller, A. C., B. S. Payne & L. R. Shaffer, 1999. A shell gape monitor to study effects of physical disturbance on freshwater mussels. Journal of Freshwater Ecology 14: 241–247.
Mincy, G. A. & M. L. McKinney, 2019. The Asian clam Corbicula fluminea as a pollution sentinel species. Journal of Shellfish Research 38: 183–190.
Mitchell, Z. A., J. McGuire, J. Abel, B. A. Hernandez & A. N. Schwalb, 2018. Move on or take the heat: can life history strategies of freshwater mussels predict their physiological and behavioural responses to drought and dewatering? Freshwater Biology 63: 1579–1591.
Molluscan eye. (n.d.). https://molluscan-eye.epoc.u-bordeaux.fr/index.php?rubrique=accueil&lang=en.
Montalvão, M. F., T. Q. Chagas & T. Gabriela da Silva Alvarez, C. Mesak, A. Pereira da Costa Araújo, A. R. Gomes, J. Emmanuela de Andrade Vieira, T. L. Rocha, & G. Malafaia, 2019. Cigarette butt leachate as a risk factor to the health of freshwater bivalve. Chemosphere 234: 379–387.
Muncaster, B. W., P. D. N. Hebert & R. Lazar, 1990. Biological and physical factors affecting the body burden of organic contaminants in freshwater mussels. Archives of Environmental Contamination and Toxicology 19: 25–34.
Nagai, K., T. Honjo, J. Go, H. Yamashita & Oh. Seok Jin, 2006. Detecting the shellfish killer Heterocapsa circularisquama (Dinophyceae) by measuring bivalve valve activity with a Hall element sensor. Aquaculture 255: 395–401.
Nagarajan, R., S. E. G. Lea & J. D. Goss-Custard, 2006. Seasonal variations in mussel, Mytilus edulis L. shell thickness and strength and their ecological implications. Journal of Experimental Marine Biology and Ecology 339: 241–250.
Naughton, G. P., Z. S. Hogan, T. Campbell, P. J. Graf, C. Farwell & N. Sukumasavin, 2021. Acoustic telemetry monitors movements of wild adult catfishes in the Mekong River, Thailand and Laos. Water 13: 641.
Nebiolo, K. P. & T. H. Meyer, 2021. High precision 3-D coordinates for JSATS tagged fish in an acoustically noisy environment. Animal Biotelemetry 9: 1–15. https://doi.org/10.1186/s40317-021-00244-0.
Newell, C. R., D. J. Wildish & B. A. MacDonald, 2001. The effects of velocity and seston concentration on the exhalant siphon area, valve gape and filtration rate of the mussel Mytilus edulis. Journal of Experimental Marine Biology and Ecology 262: 91–111.
Newton, T. J., E. M. Monroe, R. Kenyon, S. Gutreuter, K. I. Welke & P. A. Thiel, 2001. Evaluation of relocation of unionid mussels into artificial ponds. Journal of the North American Benthological Society 20: 468–485.
Nicholson, S., 1999a. Cardiac and lysosomal responses to periodic copper in the mussel Perna viridis (Bivalvia: Mytilidae). Marine Pollution Bulletin 38: 1157–1162.
Nicholson, S., 1999b. Cytological and physiological biomarker responses from green mussels, Perna viridis (L.) transplanted to contaminated sites in Hong Kong coastal waters. Marine Pollution Bulletin 39: 261–268.
Nicholson, S., 2002. Ecophysiological aspects of cardiac activity in the subtropical mussel Perna viridis (L.) (Bivalvia: Mytilidae). Journal of Experimental Marine Biology and Ecology 267: 207–222.
Nicholson, S., 2003. Cardiac and branchial physiology associated with copper accumulation and detoxication in the mytilid mussel Perna viridis (L.). Journal of Experimental Marine Biology and Ecology 295: 157–171.
Nobles, T. & Y. Zhang, 2015. Survival, growth and condition of freshwater mussels: effects of municipal wastewater effluent. PLoS ONE 10: 1–19.
Pandolfo, T. J., W. G. Cope & C. Arellano, 2009. Heart rate as a sublethal indicator of thermal stress in juvenile freshwater mussels. Comparative Biochemistry and Physiology - A Molecular and Integrative Physiology. 154: 347–352. https://doi.org/10.1016/j.cbpa.2009.07.001.
Parker, L. M., W. A. O’Connor, D. A. Raftos, H. O. Pörtner & P. M. Ross, 2015. Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE 10: 1–19.
Peck, L. S., A. D. Ansell, K. E. Webb, L. Hepburn & M. Burrows, 2004. Movements and burrowing activity in the Antarctic bivalve molluscs Laternula elliptica and Yoldia eightsi. Polar Biology 27: 357–367.
Pickens, P. E., 1965. Heart rate of mussels as a function of latitude, intertidal height, and acclimation temperature. Physiological Zoology 38: 390–405.
Powell, J. A., N. L. C. Ragg & B. J. Dunphy, 2017. Phenotypic biomarkers in selectively-bred families of the Greenshell™ mussel (Perna canaliculus): Anaerobic enzyme and shell gape behaviour as biomarkers of prolonged emersion tolerance. Aquaculture 479: 601–608.
Redmond, K. J., M. Berry, D. M. Pampanin & O. K. Andersen, 2017. Valve gape behaviour of mussels (Mytilus edulis) exposed to dispersed crude oil as an environmental monitoring endpoint. Marine Pollution Bulletin 117: 330–339. https://doi.org/10.1016/j.marpolbul.2017.02.005.
Ritto, P. A., J. G. Contreras & J. J. Alvarado-Gil, 2003. Monitoring of heartbeat by laser beam reflection. Measurement Science and Technology 14: 317–322.
Ritto, P. A., J. J. Alvarado-Gil & J. G. Contreras, 2005. Scaling and wavelet-based analyses of the long-term heart rate variability of the Eastern Oyster. Physica a: Statistical Mechanics and Its Applications 349: 291–301.
Robson, A., R. Wilson & C. G. De Leaniz, 2007. Mussels flexing their muscles: a new method for quantifying bivalve behaviour. Marine Biology 151: 1195–1204.
Robson, A. A., G. R. Thomas & C. G. de leaniz, & R. P. Wilson, 2009. Valve gape and exhalant pumping in bivalves: optimization of measurement. Aquatic Biology 6: 191–200.
Rovero, F., R. N. Hughes & G. Chelazzi, 1999. Cardiac and behavioural responses of mussels to risk of predation by dogwhelks. Animal Behaviour 58: 707–714.
Sarà, G. & M. de Pirro, 2011. Heart beat rate adaptations to varying salinity of two intertidal Mediterranean bivalves: the invasive Brachidontes pharaonis and the native Mytilaster minimus. Italian Journal of Zoology 78: 193–197.
Schmitt, F. G., M. De Rosa, G. Durrieu, M. Sow, P. Ciret, D. Tran & J. C. Massabuau, 2011. Statistical study of bivalve high frequency microclosing behavior: scaling properties and shot noise analysis. International Journal of Bifurcation and Chaos 21: 3565–3576.
Schwartzmann, C., G. Durrieu, M. Sow, P. Ciret, C. E. Lazareth & J. C. Massabuaua, 2011. In situ giant clam growth rate behavior in relation to temperature: a one-year coupled study of high-frequency noninvasive valvometry and sclerochronology. Limnology and Oceanography 56: 1940–1951.
Scott, D. M. & C. W. Major, 1972. The effect of copper (ii) on survival, respiration, and heart rate in the common blue mussel, Mytilus edulis. Biological Bulletin 143: 679–688.
Segal, E., 1956. Microgeographic variation as thermal acclimation in an intertidal mollusc. Biological Bulletin 111: 129–152.
Sénéchal, J., J. Grant & M.-C. Archambault, 2008. Experimental manipulation of suspended culture socks: growth and behavior of juvenile mussels (Mytilus spp.). Journal of Shellfish Research 27: 811–826.
Seo, E., T. Sazi, M. Togawa, O. Nagata, M. Murakami, S. Kojima & Y. Seo, 2016. A portable infrared photoplethysmograph: heartbeat of Mytilus galloprovincialis analyzed by MRI and application to Bathymodiolus septemdierum. Biology Open 5: 1752–1757.
Shaw, J. R., M. E. Pfrender, B. D. Eads, R. Klaper, A. Callaghan, R. M. Sibly, I. Colson, B. Jansen, D. Gilbert & J. K. Colbourne, 2008. Daphnia as an emerging model for toxicological genomics. Advances in Experimental Biology 2: 5–7.
Sow, M., G. Durrieu, L. Briollais, P. Ciret & J. C. Massabuau, 2011. Water quality assessment by means of HFNI valvometry and high-frequency data modeling. Environmental Monitoring and Assessment 182: 155–170.
Spann, N., E. M. Harper & D. C. Aldridge, 2010. The unusual mineral vaterite in shells of the freshwater bivalve Corbicula fluminea from the UK. Naturwissenschaften 97: 743–751. https://doi.org/10.1007/s00114-010-0692-9.
Speiser, D. I. & S. Johnsen, 2008. Scallops visually respond to the size and speed of virtual particles. Journal of Experimental Biology 211: 2066–2070.
Steinhardt, J., P. G. Butler, M. L. Carroll & J. Hartley, 2016. The application of long-lived bivalve sclerochronology in environmental baseline monitoring. Frontiers in Marine Science 3: 1–26.
Stenton-Dozey, J. M. E. & A. C. Brown, 1994. Exposure of the sandy-beach bivalve Donax serra Röding to a heated and chlorinated effluent III. Effects of temperature and chlorine on heart rate. Journal of Shellfish Research 13: 455–459.
Stickle, W. B. & T. D. Sabourin, 1979. Effects of salinity on the respiration and heart rate of the common mussel, Mytilus edulis L., and the black chiton, Katherina tunicata (Wood). Journal of Experimental Marine Biology and Ecology 41: 257–268.
Storey, M. V., B. van der Gaag & B. P. Burns, 2011. Advances in on-line drinking water quality monitoring and early warning systems. Water Research Elsevier Ltd 45: 741–747. https://doi.org/10.1016/j.watres.2010.08.049.
Sukhotin, A., N. Fokina, T. Ruokolainen, C. Bock, H. O. Pörtner & G. Lannig, 2017. Does the membrane pacemaker theory of metabolism explain the size dependence of metabolic rate in marine mussels? Journal of Experimental Biology 220: 1423–1434.
Taylor, H. D., A. Kruger, & J. J. Niemeier, 2013. Embedded electronics for a mussel-based biological sensor. 2013 IEEE Sensors Applications Symposium, SAS 2013 - Proceedings IEEE 148–151.
Thompson, R. J. & B. L. Bayne, 1972. Active metabolism associated with feeding in the mussel Mytilus edulis L. Journal of Experimental Marine Biology and Ecology Elsevier 9: 111–124.
Tran, D., P. Ciret, A. Ciutat, G. Durrieu & J.-C. Massabuau, 2003. Estimation of potential and limits of bivalve closure response to detect contaminants: application to cadmium. Environmental Toxicology and Chemistry 22: 914–920.
Tran, D., E. Fournier, G. Durrieu & J.-C. Massabuau, 2007. Inorganic mercury detection by valve closure response in the freshwater clam Corbicula fluminea: integration of time and water metal concentration changes. Environmental Toxicology and Chemistry 26: 1545–1551.
Trueman, E. R., 1967. Activity and heart rate of bivalve molluscs in their natural habitat [39]. Nature 214: 832–833.
Trueman, E. R., & G. A. Lowe, 1971. The effect of temperature and littoral exposure on the heart rate of a bivalve mollusc, Isognomum alatus, in tropical conditions. Comparative Biochemistry and Physiology -- Part A: Physiology 38: 555–564.
Trueman, E. R., J. G. Blatchford, H. D. Jones & G. A. Lowe, 1973. Recordings of the heart rate and activity of molluscs in their natural habitat. Malacologia 14: 377–383.
Turja, R., N. Höher, P. Snoeijs, J. Baršiene, L. Butrimavičiene, T. Kuznetsova, S. V. Kholodkevich, M. H. Devier, H. Budzinski & K. K. Lehtonen, 2014. A multibiomarker approach to the assessment of pollution impacts in two Baltic Sea coastal areas in Sweden using caged mussels (Mytilus trossulus). Science of the Total Environment 473–474: 398–409.
Véró, M. & J. Salánki, 1969. Inductive attentuator for continuous registration of rhythmic and periodic activity of mussels in their natural environment. Medical and Biological Engineering 7: 235–237.
White, B., M. Gilbert & R. Deininger, 2002. Biological monitors. In Hargesheimer, E., O. Conio & J. Popovicova (eds), Online Monitoring for Drinking Water Utilities American Water Works Association, Denver: 203–241.
Widdows, J., 1973. Effect of temperature and food on the heart beat, ventilation rate and oxygen uptake of Mytilus edulis. Marine Biology 20: 269–276. https://doi.org/10.1007/BF00354270.
Wilson, R., P. Reuter & M. Wahl, 2005. Muscling in on mussels: new insights into bivalve behaviour using vertebrate remote-sensing technology. Marine Biology 147: 1165–1172.
Xing, Q., Y. Li, H. Guo, Q. Yu, X. Huang, S. Wang, X. Hu, L. Zhang & Z. Bao, 2016. Cardiac performance: a thermal tolerance indicator in scallops. Marine Biology 163: 1–9.
Xing, Q., L. Zhang, Y. Li, X. Zhu, Y. Li, H. Guo, Z. Bao & S. Wang, 2019. Development of novel cardiac indices and assessment of factors affecting cardiac activity in a Bivalve Mollusc Chlamys farreri. Frontiers in Physiology 10: 1–9.
Zapitis, C., M. Huck, A. Mehra, M. T. Bulling, & A. Ramsey, 2021. Behavioural responses of Anodonta anatina and Unio pictorum to temperature and algal concentration. Journal of Molluscan Studies 87. https://academic.oup.com/mollus/article/87/4/eyab036/6470380.
Zhang, P., T. Zhao, L. Zhou, G. Han, Y. Shen & C. Ke, 2019. Thermal tolerance traits of the undulated surf clam Paphia undulata based on heart rate and physiological energetics. Aquaculture 498: 343–350. https://doi.org/10.1016/j.aquaculture.2018.08.037.
Funding
JEV was supported by the Engineering and Physical Sciences Research Council (EPSRC) [Grant Number EP/L015889/1]. DCA was supported by Corpus Christi College, Cambridge, and through a Dawson Fellowship from St Catharine’s College, Cambridge.
Author information
Authors and Affiliations
Contributions
JEV and DCA conceived the idea and designed the structure and focus of the review. JEV collated literature, produced figures and tables and drafted the manuscript. Both authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vereycken, J.E., Aldridge, D.C. Bivalve molluscs as biosensors of water quality: state of the art and future directions. Hydrobiologia 850, 231–256 (2023). https://doi.org/10.1007/s10750-022-05057-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-05057-7