Abstract
Cyanobacteria harmful algal blooms (cHABs) are increasingly becoming an emerging threat to aquatic life, ecotourism, and certain real estate investments. Their spontaneous yet sporadic occurrence has made mitigation measures a cumbersome task; moreover, current trends regarding anthropogenic activities, especially in agriculture and industry portend further undesirable events. Apart from the aesthetic degeneration they create in their respective habitats, they are equally capable of secreting toxins, which altogether present grave environmental and medical consequences. In this paper, we gave an update on factors that influence cHABs, cyanotoxin exposure routes, and environmental public health implications, especially impacts on fish, pets, and livestock. We discussed social economic impacts, risk assessment, and management problems for cHABs and, thereafter, assessed the extant management approaches including prevention, control, and mitigation of the proliferation of cyanobacterial blooms. In light of this, we suggest that more intensified research should be directed to the standardization of procedures for cyanotoxin analysis. Also, the provision of standardized reference material for the quantification of cyanotoxins is vital for routine monitoring as well as the development of strong in situ sensors capable of quantifying and detecting HABs cells and toxins in waterbodies to prevent the adverse impacts of cHABs. Also, more investigations into the natural and environmentally friendly approach to cyanobacteria management and the necessary and appropriate deployment of artificial intelligence are required. Finally, we wish to redirect the focus of public health authorities to protecting drinking water supply sources, agriculture products, and food sources from cyanotoxins contamination as well as to implement proper monitoring and treatment procedures to protect citizens from this potential health threat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microalgae are vital primary producers, as they are essential to energy flow, ecological balance, nitrogen fixation, material circulation, and pollution degradation. They are also implicated in the accumulation of contaminants or pollutants that directly result in ecosystem deterioration (Lu et al., 2021). Although most algae are beneficial, microalgae occasionally form algal blooms (ABs) especially in waterbodies (both salt and freshwater milieus) when compacted or when dense populations of algae rapidly rise under favorable growth conditions (USEPA 2017). Algal blooms are an unpleasant sight to behold; however, they are not all harmful to humans. Algal blooms can cause anoxia, form high biomass, or produce taste and odor leading to economic losses as a result of the need for water treatment. Some species capable of secreting harmful toxins are characterized as harmful algal blooms (HABs) (USEPA 2017). Harmful algal blooms occur when toxin-producing algae grow excessively in a body of water, thereby causing harm, either due to toxins or accumulated biomass that affects co-existing organisms in the environment, altering food-web dynamics (Anderson, 2009). Harmful algal blooms can be differentiated by three characteristic phenomena including intoxication of freshwater and marine life, anoxia (due to high biomass), and accumulation of toxins in the food chain (Marampouti et al., 2021). Harmful algal blooms are small plant-like organisms (mainly of cyanobacteria—blue-green algae), that give water bodies their characteristic blue-green color. Common bloom-forming cyanobacteria genera include Dolichospermum, Microcystis, Aphanizomenon, Trichodesmium, Nodularia, Cylindrospermopsis, and Planktothrix (Huisman et al., 2018). Globally, water contamination resulting from cyanobacteria blooms has been reported in lakes, lagoons, oceans, rivers, streams, water reservoirs, and wells (Massey et al., 2020).
Due to the threat of cyanobacteria harmful algal blooms (cHABs) to humans and animals, several studies on these organisms have been carried out in freshwater environments in North America, Asia, Oceania, Africa, and Europe (Huang & Zheng, 2017) and the studies indicated a global increase of cyanobacteria in the aquatic ecosystem. For instance, in 2017, a study predicted the effects of climate change on the concentration of cyanobacteria in large reservoirs in the USA using statistical modelling framework (Chapra et al., 2017). The framework utilized a forecast of climate change from five global calculation models, two cyanobacteria growth and two greenhouse gas emission scenarios coupled with a water quality/hydrologic network model of the contiguous USA. The model forecasted a likely increase in the year 2017 in the mean number of days of harmful cHABs from 7 days per year per water body to 16–23 days in 2050 and 18–39 days in 2090 due to the increase in nutrient levels as well as the rise in water temperature in the aquatic milieu (Chapra et al., 2017). The expansion of cyanobacteria blooms in Taihu Lake is currently extending across almost approximately 2400 km2 of its entire surface and this proliferation has been accompanied by serious environmental, economic, and societal challenges with long-term negative impacts on water quality, economic activities, fisheries and aesthetics, tourism, and other economic activities. Furthermore, an analysis carried out on satellite data from lake revealed that high nutrient concentrations and high temperatures promote cyanobacterial growth in spring whereas low atmospheric temperatures and low wind speeds favor the formation of surface blooms (Guo et al. 2007; Shi et al., 2017). Vieira et al. 2022 recorded cyanobacteria species in 252 of the 988 existing Spanish water reservoirs and blooms in 91 of the reservoirs most destined for water supply which demonstrated the widespread increase of potentially harmful cyanobacteria in recent times. The data were sourced from different websites of Spanish basin organizations, technical reports, and scientific papers from 1981 to 2017.
Harmful algal blooms especially cHABs alter the energy flux of the food webs in the aquatic environments, leading to loss of biodiversity and threatening the sustainability of the aquatic ecosystem (Paerl & Otten, 2013). In humans, the impacts of cHABs and other HABs include illness and death through direct exposure to their toxins, indirect exposure to organisms that amass the toxins or exposure to aerosolized toxins (Carmichael & Boyer, 2016). HAB toxins are associated with several syndromes such as paralytic shellfish poisoning (PSP), ciguatera fish poisoning (CFP), diarrheic shellfish poisoning (DSP), neurotoxic shellfish poisoning (NSP), and amnesic shellfish poisoning (ASP) (Gholami et al., 2019). Other HABs-related syndromes include palytoxin poisoning, azaspiracid poisoning (AZP) and tetrodotoxin poisoning (Tsikoti & Genitsaris, 2021), brevetoxins (BTX), okadaic acid (OA), domoic acid (DA), and numerous other toxins (Durán-Vinet et al., 2021). These toxins are transferred to humans through the consumption of surviving organisms that have bioaccumulated these toxins through sequential trophic advancement in the food web (Marampouti et al. Marampouti et al., 2021). According to the data captured by the harmful algal event database (HAEDAT) from 1987 to 2022, about 82 human mortalities, 4045 cases of toxins in seafood and 181 reports of water discoloration because of HABs (http://haedat.iode.org/). An example of the challenge of cyanobacteria blooms was the Wuxi water crisis in May 2007 which affected about 2 million inhabitants who had no access to drinking water for over a week due to massive Microcystis spp. toxin blooms (Qin et al., 2010).
Interestingly, HABs, especially cHABs, are occurring with phenomenal growth rates and depositions in unorthodox environments due to global warming and eutrophication (Huisman et al., 2018), anthropogenic actions, hydrological variables, and ecophysiological adaptation strategies. The accumulation of cyanobacteria biomass affects numerous ecosystem services and accounts for significant economic losses associated with water treatment, recreational services, and subsistence and commercial aquaculture (Le Moal et al., 2019). Cyanobacteria harmful algal blooms are known environmental threats that deteriorate the quality of different waterbodies by increasing water turbidity, depleting dissolved oxygen (DO), and in producing diverse toxic secondary metabolites (Wang et al., 2021a). In the freshwater ecosystem, notable cHABs-causing genera include diazotrophic taxa (N2-fixing), such as Cylindrospermopsis, Nodularia, and Anabaena, and non-N2-fixing taxa, such as Microcystis, Oscillatoria, and Planktothrix (Wang et al., 2021a). The availability of nitrogen (N2) is a known regulator for cHABs community structure (Lu et al., 2019), and it is commonly believed that N-limiting conditions favor the growth of N2-fixing cyanobacteria, whereas when N2 supply is abundant, non-N2-fixing cyanobacteria would outcompete slow-growing N2-fixing cyanobacteria taxa (Pearl and Otten 2016). Nevertheless, several cHABs are initiated with N2-fixers when N2 is replete; thereafter, the community structure then accommodates non-N2-fixing genera under low N2 concentrations (Fig. 1). The reverse situation has been recognized to either directly or indirectly transfer N2 from N2-fixers to non-N2-fixing cyanobacteria (Lu et al., 2019). Altogether, the various traits shared among cyanobacteria genera reveal that these organisms are a heterogeneous group reacting differently to various ecological conditions and abiotic regulators. The cycles of occurrence of cHABs are intrinsically directed by a notable change in seasonal environmental conditions (Fig. 1), accompanied by changes in zooplankton and phytoplankton populations (Zhao et al., 2021). The changes in planktonic population dynamics are attributed to the following:
-
(i)
Production of toxic metabolites, as well as competitive exclusion amongst cyanobacteria, bloom community that repress phytoplankton proliferation (Xue et al., 2018)
-
(ii)
Selective or alternative feeding of zooplankton on phytoplankton and cyanobacteria, thereby promoting either the formation or repression of cyanobacteria blooms (Zhao et al., 2021).
Cyanobacteria harmful algal blooms are produced in extremely high cell densities; however, toxin production is not always proportionate to cell density. Moreover, certain cHAB denizens secrete potent toxins at low cell densities that affect human health when contaminated seafood such as shellfish are consumed (Xie et al., 2021). Several researchers have so far detected varying concentrations of cyanobacteria toxins in fish (Greer et al., 2017; Skafi et al., 2021), environmental waters (Keliri et al., 2021; Ogungbile et al., 2021; Serrà et al., 2021; Vogiazi et al., 2021), and in soil (Chatziefthimiou et al., 2021; Maity et al., 2021; Zhang et al., 2021); however, the hotspots of human exposure to cyanobacteria toxins might not have been satisfactorily elucidated. The effect of cHABs on humans and animals and several control strategies being suggested have been well documented; however, there is yet no proven, practicable, affordable, and widely acceptable interventions adopted as global best practice to salvage this menace and this calls for more research in this direction. To better comprehend the current state of the impacts of cyanobacteria toxins, it is essential to collate, compare, and analyze the current research discoveries, determine the current state of knowledge, and characterize the effect of cyanobacteria toxins on humans and animals. We reviewed the exposure routes, socio-economic, and public health impacts and assessed the risks of harmful agal blooms to humans and animals to increase public awareness of the potential implications of cHABs. This review presents the current state of knowledge of cyanobacteria and cyanotoxins pollution research with emphasis on current developments, knowledge gaps, and suggestions to support future research.
2 Factors that Influence Harmful Algae Blooms
The overwhelming occurrence of cHABs, worldwide, threatens the safety of both rural and urban freshwater supply, water quality, and aquatic organisms. It also threatens the financial viability of properties and ecological sustainability of numerous freshwater ecosystems. Cyanobacteria, like other living organisms, do require certain factors for their optimal biomass growth and maintenance. The regulation of these factors often dictates their population dynamics and subsequently cHABs formation in any given environmental matrix. Moreover, these factors can influence sporadic and spontaneous HABs situations, either individually or interdependently. Hence, the major drivers for the formation of cHABs and other HABs can be grouped into the following:
2.1 Eutrophication
A healthy, wholesome body of water has its limits with regard to concentrations of minerals and nutrients; this ensures the proper energy distribution in the matrix and the maintenance of ecological balance. However, in some cases, this balance can be disrupted due to the influx of certain compounds and nutrients above their counterparts, thereby creating an incessantly imbalanced community. In this regard, eutrophication may be defined as the enrichment of water bodies with nutrients and minerals, particularly phosphorus (P) and/or N, usually beyond their thresholds. This resultantly causes an increase in the growth of higher forms of plants and algae to produce undesirable water conditions, water quality, and the balance of organisms in the water to form blooms (Namsaraev et al., 2020). Eutrophication and pollution are usually increased by rainfall due to the transport of contaminants and nutrient-rich run-off across freshwater environments to the marine continuum. It has been reported as a major cause of increasing cHABs occurrence and is among the major driving factor for the formation of cyanobacterial bloom (Chirico et al., 2020). The main nutrient pathways leading to eutrophication include soil erosion, wastewater discharge, leaking or overflowing domestic sewers, agricultural fertilization, and farmland drainage (Fig. 2) (Li et al., 2019). Eutrophication induces cyanobacterial growth that subsequently forms algal bloom and is becoming one of the leading challenges associated with water quality in densely populated areas. Cyanobacteria pigments in sediment cores from a survey of over 100 lakes in North America and Europe revealed an increase of almost 60% of cyanobacteria abundance compared with other phytoplankton since the industrial revolution and has accelerated since 1945 and is likely to continue in the next decades (Taranu et al., 2015).
Factors and pathways through which eutrophication can be stimulated in aquatic ecosystems.
Adapted from Ærtebjerg et al. (2003)
In the USA, about 2.2–4.6 billion dollars was estimated as an annual economic loss due to eutrophication (Namsaraev et al., 2020). With the continuous increase in the world population and anthropogenic pressure on water bodies, more deterioration of the water quality is expected. Furthermore, due to the increase of livestock droppings, increase erosion incidence, regular use of fertilizers for agricultural purposes and inflow of contaminated wastewater, eutrophication/deterioration of the water quality is expected to rise by 1.37–3.1 times (Beaulieu et al., 2019); hence, the challenge of eutrophication in large parts of inland and coastal waterbodies might only grow worse, globally.
2.2 Anthropogenic Actions
Anthropocentric day-to-day living has been reported to contribute over 80% of eutrophication, cHABs and other HABs events in marine, freshwater, and other interconnected hydrologic systems (Zhou et al., 2020). Noteworthy, anthropogenic activities that trigger HABs and cHABs include the construction of dams, river diversions, and deforestation. In addition, developments of reservoirs, land, and watershed, as well as inputs of N and P in waterbodies from non-point and point sources (Huang et al., 2012). Anthropogenic inputs of N, partial pressure of carbon dioxide (pCO2) and P emissions from both non-point and point sources significantly contribute to the development of HABs, especially cHABs that alter water quality. Anthropogenic inputs of N and P from point sources (wastewater, mining, and industrial effluent) and non-point sources (agricultural run-offs, inorganic fertilizer, and stormwater) have tremendously encouraged HAB proliferation in the freshwater-marine continuum (Paerl et al., 2015). The effect pCO2 is known to promote the cHABs. However, not much is known about the in situ mechanisms in comparison with the effects of temperature (Ji et al., 2020; Verspagen et al., 2014).
The growing rates of industrialization and urbanization have put major burdens on the rerouting of some rivers, forest resources, watersheds, wetland removal, and land development. These have worsened eutrophication and HABs dynamics on a regional level (Mccarthy et al., 2018). At the global level, the release of nutrients by agricultural, urban, and industrial settlements into rivers, reservoirs, lakes, and coastal waters increases the incidence of cHABs and other HABs. In developing countries, especially the ones without proper water and sanitation infrastructure or reticulation systems, lentic or stagnant water bodies are common habitats for cHAB. This is because they are overstretched for multiple uses, especially recreation and sanitation purposes, such as laundry and bathing. More so, nearby watersheds are deemed a perfect ground for open defecation. Since detergents and human feces are rich in N and P, adjacent water bodies might therefore have a high likelihood of nutrient infusion and overload during flash floods. Other contaminants that pollute waterbodies via accidental discharge and pipeline vandalism due to human activities that significantly encourage the processes of episodic blooms include petroleum products such as kerosene, spent lubricating oil, premium motor spirit, diesel, gasoline, automobile gas oil, and heavy metals (Nwankwegu et al., 2019). Anthropogenic activities have been reported to alter planktonic community structures, migration patterns, and species distribution that result in changes in the biogeography and HAB dynamics, especially cHABs (Müller et al., 2020).
2.3 Hydrodynamic Variables
Worldwide, extreme weather events are expected to increase due to global climate change leading to short-term hydrodynamic fluctuations and such fluctuations influence cyanobacterial blooms (Wu et al., 2013). The extent of hydrologic forces exerted on coastal waters and freshwater bodies affects phytoplankton, cHABs and HABs, nutrient dynamics, water integrity, and cell buildups (Wang et al., 2017). Aquatic environments are potentially affected by both vertical and horizontal mixes in connection with fluvial and alluvial procedures. Flushing and mixing are two hydrodynamics events that regulate the growth of cHABs and other HABs that directly influence water residence time and temperature (Mao et al., 2015). These changes in the vertical mixing of water layers driven by stratified density currents influence the formation of algal blooms (Li et al., 2020). Hydrological modifications such as water diversion and river damming increase the consequence of high nutrient loading from upstream. Similarly, the practice of blocking and impounding rivers enacts significant physical variations on the river continuum. This, in turn, releases large amounts of methane, disrupts the hydrological cycle of floodplains, and fragments habitats, eutrophication, and the formation of HABs (Xu et al., 2020). In general, hydrodynamic, meteorological conditions and nutrients are among the main factors that contribute to the formation of algal blooms. Since meteorological conditions cannot be controlled, bloom control should be primarily determined by hydrodynamic conditions and pollution loads (Mao et al., 2015). Likewise, as the majority of living biomass, suspended particles, pollutants, and dissolved gases are mixed and carried by turbulent motions, the interactions of different water quality variables may be greatly affected by fluctuating hydrodynamic conditions to form blooms (Mao et al., 2015). In riverine ecosystems, changes in hydrological conditions, such as water level, agitation, and transparency, are highly influential on algal growth, diffusion, migration, and bloom accumulation (Cheng et al., 2019).
2.4 Climate Change
Climate change alters hydrologic patterns and causes a rise in temperatures, intensity, frequency, and duration of cyanobacterial blooms. Climatic change creates a significant challenge in assessing cHABs and other HABs growth dynamics, proliferation rate, intensity, and integrity. In addition, global warming, rise in sea level, and changes in the pattern of rainfall impact cHABs and other HAB dominance. Climate change can result in extreme changes in freshwaters (rivers and lakes) and ocean circulations, flows, stratification, cyclone frequency and severity, wind direction, and speed and are strong modulations of eutrophication. It also increased average global temperature, natural disasters, extended dry periods, rising sea levels, and increased incidence of intense precipitation events (Misiou & Koutsoumanis, 2022). The impact of climate change could increase the acidification of water, which increases the dissolve CO2, enhancing photosynthesis in cyanobacteria. It also supports extreme hydrologic activities such as drought and storm-related flooding and these are crucial drivers of bloom expansions (Lin et al., 2017). Climate change also influences precipitation, irradiance, temperature increase, salination, mixing and stratification patterns, and the rate of storm events, and all these add to the factors that encourage the formation of cHABs in aquatic environments. Heavy precipitation incidents such as rainstorms and hurricanes cause a rise in surface runoff that carry excess nutrients including phosphorous and nitrogen, and precipitation is regarded as one of the major factors influencing HAB proliferation (Chapra et al., 2017).
The consequence of climate change is perceived in the form of regular and longer periods of precipitation incidents followed by longer periods of low rainfall or drought. This results in an increase in the residence time of nutrients in the environments that create ideal situations for blooms formation of cHABs and other HABs (Michalak et al., 2013). Climate change has contributed to the continuous upsurge of microalgae population (cyanobacteria) in aquatic milieu to form algal bloom. The thick layer of microalgae clusters can lead to HABs. In this condition, light is prevented from penetrating the waterbody and this cause death of aquatic plant that needs light for the photosynthetic process (Kasan et al., 2021). Other climate-driven impacts on the occurrence and distribution of bloom-forming phytoplankton groups such as cyanobacteria are salinity, dissolved oxygen, light intensity, temperature, and pH. Interestingly, these environments have been regularly characterized by the occurrence or presence of cyanotoxins (Kusumawati & Mangkoedihardjo, 2021). In the aquatic milieu, the presence of cHABs increases O2 depletion (resulting from bacterial degradation of dead biomass), reduces the water quality, releases toxins, and affects drinking water safety. The occurrence of cHABs in the water is a serious challenge for environmental protection and water resources management.
2.5 Eco-physiological Adaptation Strategies
Cyanobacteria, being a unique class of unicellular algae, are among the simplest organisms to have existed since the foundation of the earth. Due to their autotrophic way of life, extant species have occupied habitats ranging from the expected pliable and favorable niches, such as freshwater lakes and rivers to the rigid, not-so-conducive environments, such as hot springs and deserts (hot or cold). Moreover, cyanobacteria possess an array of genetically enabled physiological or morpho-functional traits that afford survival and proliferation, which are summarily listed below:
Phototaxis—this confers the ability of photosynthesis at diverse wavelengths, even at low light intensities. Here, certain species (Synechocystis sp., Cylindrospermopsis sp., and Planktothrix sp.) outcompete others based on their ability to adjust to constantly changing or inadequate light intensities. One possible reason for the disparity in photoacclimation might be cell morphology. For example, slender, disintegrated biomass, like the filamentous cyanobacterial species usually outcompeted larger masses at harnessing light.
Buoyancy—this adaptational feature is enabled by the production of gas vesicles by cyanobacteria, which allows their movement in a stratified water column, thereby providing them foremost and unrestricted access to the well-lit depths of the water column. In this regard, certain species (Microcystis sp. and Anabaena sp.) are able to shuttle between the lower nutrient-rich depths and the clearer depth higher up the water column. This attribute is responsible for the thermal stratification of the water column, which makes dissolved oxygen and light unavailable to greater depths, thereby causing a decline in resident algal, plant and animal species. However, it should be noted that buoyancy is commonly exhibited at higher temperatures; at lower temperatures, gas vesicles collapse due to carbohydrate ballast and many cyanobacteria sediment in greater depths of the water column. This collapse is not strictly attributed to the influence of temperature on the vesicles, but due to the turgor pressure realized from the difference in temperature sensitivity between respiration and photosynthesis.
Critical phosphorus stashing—it is well known that many freshwater matrices are limiting in phosphorus (P), which is a critical nutrient for algal bloom formation. However, cyanobacteria have been evinced to maneuver this environmental condition, either through the secretion of phosphatases (enzymes) that hydrolytically liberates phosphate from organic solutes for ready assimilation or through surplus P sequestration. During P-limiting conditions, certain species (Calothrix sp., Dichothrix sp., and Gloeotrichia sp.) with abundance of P, intracellulary, are able to actively divide their cells up to the fourth generation, without having to scavenge scarcely available extracellular P.
Nitrogen fixation—this is a physiological feature that positions some cyanobacteria species (Trichodesmium sp., Lyngbya sp., Anabaena sp., Planktothrix sp., and Nodularia sp.) ahead of the others when energetically inexpensive N sources are depleted. In this regard, N-fixing cyanobacteria are among the dominant population of cHABs at N-limiting conditions, as they do so with the aid of nitrogenase (enzyme).
Akinete formation—as a survival strategy during prolonged unfavorable changes in temperature, light intensity, and nutrient distribution, and also especially during the dry season (usually characterized by intense desiccation), some cyanobacteria taxa (Anabaena, Nodularia, Cylindrospermopsis and Gloeotrichia) form akinetes or thick-walled resting cells, which are denser than the vegetative cells and therefore sediment. At this stage, the non- or meagre requirement of the cells permits cyanobacteria to survive the unavailing conditions of bottom sediments for long periods. Interestingly, the sunken cells can tolerate harsh environmental conditions, such as high temperatures, and begin to germinate when favorable conditions are restored.
Ultimately, the aforementioned unique characteristics, which make cyanobacteria a strong competitor in their environment and equally environmentally robust are enabled by the presence of certain sets of genes (either constitutive or inducible). In other to understand the genetic input of cyanobacterial adaptation, Chen et al. (2021a) did a comparative genomic analysis of 650 cyanobacterial genomes (obtained from terrestrial, marine, and freshwater habitats). Here, they surmised that successful cyanobacteria adaptation was not only based on the expression of certain genes for the features discussed above but also on their ability to acquire and maintain foreign genes in relation to their specific habitat. Correspondingly, Prabha et al. (2016) conducted a functional profiling of cyanobacterial genomes and observed that variations among the functional categories were caused by ecological adaptation strategies shaped by habitat or niche. Therefore, understanding their genetic machinery might help in the exploitation and further attenuation of these organisms toward ecological balance, and human-driven environmental regulation.
3 Cyanobacteria and Their Toxins
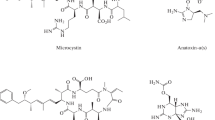
Although not mentioned earlier as an adaptational strategy, cyanobacteria species may secrete certain toxic metabolites to enact competitive exclusion of potential opponents during the campaign for marginal availability of nutrients and light. Regrettably, these toxins do not only unleash harm on their primary target (competitors) but also pose a threat to higher animals and humans. The toxic metabolites released by many cyanobacteria species are called cyanotoxins (CTs) and they may be broadly categorized as (Table 1):
-
(i)
Cytotoxins (cylindrospermopsin): these are implicated in the inhibition of glutathione and protein synthesis and are responsible for genetic and necrotic damage (Moreira et al., 2013).
-
(ii)
Neurotoxins (anatoxin-a, saxitoxins, anatoxin-a(s), analogues, and β-methylamino-l-alanine): these are low molecular weight alkaloids that block sodium channels by preventing nerve conduction.
-
(iii)
Hepatotoxins (nodularins and microcystins): this class of toxins are involved in the inhibition of phosphate proteins 1A and 2A, which cause cancer promotion, deformation of hepatocytes, liver damage and hyperphosphorylation of cytoskeletal filaments (Catherine et al., 2017).
-
(iv)
Dermatoxins (lyngbyatoxin, debromoaplysiatoxin and aplysiatoxins), and
-
(v)
Irritating toxins (lipopolysaccharide endotoxins): these are responsible for inflammation of the gastrointestinal tract and skin irritation (Miglione et al., 2021).
Hepatotoxins, lipopolysaccharide, and neurotoxins are the three main classes of toxins produced by freshwater cyanobacteria. Hepatotoxins (microcystins) are produced by Oscillatoria species, Microcystis species, and Anabaena species, while neurotoxins are produced by Anabaena species, Oscillatoria species, and Aphanizomenon species (Kasan et al., 2021). The third major group of toxins produced by cyanobacteria is lipopolysaccharide (LPS). LPS is produced by numerous cyanobacteria species, and it causes skin discomfort as well as gastroenteritis (Barsanti & Gualtieri, 2014). Other harmful toxins produced by cyanobacteria include irritants and/or gastrointestinal toxins (Cheung et al., 2013), some of which have been involved in the deaths of aquatic and terrestrial wildlife (Wood, 2016). Common cyanobacteria genera within the Cyanophyceae that can form cHABs include Microcystis, Nodularia, Cylindrospermopsis, Dolichospermum, and Planktothrix (Huisman et al., 2018). In tropical waters, Microcystis and Raphidiopsis are the two known cyanobacteria genera that usually form blooms associated with a variety of toxins that degrade water quality. Oftentimes, CTs produced by some cyanobacteria species are secreted intracellularly and are later released into the environment when the cells rupture or die during periods of extreme salinity. Conversely, toxins might be secreted or released at the end of the cyanobacteria bloom’s lifecycle. Neurotoxins, hepatotoxins, and dermatoxins produced by cyanobacteria species can cause acute and chronic human health effects (Smucker et al., 2021).
The occurrence and production of cyanobacteria toxin is largely influenced by some environmental factors including warmer water temperatures, strong nitrogen–phosphorus limitation (N:P), low salinity, and higher water transparency. However, these factors are still open to debate as they vary with geographical settings and seasons. Tarafdar et al. observed that warmer water temperatures (25.31–32.48 °C), strong P– imitation (N:P ratio 138.47–246.86), low salinity (5.45–9.15), and higher water transparency (46.62–73.38 cm) are the major environmental factors that triggered Microcytis outbreak in India’s largest brackish water coastal lagoon, Chilika.
4 Cyanotoxins Exposure Routes and Public Health Impacts
Cyanobacteria toxins are among the most powerful known poisons with significant harmful effects against humans and animals. However, several studies have reported various chemoprotectants with relevant effects against MCs in vivo, especially in mammals (mice) and aquatic organisms (Guzman-Guillen et al. 2017).
Apart from the understanding scientists have about the threats associated with exposure to CTs, it is gradually gaining attention in the media and government agencies (Carmichael & Boyer, 2016). The health threats presented by CTs rank highly among the challenges faced in the treatment of drinking water (Codd et al., 2020).
CTs exposure routes (dermal, oral, inhalation, and parenteral), exposure media (foodstuffs, water, dietary supplements, aerosols, and dust) and poisoning cases present an awareness of the threat to human and animals health (Codd et al., 2020). In recreational events, human exposure to cyanotoxins can occur via accidental ingestion of contaminated water or dermal contact with water, containing cyanobacteria cells (Funari & Testai, 2008). Human exposure to cyanobacteria toxins could also occur through the consumption of seafood (e.g. shellfish) or ingestion of drinking water containing cyanotoxins. Another potential exposure route to cyanotoxins is through inhalation of aerosolized toxins during swimming or boating (Backer et al., 2010). Inhalation of airborne microalgae and cyanobacteria toxins can lead to bronchitis, asthma, allergies, rhinitis, and dermatitis (Hofbauer, 2021). In addition to all these exposure routes, aerosolized CTs represent an essential possible route of exposure and should be incorporated in risk assessments for persons living near affected waterbodies.
Worldwide, there have been several reports of illnesses or even deaths associated with human exposure to cyanotoxins after swimming or bathing in contaminated water (Nguyen et al., 2021).
Threats due to human exposure to CTs may range from a minor infection to severe illness or in some cases, death. Some health effects associated with human exposure to cyanobacteria toxins include hives; conjunctivitis (Jia-Fong et al., 2021); flu-like symptoms; vomiting; blistered mouth; rashes; abdominal pain; fever; eye, ear, and skin irritation; visual disturbances; hepatic failure; and death (Brown et al., 2018; Kubickova et al., 2019). Symptoms of exposure to ciguatera fish poisoning (CFP) include numbness of the mouth that can last for months. Eye and ear irritation, skin rash, blisters, and conjunctivitis are signs of human exposure to cyanotoxins by direct contact (Schaefer et al., 2020). Some health impacts associated with human exposure to cyanobacteria toxins in relation to different syndromes include gastrointestinal disorders (nausea, vomiting, and diarrhea), neurological, and muscular conditions. In the Pacific Island of Guam, BMAA produced by cyanobacteria has been reported to be implicated in fatal progressive neurodegenerative disease among Chamorro people due to the ingestion of contaminated traditional foods (Davis et al., 2020). Paralytic shellfish poisoning (PSP) syndrome and its intoxication can cause paralysis of the abdominal muscles and chest muscles as well as death (Marampouti et al., 2021). Another HAB toxin-associated syndrome called amnesic shellfish poisoning (ASP) can also cause headaches, dizziness, disorientation, motor deficiency, short-term memory loss, and confusion (Kudela et al. 2015).
Microcystins (CTs) such as microcystins (MCs) are toxins produced by some groups of cyanobacteria. These toxins are powerful (phosphatases (PP) 1 and 2A) and are specific inhibitors of protein in mammals and target liver cells (Trinchet et al., 2013). The presence of MCs in the cell inhibits PP1 or PP2A, and this leads to hyperphosphorylation of regulated proteins, disruption of cellular processes, and cell damage (Pham & Utsumi, 2018). MCs are known to be the most hazardous and widespread compounds from cyanobacterial (Pham & Utsumi, 2018). These compounds have been found in numerous countries including China, Spain, Sweden, the USA, Australia, Germany, New Zealand, Romania, Turkey, and Italy (Catherine et al., 2017). Symptoms of acute illnesses due to short-term exposure to CTs from cyanobacteria during recreational events or activities include skin rashes, hay fever-like symptoms, and respiratory and gastrointestinal distress (USEPA, 2015a). Other severe conditions such as liver and kidney damage have also been reported in humans following long-term exposure to high concentrations of CTs (microcystin and cylindrospermopsin) in drinking water (USEPA, 2015a). Microcystins (general structure: cyclo (-D-Ala1-L-X2-D-erythro-β-methylAsp(iso-linkage)3-L-Z4-Adda5-DGlu(iso-linkage)6-N-methyldehydro-Ala7) (Catherine et al., 2017) are group of about 279 described congeners produced by several cyanobacteria genera (Chen et al., 2021a, 2021b, 2021c; Wan et al., 2020).
According to a compilation of murine model studies, their lethal doses (LD50) are a minimum of 50 µg kg−1 and can even go beyond 1200 µg kg−1 (Bouaïcha et al., 2019). In natural waters, the three most predominant MC congeners are MC-LR, MC-RR, and MC-YR (R, Y, and L are the short forms of arginine, tyrosine, and leucine) (Li et al., 2017). In water, MCs are quite stable and are difficult to *remove by conventional water treatment plants process (Zamyadi et al., 2012). Therefore, the occurrence of these MCs in drinking water bodies poses a serious risk to human health (Zhao et al., 2015). According to the Bulgarian legislature, there is no acceptable limit on cyanotoxins in water (Ilieva et al., 2019). However, the United States Environmental protection agency (US EPA) recommended 0.3 μg/L for microcystins and 0.7 μg/L cylindrospermopsin in drinking water for children less than six years old and 1.6 μg/L for microcystins and 3.0 μg/L cylindrospermopsin for adults and school-age children (US EPA, 2019). This is because young children are more susceptible than older children and adults as they consume more water relative to their body weight.
Worldwide, cyanobacteria and cyanotoxins have been detected in water sources and gastrointestinal (GI) infection is the most widely reported symptom following human exposure to cyanotoxin through drinking water (Wu et al., 2021). The first notable case involving human exposure to cyanotoxins resulting in gastrointestinal symptoms was between 1930 and 1931 in West Virginia, Charleston, and other metropolises along the Ohio River, USA (Veldee, 1931). Other cases of infection arising from drinking water sources containing cyanotoxins have been reported in many countries including Zimbabwe, Australia, the Philippines (Wood, 2016), Sweden (Annadotter et al., 2001), and China (Bláha et al., 2009). Besides drinking water exposure, human exposure to CTs via recreational waters can result in multiple non-specific severe illnesses such as otic, dermal, respiratory, neurological, musculoskeletal, GI, and other signs such as pyrexia and anorexia (Hilborn et al., 2014). In 1996 according to the report of Pouria et al. (1998), in Caruaru Brazil, 60 out of 126 patients with symptoms of subacute hepatotoxicity and acute neurotoxicity died due to exposure to contaminated water. Chen et al. (2016) also revealed that human exposure to cyanobacteria toxins could also cause 11 different kinds of illnesses in the forms of hepatoxicity, cancer, and neurotoxicity. In Australia, Florida, and Hawaii, gastrointestinal symptoms, rashes, and respiratory infections were the symptoms observed among recreational fishermen and swimmers expose to algal blooms-contaminated coastal waters (Schaefer et al., 2020). In another incident in Australia, 800 persons were reported to have oral ulcers, skin rash, and eye infections within seven days after accidental ingestion and cutaneous exposure to an active bloom (Schaefer et al., 2020). In Carrasco, Uruguay, a case of acute liver failure and death was reported following recreational exposure to high concentrations of MCs (Vidal et al., 2017
Globally, there are over 1118 reported important cyanotoxins identified in 869 freshwater environments from 66 countries (Svirčev et al. (2019), in the UK, Sweden, Belgium, Finland, France, Greece, Serbia, Italy, Norway, Denmark, Spain, Netherlands, Hungary, and Switzerland. Poisoning incidents or cases have been reported as a result of polluted freshwater environments (Drobac et al., 2021). In general, 80% of persons exposed to cyanotoxins are caused by contaminated drinking water and the rest were caused by consumption of contaminated vegetables and aquaculture especially mussels (Jiménez et al., 2020). Due to human consumption of algal toxins, around 2000 human poisoning cases have been reported yearly (Pal et al., 2021). The health impacts and symptoms associated with human exposure to cyanobacteria toxins are dependent on the kinds of toxin, toxin concentration, and the exposure route. Furthermore, exposure may also have both short and long-term impacts (Lopez et al., 2008). Major target organs of toxicity as well as other organs/systems affected by toxins produced by cyanobacteria are as shown in Fig. 3 (Kubickova et al., 2019), while Table 2 contains some information on selected toxins and their potential short- and long-term health impacts.
5 Impacts of Cyanotoxins on Fish, Pets, and Livestock
In over 50 countries around the globe, high cyanotoxin loads from cyanobacteria blooms have been reported to be associated with animal sickness and death (Lim et al., 2020). Cyanobacteria toxins are common in freshwater and marine milieus and are toxic to aquatic organisms (Shahmohamadloo et al., 2020). Among the CTs, the MC and some specific variants (i.e., MC-LR, MC-RR, and their demethylated forms) are the most frequently investigated or detected. There is increasing and strong evidence of CTs adverse effects on animal health (Christensen & Khan, 2020).
Cyanotoxins have been reported to cause disease and death in pets, livestock, and wildlife (Backer et al., 2013; Vidal et al., 2017), even so, cyanobacteria toxin poisoning in livestock and pets has been shown to be significantly under-reported (Trevino-Garrison et al., 2015). In some aquatic animals, chronic symptoms associated with long-term exposure to cyanobacteria toxins include diminished fecundity, limited growth, tissue and cellular damage, behavioral, and biochemical alterations (Mehinto et al., 2021). Toxins produced by toxic freshwater and marine algae directly affect the food web and aquatic ecosystems. Some organisms such as bivalves or fish can accumulate these toxins, after long-term exposure, which could biomagnify in the food web, or also cause fish death (Durán-Vinet et al., 2021). CTs can precisely affect the target system/organs of aquatic organisms and cause diverse kinds of toxicity including neurotoxicity, immunotoxicity, hepatotoxicity, and endocrine disruption, which might lead to death (Yu et al., 2021). In Doñana National Park, Spain, cyanotoxins were reported to be associated with the death of thousands of birds and fish (Mehinto et al., 2021). Skafi et al. (2021), reported a case of massive fish deaths in their hundreds to thousands after exposure to toxins not only from toxins ingestion but also from gill irritation and apoxia. Fish exposure to cyanobacteria toxins, such as from toxic Microcystis strains can cause histopathological changes in the kidney and liver, growth inhibition and compromised immune function (Li et al., 2021; Liu et al., 2014). Fish deaths associated with cyanotoxins may occur during or after the blooming of different bloom-forming cyanobacteria taxa or genera (Svirčev et al., 2019). The first report describing harmful CTs was by Hald in 1833 to the Danish government where associated cattle and fish death were reported (Moestrup, 1996). Apart from the report of Mehinto et al. (2021), there are several other reported cases of mass fish mortalities associated with cyanobacteria toxins, worldwide, which include brown trout mass mortality in Loch Leven, Scotland (Rodger et al., 1994), mass mortality of tilapias in Lago Paranoa, Brazil (Starling et al., 2002) and stickleback mass death in the Gulf of Finland, Baltic Sea (Kankaanpää et al., 2001). A large-scale record of fish deaths that largely affect adult Dasyatis sabina and Sciaenops ocellatus alongside different baitfish, such as Dorosoma species and Brevoortia species was reported in 2010 at the St. Johns River, Florida, USA (Landsberg et al., 2020). In Eastern Asia countries (Japan, China, and Korea), there were reported cases of finfish mortalities caused by Karenia, Chattonella, Karlodinium and Margalefidinum, and shellfish deaths caused by Heterocapsa circularisquama (Sakamoto et al., 2021). In Chile, salmon deaths were reported to be associated with Alexandrium catenella and Pseudochattonella verruculosa and in the Mexican Pacific, tuna deaths were reported to be associated with Chattonella and Tripos furca and these losses were among the greatest economic loss (Sunesen et al., 2021). In Malaysia and the Philippines, the occurrence of fish-killing algal blooms such as Karlodinium, Prorocentrum cordatum, Chattonella, and Margalefidinium (Cochlodinium) polykrikoides are recent crisis or problems in these areas (Pitcher & Louw, 2021). Between 1991 and 2019 in the Kattegat-Skagerrak, Norway, major fish farm mortalities were caused by Chrysochromulina leadbeateri (Karlson et al., 2021). Massive fish death event was reported in July 2017 due to potentially toxic cyanobacteria Planktothrix sp toxin (microcystin). observed in Beni-Haroun reservoir in Algeria (Benayache et al., 2022). Other than fish mortalities and human toxicity, HABs and cHABs also cause animals (mammal) deaths (Farrer et al., 2015). Domestic animals and livestock usually suffer more fatal CTs poisoning than man since they are liable to unrestrictedly drink and swim in ponds with active cyanobacterial blooms even though the waters may have a bad smell or surface scum (Backer et al., 2013). Many cases of CT intoxication in domestic animals and livestock leading to mass mortalities in mammals, marine invertebrates, and seabirds have been reported in numerous countries (Turner et al., 2021). CTs contained in cyanophyte cells can cause serious effects on vertebrates ranging from minor skin irritations, impaired system, liver injury to death (Vantarakis 2021). Birds get exposed to CTs by eating detached cyanobacteria cells from mats during feeding or drinking water (Krienitz et al., 2003). The report of Lopez-Rodas et al. (2008), also reveals that about 6000 birds belonging to 47 different species died due to microcystin intoxication at the Donana National Park, Spain.
Saxitoxins (STXs) are another toxin produced by cyanobacteria that have been reported to cause mass mortality of birds after consuming STXs-contaminated fish (Papadimitriou et al., 2018). In Australia, and Germany, there were reports of several poisoning cases and deaths of dogs, horses, pigs, sheep, cattle, and guinea pigs due to the consumption of nodularin (Chen et al., 2021b). In Africa, CTs have often been the main suspected cause of mass deaths of both large-sized and medium terrestrial mammals such as livestock (sheep and cattle) as well as non-wading mammals (zebra, impalas, blue wildebeests, giraffes, and white rhinoceros) (Wang et al., 2021b). In Botswana, between May and June 2020, the mass deaths of over 330 African elephants were supposed to be cyanobacteria related (biotoxins) (Wang et al., 2021b). The report of Backer et al. (2013) has also revealed that cats, dogs, and other mammals have also died from anatoxin-a and microcystin poisoning after exposure to contaminated water. Besides the harmful impacts of cyanobacteria producing toxins on aquatic organisms or animals, non-toxic algae can also cause adverse effects in the aquatic environments by reducing dissolved oxygen, suffocating benthic fauna and flora and blocking fish gills leading to fish death (Davidson 2014).
6 Socio-economic Impacts of Cyanobacterial Harmful Algal Blooms
The most important difference involving the methods used to assess the economic impacts of HABs is whether the implications are measured as changes in the market or nonmarket value. Market-based approaches estimate losses linked with changes in the number of goods or services demanded or sold and/or the values of those goods. Non-market estimation methods are used to assess the value of goods and services, often recreational in nature that are not exchanged within an existing market and as such have no market value (Qiao & Saha, 2021). Cyanobacteria harmful algal blooms have adverse effects on economies through the closure of water-related industries, decrease in tourism activities and increased water treatment costs. Cyanobacteria harmful algal blooms affect wastewater and drinking water treatment plants by requiring further treatment or in extreme cases resulting in treatment failure or plant closure (Rashidi et al., 2021).
Core socio-economic sectors such as commercial fisheries farms, tourism, public health, recreational centers, monitoring and management have been adversely affected by the impact of cHABs and other HABs. Cyanobacteria harmful algal blooms have been reported to cause severe societal impacts such as disruption to social and cultural practices, economic loss (Moore et al., 2020), adverse health effects (Backer & Moore, 2012), and losses to both private and public wellbeing (Willis et al., 2018).
Other socioeconomic impacts caused by cHABs include a decline in recreational uses of waterways of habitat for mosquitoes, microbial pathogens, snails as vectors for schistosomiasis, and reduced waterfront real estate values. For example, in six years (2009–2015), algal blooms at Lake Erie and two other lakes in Ohio accounted for a $152 million loss to homeowners (Ohio State University 2017). This was due to a fall in property value due to individual perception of lack of aesthetic appeal of the adjacent lakes, the health concerns they may pose and the decreasing likelihood of recreational activities, which result in a loss in sales of recreational fishing licenses. A similar trend was observed in the coastal US (Florida), where properties in some towns recorded 20.3% price reductions during blooms (red tide) that sometimes were extensively persistent (Bechard, 2021). Smith et al. estimated in 2015 estimated that an annual cost of $271 million Canadian prices could be lost in tourism and recreational activities over 30 years if algal blooms are left unchecked (Smith et al., 2019). The safety of drinking water can also be threatened by the HAB toxins in portable water due to the source contamination of potable water reservoirs. An outbreak of hepatoenteritis that lead to the hospitalization of 140 children and 10 adults linked to Solomon Dam was caused by toxic cyanobacterium Cylindrospermopsis raciborskii (Griffiths, D.J. and Saker. 2003; Poniedziałek et al., 2012). Also, in 1996, a hemodialysis center at Caruaru, Brazil, recorded an episode of cyanotoxin (microcystins and cylindrospermopsin) contamination of water due to a lack of reverse osmosis in the filtration system. One-hundred and thirty-one patients were affected, and 100 patients developed acute liver failure and more than half of them died (Azevedo et al., 2001). These incidents speak to the deleterious of human exposure to cyanotoxins and the need for the strategic management of algal blooms to neutralize toxins from water (Lad et al., 2022).
Another incidence of HAB events was linked to human illnesses and deaths of animals (Trevino-Garrison et al., 2015), as well as the emergency caution “Do not drink” for the residents of Northwest Ohio in the USA in 2014 (Jetoo et al., 2015).
Direct impacts include costs of medical treatments for incidents of human illness, loss of revenue in marine business due to shellfish closure, fish scarcity, decrease in consumers’ demand for fish due to anxiety or fear of SFP, increase in fish price, costs of algae remover from the waterbody or remover of dead fishes from the beach, and investment costs in monitoring and preventing cHABs. Indirect impacts include a substantial decline in tourists in regions or areas affected by cHABs, loss of revenue from trades or businesses such as hotels that serve the industry, and reduction in recreation use of sea, oceans, and lakes (Sanseverino et al., 2016).
Public health effects or impacts are medical costs due to cHABs’ intoxication and allelopathy such as hospitalization costs and the cost of transportation to the hospital (Nwankwegu et al., 2019). Globally, due to the occurrence of HAB, the economic or financial loss incurred is over millions of US dollars (Pal et al., 2021). Despite the known impacts of cHABS on specific industries in different geographic locations or settings, there is still a lot to unveil in terms of the long-term and short-term socio-economic impacts of algae bloom events (Qiao & Saha, 2021). According to the report of Adams et al. (2018), future studies should explore the lagged impacts of cHABs (both in time and space) as well as techniques for assessing the indirect economic impacts of cHABs. In addition, the growing research trend on the public health impacts, seafood consumption impacts, risk preferences, risk assessment, and risk awareness of cHABs can be used to comprehend the complexities involved in knowing the associations between coastal, marine, freshwater resources, and human society.
7 Risk Assessment and Management Approaches for Harmful Algae Blooms
Understanding the environmental conditions that encourage blooms formation is a useful basis for risk assessment and management. Thus, effective water monitoring and management strategies are needed to protect consumers (WHO, 2021). In both raw and drinking water sources, monitoring the presence of algal bloom indicators and their toxins will help provide early warnings of cHAB events and allow water managers to take proper actions when these events threaten the water sources (USEPA, 2015b). Hence, various strategies have been adopted by many countries and commercial enterprises to manage and monitor cHABs and other HABs in coastal waters (Anderson 2012). According to Corcoran and Hunt (2021), management approaches for cHABs and other HABs can be divided into three different categories including prevention, control, and mitigation.
-
(i)
Prevention: in management approaches for cHABs, prevention refers to the actions taken to reduce the occurrence and severity of blooms or to keep cHABs from occurring or directly impacting certain resources such as chemical-algaecides addition, nutrient-loads reduction, water management, biological competition, and hydrodynamics regulation (Zhu et al., 2021). Other effective approaches to prevent HABs including cHABs are to reduce nutrient loads in sewage effluents and prevent fertilizer overload in agricultural soils, groundwater control and stormwater runoff by nature-based solutions such as sedimentation, biofiltration systems, ecotone zones, and denitrification barriers, (Morón-López, 2021; Paerl & Barnard, 2020).
-
(ii)
Control: from a management perspective, control refers to the measures taken to kill or destroy cHABs and other HABs to quickly interrupt bloom formation (Anderson, 2009). In controlling ABs, five common strategies are employed to combat insidious or harmful species, and these include chemical, mechanical, genetic, biological, and environmental control (Qu et al. 2021). Control methods include harvest, flocculation and settling, mixing, flushing, the use of chemical treatment, algicidal bacteria, filter-feeding organisms, cyanophage, plasma discharge technology, UV/Fenton system, and genetic engineering (Bhatt et al. 2023Corcoran and Hunt 2021; El-Sheekh et al, 2023; Li et al., 2023).
-
(iii)
Mitigation: mitigation refers to the measures taken to reduce the negative effects of cHABs and other HABs on the ecosystem, human health, and the economy. Examples of mitigation procedures include monitoring programs, drinking water treatment, closures of shellfish harvest areas, movement of fish or shellfish product away from blooms areas, closures of beaches, lakes, and the use of predictive models (Corcoran & Hunt, 2021).
7.1 Potential Application of Artificial Intelligence in the Risk Assessment and Management of Harmful Algae Blooms
Application of advanced computer programming techniques including artificial intelligence modelling, machine learning, and artificial neural networks has demonstrated promising capacity for the management and assessment of risks associated with HABs to achieve technological and artificial intelligence. Several studies have engaged artificial intelligence for the surveillance and detection of cHABs which can support informed management decisions from field observations complemented by satellite technologies in the US and South Korea (Whitman et al., 2022; Kim et al. 2022). For instance, the RF (random forest) models were deployed for microcystins (MCs) prediction using four identified drivers including total carbon, rainfall, ammonium, and chlorophyll-a while total carbon, chloride, nitrate, and rainfall for CYN (Cylindrospermopsin) predictions. A better understanding of the potential relationships between cyanotoxins and environmental variables can assist policy and decision-making with useful information (You et al., 2022). Artificial intelligence was also deployed to develop a novel analytical algorithm in Israel and applied to satellite imageries to detect and quantify cyanobacterial blooms and to monitor the spatiotemporal variability by estimating the concentration of common phytoplankton pigment chlorophyll a (Chl a) and cyanobacteria-specific pigment phycocyanin from hyperspectral remote sensing data (Dev et al., 2022). Furthermore, a prediction model to predict accurate cyanobacteria blooms concentration was developed through machine learning techniques applicable to water environments with an inconvenient geographical location or frequent sensor failures where enough historical data might be lacking (Ni et al., 2022). Similarly, Cao et al. (2022) developed an integrated convolutional neural networks-long short-term memory networks (CNN-LSTM) for predicting cHABs in Taihu Lake in China using four identified drivers including temperature, wind speed, relative humidity, and precipitation. This technology, if well harnessed and properly deployed, could offer a more reliable solution and guide in decision-making.
8 Challenges Facing the Management of Harmful Algal Blooms
Consistent monitoring is the basis for decision-making, as it gives significant information about the physicochemical parameters of the waterbody, the presence of HABs and emerging pollutants. The problems caused by cHABs and other HABs are serious and in some parts of the world, they are growing worse, but competence and knowledge-based research have emerged in a bid to reduce their effects and protect public health and marine resources. However, efforts made by management and the scientific community are struggling to match the pace of the rapidly evolving world and the diverse challenges it presents, such as population explosion and its peculiar issues (pollution, climate change, global economic crisis), which might affect the future management, especially due to stiff competition with research funding (Anderson 2014). Moreover, despite the incidence and the widespread reports of cHABs in waterbodies throughout the world, and the known health and ecological risks of these blooms, many countries (especially developing countries) do not have proper surface water algal toxins or HAB species monitoring programs. The lack of established monitoring procedures for HAB–related pollutants of emerging concern make it hard to assess the risks posed to the environment and to humans (Brooks et al. 2016). In different countries, the lack of documented water quality standards for algal toxins monitoring makes it difficult to ascertain the impending risk it poses to humans and the environment. In addition, reliable analytical standardized procedures for toxins monitoring are not commonly available, which inherently leads to nonroutine surface water monitoring (Brooks et al. 2016). Likewise, the diversity of blooms and the toxin types they produce present a huge challenge for cHABs control (Anderson, 2009). Presently, one of the major techniques used for cHABs detection and monitoring is the use of satellite, airborne remote sensing, and on-site automatic monitoring networks (Shen et al., 2012). Nevertheless, these current methods are costly and cannot effectively track cHABs’ dynamics involved in the rapid formation, dissipation, and migration practices (Qu et al., 2019). Similarly, the five common strategies that can be employed to control harmful blooms (chemical, mechanical, genetic, biological, and environmental) are not consistently effective because of spatial and temporal distributions and the quick change of cHABs depending on the water current, wind, and cyanobacterial population (Qu et al., 2019). According to the report of Paerl et al. (2016), an increasing warming climate is another predicted future management challenge in controlling the incidence and expansion of cHABs and other HABs than it is at present.
9 Future Perspectives
The increasing number of reports regarding detections of cyanobacteria and their toxins in water pose a high risk to the aquatic ecosystems and endanger public water suppliers (Sukenik & Kaplan, 2021). Managing, controlling, and treating cyanobacteria and cyanotoxins in drinking, recreational, and raw water sources are important in protecting public health, aquatic organisms, and to protect living. In preventing the formation of blooms in the environment, proper conventional treatment plants should be designed to reduce turbidity, remove intact algal cells, and reduce the levels of toxins. Also, it should be recommended that a proper plan for nutrient capture, immobilization and/or immediate repurposing be considered before certain industrial activities (e.g., mining, detergent manufacture and farming) commence full scale. Regular monitoring of water sources and public awareness regarding the economic impacts of cHABs and other HABs would be appreciated to promote precautionary measures principally when risk assessments lead to restrictions of its use. This will help in safeguarding water resources, public health, and making predictions of the occurrence of algal blooms (Schmale et al., 2019). Similarly, to help prevent the impacts of cHABs, public-health strategies should include rapid diagnosis, surveillance of exposure, infection, seafood testing, and treatment of HAB-related infections. From a community perspective in preventing the impacts of cHABs, the following strategies should be adopted.
-
(i)
To create other recreational centers to keep tourism and this will help local businesses during bloom incidence.
-
(ii)
To develop private or public programs that will provide social and economic support to temporarily unemployed workers such as fishermen.
-
(iii)
To develop educational programs that will help ethnically diverse residents to avoid exposure.
For resource management measures, effective, proper, and continuous water monitoring and management approaches should be adopted to help protect consumers, animals, the public, and fisheries as well as to help prevent risks associated with cHABs. From a preventive perspective in regions where cHABs and other HABs have been connected to anthropogenic sources of nutrients, compulsory nutrient management plans will be significant to help reduce the nutrient load in the environment. In this regard sanctions and incentives must be given to ensure the compliance of nutrient management below eutrophication thresholds. Similarly, providing guidance with regards to cyanotoxins standard levels in waters and seafood, regular risk assessment concerning recreational and commercial waters and seafood safety is important and necessary. However, a critical problem for risk assessment is the rapid change of planktonic HABs biomass based on the resilience of the blooms, wind direction, and currents, thereby exposing the insignificance of snapshot cHAB monitoring. Hence, developing proper approaches to constantly monitor water sources for the detection of cyanobacteria and cyanotoxins would support more suitable risk assessment procedures. For example, the installation of on-site bioelectrochemical sensors would be able to accurately capture vital information regarding the behaviour of a water body, which could be used to prevent the early stages of algae formation. In addition to the basic knowledge of risk assessment approaches, other new approaches need to be further developed for affordable practical purposes. Also, more investigation into the natural and environmentally friendly approach to cyanobacteria management is necessary. This has been demonstrated by the multiple roles and applicability of terrestrial bamboo as cyanobacteria bloom regulator in waterbodies was investigated. First, it was observed that extract treatment suppressed Microcystis aeruginosa growth but had little effect on green alga (Scenedesmus obliqus). Also, artificially installed poles or bamboo forest stands along the banks can enhance competitors’ growth especially diatoms that can invade cyanobacteria colonies (Hao et al., 2022). Similarly, standardization of procedures for cyanotoxins analysis as well as the provision of standardized reference material for their quantification is vital for regular or routine water monitoring (Welker et al., 2021). Apart from the earlier mentioned electrochemical sensors, the development of strong in situ sensors capable of quantifying and detecting HABs cells and toxins in waterbodies could also help prevent the outbreak of HABs and appropriate deployment of artificial intelligence. Furthermore, proactive risk avoidance as well as creating public awareness via publication, news media, and placement of warning and advisory signs where CHABs and other HABs have been reported will be significant to preventing human and domestic animal poisoning risk of HABs.
10 Conclusion
cHAB formation and their respective toxin is an environmental issue that has received global concerns, yet inadequately solved. Sadly, as human advancements in agriculture and pop culture continue unregulated, the spontaneity of cHAB formation might remain an overwhelming phenomenon. To sensitize the issue, the detection of cyanotoxins, such as microcystins in the vadose zone, and in drinking water sources from groundwater, watersheds suggest that cyanobacteria toxins may be transferred or transported from cyanobacterial blooms in lakes to groundwater via natural hydrologic processes. In this regard, cHABs might be a greater threat than perceived, though knowledge of the ecotoxicology of different cyanotoxins contaminants in the environment is still fragmentary. Also, more investigations into natural, environmentally friendly approach and affordable technology applicable to cyanobacteria management are required. In conclusion, public health authorities should be alerted to cyanotoxins contamination in drinking water supply sources, food sources, and agricultural products as well as implement proper monitoring using artificial intelligence and treatment procedures to protect citizens from this potential health threat.
Data Availability
Sources of data collected have been mentioned in the text.
References
Adams, C. M,, Larkin, S. L., Hoagland, P., Sancewich, B. (2018). Assessing the economic consequences of harmful algal blooms: A summary of existing literature, research methods, data, and information gaps. Harmful Algal Blooms: A Compendium Desk Reference, pp 337–354
Ærtebjerg, G., Anderson, J. H., Hanson, O. S. (Eds.) (2003). Nutrients and eutrophication in danish marine waters. A challenge for science and management. Danish Environmental Protection Agency & National Environmental Research Institute. pp 126.
Anderson, D. M. (2009). Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean and Coastal Management, 52(7), 342–347.
Anderson, D. M., Cembella, A. D., & Hallegraeff, G. M. (2012). Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4, 143–176.
Annadotter, H., Cronberg, G., Lawton, L., Hansson, H.-B., Göthe, U., & Skulberg, O. (2001). An extensive outbreak of gastroenteritis associated with the toxic cyanobacterium Planktothrix agardhii (Oscillatoriales, Cyanophyceae) in Scania, south Sweden. In I. Chorus (Ed.), Cyanotoxins: Occurrence, causes, consequences (pp. 200–208). Springer-Verlag.
Azevedo, S. M., Carmichael, W., Jochimsen, E. M., Rinehart, K. L., Lau, S., Shaw, G. R., & Eaglesham, G. (2001). Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology, 164(1–3), 32–32.
Backer, L. C., & Moore, S. K. (2012). Harmful algal blooms: Future threats in a warmer world. In A. E. Nemr (Ed.), Environmental pollution and its relation to climate change (pp. 485–512). Nova Science Publishers.
Backer, L. C., McNeel, S. V., Barber, T., Kirkpatrick, B., Williams, C., Irvin, M., Zhou, Y., Johnson, T. B., Nierenberg, K., Aubel, M., et al. (2010). Recreational exposure to microcystins during algal blooms in two California lakes. Toxicol, 55, 909–921.
Backer, L. C., Landsberg, J. H., Miller, M., Keel, K., & Taylor, T. K. (2013). Canine cyanotoxin poisonings in the United States (1920s–2012): Review of suspected and confirmed cases from three data sources. Toxins, 5(9), 1597–1628.
Barsanti, L., & Gualtieri, P. (2014). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Beaulieu, J. J., DelSontro, T., & Downing, J. A. (2019). Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nature Communications, 10, 1375.
Bechard, A. (2021). Gone with the wind: Declines in property values as harmful blooms are blown towards the shore. The Journal of Real Estate Finance and Economics, 62, 242–257.
Benayache, N. Y., Afri-Mehennaoui, F. Z., Kherief-Nacereddine, S., Vo-Quoc, B., Hushchyna, K., Nguyen-Quang, T., & Bouaïcha, N. (2022). Massive fish death associated with the toxic cyanobacterial Planktothrix sp. bloom in the Béni-Haroun Reservoir (Algeria). Environmental Science and Pollution Research, 29(53), pp 80849–80859.
Bhatt, P., Engel, B.A., Reuhs, M. and Simsek, H. (2023). Cyanophage technology in removal of cyanobacteria mediated harmful algal blooms: A novel and eco-friendly method. Chemosphere, p. 137769
Bláha, L., Babica, P., & Maršálek, B. (2009). Toxins produced in cyanobacterial water blooms–Toxicity and risks. Interdisciplinary Toxicology, 2(2), 36.
Bouaïcha, N., Miles, C. O., Beach, D. G., Labidi, Z., Djabri, A., Benayache, N. Y., & Nguyen-Quang, T. (2019). Structural diversity, characterization and toxicology of microcystins. Toxins, 11(12), 714.
Brown, A., Foss, A., Miller, M. A., & Gibson, Q. (2018). Detection of cyanotoxins (microcystins/nodularins) in livers from estuarine and coastal bottlenose dolphins (Tursiops truncatus) from Northeast Florida. Harmful Algae, 76, 22–34.
Cao, H., Han, L., & Li, L. (2022). A deep learning method for cyanobacterial harmful algae blooms prediction in Taihu Lake China. Harmful Algae, 113, 102189.
Carmichael, W. W., & Boyer, G. L. (2016). Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae, 54, 194–212.
Catherine, A., Bernard, C., Spoof, L., & Bruno, M. (2017). Microcystins and nodularins. Handbook Cyanobacterial Monitoring Cyanotoxin Analysis, 1, 107–126.
Chapra, S. C., Boehlert, B., Fant, C., Bierman, V. J., Jr., Henderson, J., Mills, D., Mas, D. M., Rennels, L., Jantarasami, L., Martinich, J., & Strzepek, K. M. (2017). Climate change impacts on harmful algal blooms in US freshwaters: A screening-level assessment. Environmental Science and Technology, 51(16), 8933–8943.
Chatziefthimiou, A. D., Banack, S. A., & Cox, P. A. (2021). Biocrust-produced cyanotoxins are found vertically in the desert soil profile. Neurotoxicity Research, 39(1), 42–48.
Chen, L., Chen, J., Zhang, X., & Xie, P. (2016). A review of reproductive toxicity of microcystins. Journal of Hazardous Materials, 301, 381–399.
Chen, L., Giesy, J. P., Adamovsky, O., Svirčev, Z., Meriluoto, J., Codd, G. A., Mijovic, B., Shi, T., Tuo, X., Li, S. C., & Pan, B. Z. (2021). Challenges of using blooms of Microcystis spp in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Science of The Total Environment, 764, 142319.
Chen, M.-Y., Teng, W.-K., Zhao, L., Hu, C.-X., Zhou, Y.-K., Han, B.-P., Song, L.-R., & Shu, W.-S. (2021b). Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation. ISME Journal, 15, 211–227.
Chen, G., Wang, L., Wang, M., & Hu, T. (2021c). Comprehensive insights into the occurrence and toxicological issues of nodularins. Marine Pollution Bulletin, 162, 111884.
Cheng, B., Xia, R., Zhang, Y., Yang, Z., Hu, S., Guo, F., & Ma, S. (2019). Characterization and causes analysis for algae blooms in large river system. Sustainable Cities and Society, 51, 101707.
Cheung, M. Y., Liang, S., & Lee, J. (2013). Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. Journal of Microbiology, 51(1), 1–10.
Chirico, N., António, D. C., Pozzoli, L., Marinov, D., Malagó, A., Sanseverino, I., Beghi, A., Genoni, P., Dobricic, S., & Lettieri, T. (2020). Cyanobacterial blooms in Lake Varese: Analysis and characterization over ten years of observations. Water, 12(3), 675.
Christensen, V. G., & Khan, E. (2020). Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Science of the Total Environment, 736, 139515.
Codd, G. A., Testai, E., Funari, E., Svirčev, Z. (2020). Cyanobacteria, cyanotoxins, and human health. In: Water treatment for purification from cyanobacteria and cyanotoxins, Anastasia E. Hiskia, Theodoros M. Triantis, Maria G. Antoniou, Triantafyllos Kaludis, Dionysios D. DIonysios, Editors, pp 37–68. John Wiley & Sons
Corcoran, A. A., & Hunt, R. W. (2021). Capitalizing on harmful algal blooms: From problems to products. Algal Research, 55, 102265.
Davis, D. A., Cox, P. A., Banack, S. A., Lecusay, P. D., Garamszegi, S. P., Hagan, M. J., Powell, J. T., Metcalf, J. S., Palmour, R. M., Beierschmitt, A., & Bradley, W. G. (2020). L-serine reduces spinal cord pathology in a vervet model of preclinical ALS/MND. Journal of Neuropathology & Experimental Neurology, 79(4), 393–406.
Dev, P. J., Sukenik, A., Mishra, D. R., & Ostrovsky, I. (2022). Cyanobacterial pigment concentrations in inland waters: Novel semi-analytical algorithms for multi-and hyperspectral remote sensing data. Science of the Total Environment, 805, 150423.
Dittmann, E., Fewer, D. P., & Neilan, B. A. (2013). Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiology Reviews, 37(1), 23–43.
Drobac, B. D., Tokodi, N., Marinović, Z., Lujić, J., Dulić, T., Simić, S. B., Đorđević, N. B., Kitanović, N., Šćekić, I., Urbányi, B., & Meriluoto, J. (2021). Cyanobacteria, cyanotoxins, and their histopathological effects on fish tissues in Fehérvárcsurgó reservoir, Hungary. Environmental Monitoring and Assessment, 193(9), 1–14.
Durán-Vinet, B., Araya-Castro, K., Chao, T. C., Wood, S. A., Gallardo, V., Godoy, K., & Abanto, M. (2021). Potential applications of CRISPR/Cas for next-generation biomonitoring of harmful algae blooms: A review. Harmful Algae, 103, 102027.
El-Sheekh, M. M., Abd Al-Halim, M. A., Mohammed, S. A., (2023). Algae processing by plasma discharge technology: A review. Algal Research, 70, 102983.
Farrer, D., Counter, M., Hillwig, R., & Cude, C. (2015). Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins, 7, 457–477.
Fujiki, H., Suganuma, M., Suguri, H., Yoshizawa, S., Takagi, K., Nakayasu, M., Ojika, M., Yamada, K., Yasumoto, T., Moore, R. E., & Sugimura, T. (1990). New tumor promoters from marine natural products. In S. Hall & G. Strichartz (Eds.), Marine toxins: Origin, structure and molecular pharmacology (pp. 232–240). American Chemical Society.
Funari, E., & Testai, E. (2008). Human health risk assessment related to cyanotoxins exposure. Critical Reviews in Toxicology, 38(2), 97–125.
Ger, K. A., Hansson, L. A., & Lürling, M. (2014). Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biology, 59(9), 1783–1798.
Ghaffar, S., Stevenson, R. J., & Khan, Z. (2016). Cyanobacteria dominance in lakes and evaluation of its predictors: A study of Southern Appalachians Ecoregion, USA. Matec Web Conf, 60, 02001.
Gholami, Z., Mortazavi, M. S., & Karbassi, A. (2019). Environmental risk assessment of harmful algal blooms case study: Persian Gulf and Oman Sea located at Hormozgan Province, Iran. Human and Ecological Risk Assessment, 25(1–2), 271–296.
Greer, B., Maul, R., Campbell, K., & Elliott, C. T. (2017). Detection of freshwater cyanotoxins and measurement of masked microcystins in tilapia from Southeast Asian aquaculture farms. Analyt Bioanalyt Chem, 409(16), 4057–4069.
Griffiths, D. J., & Saker, M. L. (2003). The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environmental Toxicology: An International Journal, 18(2), 78–93.
Guo, L. (2007). Doing battle with the green monster of Taihu Lake. Science, 317(5842), 1166–1166.
Guzmán-Guillén, R., Puerto, M., Gutiérrez-Praena, D., Prieto, A. I., Pichardo, S., Jos, Á., Campos, A., Vasconcelos, V., & Cameán, A. M. (2017). Potential use of chemoprotectants against the toxic effects of cyanotoxins: A review. Toxins, 9(6), 175.
Han, B. N., Liang, T. T., Keen, L. J., Fan, T. T., Zhang, X. D., Xu, L., Zhao, Q., Wang, S. P., & Lin, H. W. (2018). Two marine cyanobacterial aplysiatoxins polyketides, neo-debromoaplysiatoxin A and B, with K+ channel inhibition activity. Organic Letters, 20, 578–581.
Hao, A., Su, M., Kobayashi, S., Zhao, M., & Iseri, Y. (2022). Multiple roles of bamboo as a regulator of cyanobacterial bloom in aquatic systems. Scientific Reports, 12(1), 1–12.
Hilborn, E. D., Roberts, V. A., Backer, L., DeConno, E., Egan, J. S., Hyde, J. B., Nicholas, D. C., Wiegert, E. J., Billing, L. M., DiOrio, M., & Mohr, M. C. (2014). Algal bloom–associated disease outbreaks among users of freshwater lakes—United States, 2009–2010. Morbidity and Mortality Weekly Report, 63(1), 11.
Hofbauer, W. K. (2021). Toxic or otherwise harmful algae and the built environment. Toxins, 13(7), 465.
Huang, J. D., & Zheng, H. (2017). Current trend of metagenomic data analytics for cyanobacteria blooms. Journal of Geoscience and Environment Protection, 5(06), 198–213.
Huang, Y. L., Huang, G. H., Liu, D. F., Zhu, H., & Sun, W. (2012). Simulation-based inexact chance-constrained nonlinear programming for eutrophication management in the Xiangxi Bay of Three Gorges Reservoir. Journal of Environmental Management, 108, 54–65.
Huisman, J., Codd, G. A., Paerl, H. W., Ibelings, B. W., Verspagen, J. M., & Visser, P. M. (2018). Cyanobacterial blooms. Nature Reviews Microbiology, 16(8), 471–483.
Ilieva, V., Kondeva-Burdina, M., Georgieva, T., & Pavlova, V. (2019). Toxicity of cyanobacteria Organotropy of cyanotoxins and toxicodynamics of cyanotoxins by species. Pharmacia, 66, 91.
Jetoo, S., Grover, V. I., & Krantzberg, G. (2015). The Toledo drinking water advisory: Suggested application of the water safety planning approach. Sustainability, 7(8), 9787–9808.
Ji, X., Verspagen, J. M., Van de Waal, D. B., Rost, B., & Huisman, J. (2020). Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Science Advances, 6(8), eaax2926.
Jia-Fong, H., Ouddane, B., Hwang, J. S., & Dahms, H. U. (2021). In silico assessment of human health risks caused by cyanotoxins from cyanobacteria. Biocell, 45(1), 65.
Jiménez, L. D. Q., Guzmán-Guillén, R., Cătunescu, G. M., Campos, A., Vasconcelos, V., Jos, Á., & Cameán, A. M. (2020). A new method for the simultaneous determination of cyanotoxins (Microcystins and Cylindrospermopsin) in mussels using SPE-UPLC-MS/MS. Environmental Research, 185, 109284.
Kankaanpää, H. T., Sipia, V. O., Kuparinen, J. S., Ott, J. L., & Carmichael, W. W. (2001). Nodularin analyses and toxicity of a Nodulariaspumigena (Nostocales, Cyanobacteria) water-bloom in the western Gulf of Finland, Baltic Sea, in August 1999. Phyciologia, 40, 268–274.
Karlson, B., Andersen, P., Arneborg, L., Cembella, A., Eikrem, W., John, U., West, J. J., Klemm, K., Kobos, J., Lehtinen, S., & Lundholm, N. (2021). Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae, 102, 101989.
Kasan, N. A., Yusof, S. Z. M., Manan, H., Khairul, W. M., & Zakeri, H. A. (2021). Inhibitory effect of thiourea derivatives on the growth of blue-green algae. Journal of Environmental Management, 294, 113008.
Keliri, E., Paraskeva, C., Sofokleous, A., Sukenik, A., Dziga, D., Chernova, E., Brient, L., & Antoniou, M. G. (2021). Occurrence of a single-species cyanobacterial bloom in a lake in Cyprus: Monitoring and treatment with hydrogen peroxide-releasing granules. Environmental Sciences Europe, 33(1), 1–14.
Kim, J., Jonoski, A., & Solomatine, D. P. (2022). A classification-based machine learning approach to the prediction of cyanobacterial blooms in Chilgok Weir South Korea. Water, 14(4), 542.
Krienitz, L., Ballot, A., Kotut, K., Wiegand, C., Pütz, S., Metcalf, J. S., Codd, G. A., & Stephan, P. (2003). Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiology Ecology, 43(2), 141–148.
Kubickova, B., Babica, P., Hilscherová, K., & Šindlerová, L. (2019). Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environmental Sciences Europe, 31(1), 1–27.
Kudela, R., Berdalet, E., Urban, E. (2015) Harmful algal blooms: A scientific summary for policy makers. IOC/UNESCO, Paris (IOC/INF-1320)
Kusumawati, D. I., & Mangkoedihardjo, S. (2021). Problems and use of cyanobacteria for environmental improvement–A. Journal of Aridland Agriculture, 7, 9–14.
Lad, A., Breidenbach, J. D., Su, R. C., Murray, J., Kuang, R., Mascarenhas, A., Najjar, J., Patel, S., Hegde, P., Youssef, M., & Breuler, J. (2022). As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life, 12(3), 418.
Lago, J., Rodríguez, P. L., Blanco, L., Vieites, J. M., & Cabado, A. G. (2015). Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Marine Drugs, 13, 6384–6406.
Landsberg, J. H., Hendrickson, J., Tabuchi, M., Kiryu, Y., Williams, B. J., & Tomlinson, M. C. (2020). A large-scale sustained fish kill in the St. Johns River, Florida: A complex consequence of cyanobacteria blooms. Harmful Algae, 92, 101771.
Le Moal, M., Gascuel-Odoux, C., Ménesguen, A., Souchon, Y., Étrillard, C., Levain, A., Moatar, F., Pannard, A., Souchu, P., Lefebvre, A., & Pinay, G. (2019). Eutrophication: A new wine in an old bottle? Science of the Total Environment, 651, 1–11.
Li, J., Li, R., & Li, J. (2017). Current research scenario for microcystins biodegradation-a review on fundamental knowledge, application prospects and challenges. Science of the Total Environment, 595(Suppl C), 615–632.
Li, S. M., Liu, J. P., Song, K. S., Liang, C., & Gao, J. (2019). Analysis of temporal and spatial variation characteristics and driving factors of blue alga blooms in Chaohu Lake based on Landsat images. Resources and Environment in the Yangtze Basin, 28, 205–213.
Li, X., Liu, B., Wang, Y., Yang, Y., Liang, R., Peng, F., Xue, S., Zhu, Z., & Li, K. (2020). Hydrodynamic and environmental characteristics of a tributary bay influenced by backwater jacking and intrusions from a main reservoir. Hydrology and Earth System Sciences, 24(11), 5057–5076.
Li, H., Gu, X., Chen, H., Mao, Z., Zeng, Q., Yang, H., & Kan, K. (2021). Comparative toxicological effects of planktonic Microcystis and benthic Oscillatoria on zebrafish embryonic development: Implications for cyanobacteria risk assessment. Environmental Pollution, 274, 115852.
Li, L., Zhang, H., Mubashar, M., Chen, L., Cheng, S., & Zhang, X. (2023). Parallel filtration for solid-liquid separation: A case study of highly-efficient algal removal under parallel configuration driven by magnetic force. Separation and Purification Technology, 310, 123098.
Lim, M. H., Tay, H. S. M., Devotta, D. A., Mowe, M. A., & Mitrovic, S. M. (2020). Risk management of cyanotoxins in Singapore. Journal of Water Resource and Protection, 12(6), 512–525.
Lin, Q., Wu, Z., Singh, V. P., Sadeghi, S. H. R., He, H., & Lu, G. (2017). Correlation between hydrological drought, climatic factors, reservoir operation, and vegetation cover in the Xijiang Basin, South China. Journal of Hydrology, 549, 512–524.
Liu, W., Qiao, Q., Chen, Y., Wu, K., & Zhang, X. (2014). Microcystin-LR exposure to adult zebrafish (Danio rerio) leads to growth inhibition and immune dysfunction in F1 offspring, a parental transmission effect of toxicity. Aquatic Toxicology, 155, 360–367.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., Barsanti, L., & Gualtieri, P. (2005). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., Hudnell, H. K. (2008). Scientific assessment of freshwater harmful algal blooms. Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology. Washington, D.C., USA
Lopez-Rodas, V., Maneiro, E., Lanzarot, M. P., Perdigones, N., & Costas, E. (2008). Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Veterinary Record, 162(10), 317.
Lu, J., Zhu, B., Struewing, I., Xu, N., & Duan, S. (2019). Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Science and Reports, 9(1), 1–11.
Lu, T., Zhang, Q., Zhang, Z., Hu, B., Chen, J., Chen, J., & Qian, H. (2021). Pollutant toxicology with respect to microalgae and cyanobacteria. Journal of Environmental Sciences, 99, 175–186.
Ma, M., Wang, X., Veroustraete, F., & Dong, L. (2007). Change in area of Ebinur Lake during the 1998–2005 period. International Journal of Remote Sensing, 28, 5523–5533.
Maity, S., Guchhait, R., Chatterjee, A., & Pramanick, K. (2021). Co-occurrence of co-contaminants: Cyanotoxins and microplastics, in soil system and their health impacts on plant–A comprehensive review. Science of the Total Environment, 794, 148752.
Manning, S. R., & Nobles, D. R. (2017). Impact of global warming on water toxicity: Cyanotoxins. Current Opinion in Food Science, 18, 14–20.
Mao, J., Jiang, D., & Dai, H. (2015). Spatial–temporal hydrodynamic and algal bloom modelling analysis of a reservoir tributary embayment. Journal of Hydro-Environment Research, 9(2), 200–215.
Marampouti, C., Buma, A. G., & de Boer, M. K. (2021). Mediterranean alien harmful algal blooms: Origins and impacts. Environmental Science and Pollution Research, 28(4), 3837–3851.
Massey, I. Y., Al Osman, M., Yang, F. (2020). An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Reviews, 41(1), 326–346.
Mccarthy, F. M. G., Riddick, N. L., Volik, O., Danesh, D. C., & Krueger, A. M. (2018). Algal palynomorphs as proxies of human impact on freshwater resources in the Great Lakes region. Anthropocene, 21, 16–31.
Mehinto, A. C., Smith, J., Wenger, E., Stanton, B., Linville, R., Brooks, B. W., Sutula, M. A., & Howard, M. D. (2021). Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats–Evaluating the basis for developing thresholds protective of aquatic life in the United States. Science of the Total Environment, 795, 148864.
Méjean, A., Paci, G., Gautier, V., & Ploux, O. (2014). Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon, 91, 15–22.
Michalak, A. M., Anderson, E. J., Beletsky, D., Boland, S., Bosch, N. S., Bridgeman, T. B., DePinto, J. V., Evans, M. A., Fahnenstiel, G. L., & He, L. (2013). Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proceedings of the National Academy of Sciences of the United States of America, 110(16), 6448–6452.
Miglione, A., Napoletano, M., & Cinti, S. (2021). Electrochemical biosensors for tracing cyanotoxins in food and environmental matrices. Biosensors, 11(9), 315.
Misiou, O., Koutsoumanis, K. (2022). Climate change and its implications for food safety and spoilage. Trends in Food Science and Technology, 126, 142–152.
Moestrup, Ø. (1996). Toxic blue-green algae (cyanobacteria) in 1833. Phycologia, 35(sup6), 5–5.
Moore, S. K., Dreyer, S. J., Ekstrom, J. A., Moore, K., Norman, K., Klinger, T., Allison, E. H., & Jardine, S. L. (2020). Harmful algal blooms and coastal communities: Socioeconomic impacts and actions taken to cope with the 2015 US West Coast domoic acid event. Harmful Algae, 96, 01799.
Moreira, C., Azevedo, J., Antunes, A., & Vasconcelos, V. (2013). Cylindrospermopsin: Occurrence, methods of detection and toxicology. Journal of Applied Microbiology, 114(3), 605–620.
Moreira, C., Ramos, V., Azevedo, J., & Vasconcelos, V. (2014). Methods to detect cyanobacteria and their toxins in the environment. Applied Microbiology and Biotechnology, 98(19), 8073–8082.
Morón-López, J. (2021). A holistic water monitoring approach for effective ecosystem management. Ecohydrology and Hydrobiology, 21(3), 549–554.
Müller, M. N., Mardones, J. I., & Dorantes-Aranda, J. J. (2020). Harmful algal blooms (HABs) in Latin America. Frontiers in Marine Science, 7, 34.
Namsaraev, Z., Melnikova, A., Komova, A., Ivanov, V., Rudenko, A., & Ivanov, E. (2020). Algal bloom occurrence and effects in Russia. Water, 12(1), 285.
Nguyen, T. A. D., Nguyen, L. T., Enright, A., Pham, L. T., Tran, H. Y. T., Tran, T. T., Nguyen, V. H. T., & Tran, D. N. (2021). Health risk assessment related to cyanotoxins exposure of a community living near Tri An Reservoir, Vietnam. Environ Sci Poll Res, 28(40), 56079–56091.
Ni, J., Liu, R., Li, Y., Tang, G., & Shi, P. (2022). An improved transfer learning model for cyanobacterial bloom concentration prediction. Water, 14(8), 1300.
Nwankwegu, A. S., Li, Y., Huang, Y., Wei, J., Norgbey, E., Sarpong, L., Lai, Q., & Wang, K. (2019). Harmful algal blooms under changing climate and constantly increasing anthropogenic actions: the review of management implications. Biotech 3, 9(12), 1–19.
Ogungbile, A. O., Ashur, I., Icin, I., Shapiro, O. H., & Vernick, S. (2021). Rapid detection and quantification of microcystins in surface water by an impedimetric immunosensor. Sensors and Actuators b: Chem, 348, 130687.
Ohio State University. (2017). Algal blooms cost Ohio homeowner $152 million over six years: property values fall, as do fishing license sales, two studies find. ScienceDaily. ScienceDaily, 17 August 2017. sciencedaily.com/releases/2017/08.170817141720.htm Accessed 13 Mar 2022.
Paerl, H. W. (2018). Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climatic pressures. Toxins, 10, 1–16.
Paerl, H. W., & Barnard, M. A. (2020). Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically altered world. Harmful Algae, 96, 101845.
Paerl, H. W., & Otten, T. G. (2013). Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial Ecology, 65, 995–1010.
Paerl, H. W., & Otten, T. G. (2016). Duelling ‘CyanoHABs’: Unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environmental Microbiology, 18(2), 316–324.
Paerl, H. W., Xu, H., Hall, N. S., Rossignol, K. L., Joyner, R., Zhu, G., & Qin, B. (2015). Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: Implications for controlling eutrophication and harmful algal blooms. Journal of Freshwater Ecology, 30, 37–41.
Paerl, H. W., Gardner, W. S., Havens, K. E., Joyner, A. R., McCarthy, M. J., Newell, S. E., Qin, B., & Scott, J. T. (2016). Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae, 54, 213–222.
Pal, M., Yesankar, P. J., Dwivedi, A., & Qureshi, A. (2021). Biotic control of harmful algal blooms (HABs): A brief review. Journal of Environmental Management, 268, 110687.
Papadimitriou, T., Katsiapi, M., Vlachopoulos, K., Christopoulos, A., Laspidou, C., Moustaka-Gouni, M., & Kormas, K. (2018). Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Environmental Pollution, 234, 779–787.
Petterson, L. H., Pozdnyakov, D. (2013). Quantification, species variety, and consequences of harmful algal blooms (HABs). Chapter 1 in: Monitoring of harmful algal blooms. Springer-Praxis, Berlin, pp 1–24.
Pham, T. L., & Utsumi, M. (2018). An overview of the accumulation of microcystins in aquatic ecosystems. Journal of Environmental Management, 213, 520–529.
Pitcher, G. C., & Louw, D. C. (2021). Harmful algal blooms of the Benguela Eastern Boundary upwelling system. Harmful Algae, 102, 101898.
Poniedziałek, B., Rzymski, P., & Kokociński, M. (2012). Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environmental Toxicology and Pharmacology, 34(3), 651–660.
Pouria, S., de Andrade, A., Barbosa, J., Cavalcanti, R. L., Barreto, V. T., Ward, C. J., & Codd, G. A. (1998). Fatal microcystin intoxication in haemodialysis unit in Caruaru Brazil. Lancet, 352(9121), 21–26.
Prabha, R., Singh, D. P., Somvanshi, P., & Rai, A. (2016). Functional profiling of cyanobacterial genomes and its role in ecological adaptations. Genomics Data, 9, 89–94.
Qiao, X., Saha., B. (2021). Quantifying the socio-economic impacts of harmful algal blooms in South-West Florida in 2018. Food and resource economics department, University of Florida institute of food and agricultural sciences. Accessed 16 Mar 2022.
Qin, B., Zhu, G., Gao, G., Zhang, Y., Li, W., Paerl, H. W., & Carmichael, W. W. (2010). A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environmental Management, 45(1), 105–112.
Qu, M., Anderson, S., Lyu, P., Malang, Y., Lai, J., Liu, J., Jiang, B., Xie, F., Liu, H. H., Lefebvre, D. D., & Wang, Y. S. (2019). Effective aerial monitoring of cyanobacterial harmful algal blooms is dependent on understanding cellular migration. Harmful Algae, 87, 101620.
Rashidi, H., Baulch, H., Gill, A., Bharadwaj, L., & Bradford, L. (2021). Monitoring, managing, and communicating risk of harmful algal blooms (HABs) in recreational resources across Canada. Environmental Health Insights, 15, 11786302211014400.
Rodger, H. D., Turnbull, T., Edwards, C., & Codd, G. A. (1994). Cyanobacterial (blue-green-algal) bloom associated pathology in brown trout, Salmo trutta L., in Loch Leven, Scotland. The Journal of Fish Disease, 17, 177–181.
Sakamoto, S., Lim, W. A., Lu, D., Dai, X., Orlova, T., & Iwataki, M. (2021). Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan Korea and Russia. Harmful Algae, 102, 101787.
Sanseverino, I., Conduto, D., Pozzoli, L., Dobricic, S., Lettieri, T. (2016). Algal bloom and its economic impact. European Commission, Joint Research Centre Institute for Environment and Sustainability, EUR 27905 EN. https://doi.org/10.2788/660478
Schaefer, A. M., Yrastorza, L., Stockley, N., Harvey, K., Harris, N., Grady, R., Sullivan, J., McFarland, M., & Reif, J. S. (2020). Exposure to microcystin among coastal residents during cyanobacteria bloom in Florida. Harmful Algae, 92, 101769.
Schmale, D. G., Ault, A. P., Saad, W., Scott, D. T., & Westrick, J. A. (2019). Perspectives on harmful algal blooms (HABs) and the cyberbiosecurity of freshwater systems. Front Bioeng Biotechnol, 7, 128.
Serrà, A., Philippe, L., Perreault, F., & Garcia-Segura, S. (2021). Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Research, 188, 116543.
Shahmohamadloo, R. S., Poirier, D. G., Almirall, X. O., Bhavsar, S. P., & Sibley, P. K. (2020). Assessing the toxicity of cell-bound microcystins on freshwater pelagic and benthic invertebrates. Ecotoxicology and Environmental Safety, 188, 109945.
Shen, L., Xu, H., & Guo, X. (2012). Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors (basel), 12, 7778–7803.
Shi, K., Zhang, Y., Zhou, Y., Liu, X., Zhu, G., Qin, B., & Gao, G. (2017). Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Scientific Reports, 7(1), 1–16.
Skafi, M., Duy, S. V., Munoz, G., Dinh, Q. T., Simon, D. F., Juneau, P., & Sauvé, S. (2021). Occurrence of microcystins, anabaenopeptins and other cyanotoxins in fish from a freshwater wildlife reserve impacted by harmful cyanobacterial blooms. Toxicon, 194, 44–52.
Smith, R. B., Bass, B., Sawyer, D., Depew, D., & Watson, S. B. (2019). Estimating the economic costs of algal blooms in the Canadian Lake Erie Basin. Harmful Algae, 87, 101624.
Smucker, N. J., Beaulieu, J. J., Nietch, C. T., & Young, J. L. (2021). Increasingly severe cyanobacterial blooms and deep-water hypoxia coincide with warming water temperatures in reservoirs. Global Change Biology, 27(11), 2507–2519.
Starling, F., Lazzaro, X., Cavalcanti, C., & Moreira, R. (2002). Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshwater Biology, 47, 2443–2452.
Sukenik, A., & Kaplan, A. (2021). Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms, 9(7), 1472.
Sunesen, I., Méndez, S. M., Mancera-Pineda, J. E., Bottein, M. Y. D., & Enevoldsen, H. (2021). The Latin America and Caribbean HAB status report based on OBIS and HAEDAT maps and databases. Harmful Algae, 102, 101920.
Svirčev, Z., Lalić, D., Bojadžija Savić, G., Tokodi, N., Drobac Backović, D., Chen, L., Meriluoto, J., & Codd, G. A. (2019). Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of Toxicology, 93, 2429–2481.
Tarafdar, L., Mohapatra, M., Muduli, P. R., Kumar, A., Mishra, D. R., & Rastogi, G. (2023). Co-occurrence patterns and environmental factors associated with rapid onset of Microcystis aeruginosa bloom in a tropical coastal lagoon. Journal of Environmental Management, 325, 116580.
Taranu, Z. E., Gregory-Eaves, I., Leavitt, P. R., Bunting, L., Buchaca, T., Catalan, J., Domaizon, I., Guilizzoni, P., Lami, A., McGowan, S., & Moorhouse, H. (2015). Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecology Letters, 18(4), 375–384.
Trevino-Garrison, I., DeMent, J., Ahmed, F. S., Haines-Lieber, P., Langer, T., Ménager, H., Neff, J., Van der Merwe, D., & Carney, E. (2015). Human illnesses and animal deaths associated with freshwater harmful algal blooms—Kansas. Toxins, 7(2), 353–366.
Trinchet, I., Cadel-Six, S., Djediat, C., Marie, B., Bernard, C., Puiseux-Dao, S., Krys, S., & Edery, M. (2013). Toxicity of harmful cyanobacterial blooms to bream and roach. Toxicon, 71, 121–127.
Tsikoti, C., & Genitsaris, S. (2021). Review of harmful algal blooms in the Coastal Mediterranean Sea, with a focus on Greek waters. Diversity, 13(8), 396.
Turner, A. D., Lewis, A. M., Bradley, K., & Maskrey, B. H. (2021). Marine invertebrate interactions with harmful algal blooms–Implications for one health. Journal of Invertebrate Pathology, 186, 107555.
USEPA, (2015a). Drinking water health advisories for two cyanobacterial toxins. Office of Water, 820F15003.
USEPA. (2015b). Recommendations for public water systems to manage cyanotoxins in drinking water. Office of Water (4606M), EPA 815-R-15-010.
USEPA Harmful Algal Blooms (2017): www.epa.gov/nutrientpollution/harmful-algal-blooms
USEPA. (2019). Cyanobacteria and cyanotoxins: Information for drinking water systems. United State Environmental Protection Agency. Office of Water, EPA-810F11001. https://www.epa.gov/sites/default/files/201907/documents/cyanobacteria_and_cyanotoxins_fact_sheet_for_pws_final_06282019.pdf.pdf
USEPA. (2022a). United States environmental protection agency. Learn about Cyanobacteria and Cyanotoxins. https://www.epa.gov/cyanohabs/learn-about-cyanobacteria-and-cyanotoxins
USEPA. (2022b). United States environmental protection agency. Health effects from Cyanotoxins. https://www.epa.gov/cyanohabs/health-effects-cyanotoxins
Veerman, J., Kumar, A., & Mishra, D. R. (2022). Exceptional landscape-wide cyanobacteria bloom in Okavango Delta, Botswana in 2020 coincided with a mass elephant die-off event. Harmful Algae, 111, 102145.
Veldee, M. V. (1931). An epidemiological study of suspected water-borne gastroenteritis. American Journal of Public Health, 21(11), 1227–1235.
Verspagen, J. M., Van de Waal, D. B., Finke, J. F., Visser, P. M., Van Donk, E., & Huisman, J. (2014). Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. Plos One, 9(8), e104325.
Vidal, F., Sedan, D., D’Agostino, D., Cavalieri, M. L., Mullen, E., Parot Varela, M. M., Flores, C., Caixach, J., & Andrinolo, D. (2017). Recreational exposure during algal bloom in Carrasco Beach, Uruguay: A liver failure case report. Toxins, 9(9), 267.
Vieira-Lanero, R., Barca, S., Cobo, M. C., & Cobo, F. (2022). Occurrence of freshwater cyanobacteria and bloom records in Spanish reservoirs (1981–2017). Hydrobiology, 1(1), 122–136.
Vogiazi, V., de la Cruz, A. A., Varughese, E. A., Heineman, W. R., White, R. J., & Dionysiou, D. D. (2021). Sensitive electrochemical detection of microcystin-LR in water samples via target-induced displacement of aptamer associated [Ru (NH3) 6] 3+. ACS ES&T Engineering, 1(11), 1597–1605.
Wan, X., Steinman, A. D., Gu, Y., Zhu, G., Shu, X., Xue, Q., Zou, W., & Xie, L. (2020). Occurrence and risk assessment of microcystin and its relationship with environmental factors in lakes of the eastern plain ecoregion China. Environmental Science and Pollution Research, 27(36), 45095–45107.
Wang, C., Feng, T., Wang, P., Hou, J., & Qian, J. (2017). Understanding the transport feature of bloom-forming Microcystis in a large shallow lake: A new combined hydrodynamic and spatially explicit agent-based modelling approach. Ecological Engineering, 343, 25–38.
Wang, H., Li, H., Sun, K., Huang, H., Zhu, P., & Lu, Z. (2020). Impact of exogenous nitrogen on the cyanobacterial abundance and community in oil-contaminated sediment: A microcosm study. Science of the Total Environment, 710, 136296.
Wang, K., Mou, X., Cao, H., Struewing, I., Allen, J., & Lu, J. (2021a). Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environmental Pollution, 288, 117682.
Wang, H., Xu, C., Liu, Y., Jeppesen, E., Svenning, J. C., Wu, J., Zhang, W., Zhou, T., Wang, P., Nangombe, S., & Ma, J. (2021b). From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize African megafauna. The Innovation, 2(2), 100092.
Welker, M., Chorus, I., Scheffer, B. and Urquhard E. (2021). Planning monitoring programmes for cyanobacteria and cyanotoxins. In: Toxic cyanobacteria in water, pp 641–668 CRC Press.
Whitman, P., Schaeffer, B., Salls, W., Coffer, M., Mishra, S., Seegers, B., Loftin, K., Stumpf, R., & Werdell, P. J. (2022). A validation of satellite derived cyanobacteria detections with state reported events and recreation advisories across US lakes. Harmful Algae, 115, 102191.
WHO. (2010). IARC Monographs on the evaluation of carcinogenic risks to humans, ingested nitrate and nitrite, and cyanobacterial peptide toxins (Vol. 94, p. 450). World Health Organization.
Willis, C., Papathanasopoulou, E., Russel, D., & Artioli, Y. (2018). Harmful algal blooms: The impacts on cultural ecosystem services and human well-being in a case study setting, Cornwall. UK. Mar. Policy, 97, 232–238.
Wood, R. (2016). Acute animal and human poisonings from cyanotoxin exposure—A review of the literature. Environment International, 91, 276–282.
World Health Organization (2021). WHO guidelines on recreational water quality: Volume 1: Coastal and fresh waters. World Health Organization (WHO).
Wu, T., Qin, B., Zhu, G., Luo, L., Ding, Y., & Bian, G. (2013). Dynamics of cyanobacterial bloom formation during short-term hydrodynamic fluctuation in a large shallow, eutrophic, and wind-exposed Lake Taihu China. Environmental Science and Pollution Research, 20(12), 8546–8556.
Wu, J., Hilborn, E. D., Schaeffer, B. A., Urquhart, E., Coffer, M. M., Lin, C. J., & Egorov, A. I. (2021). Acute health effects associated with satellite-determined cyanobacterial blooms in a drinking water source in Massachusetts. Environmental Health, 20(1), 1–13.
Xie, H., Fischer, A. M., & Strutton, P. G. (2021). Generalized linear models to assess environmental drivers of paralytic shellfish toxin blooms (Southeast Tasmania, Australia). Continental Shelf Research, 223, 104439.
Xu, H., Long, L., Yan, M., Ma, J., Ji, D., Liu, D., & Yang, Z. (2020). Modelling the effects of hydrodynamics on thermal stratification and algal blooms in the Xiangxi Bay of Three Gorges Reservoir. Frontiers in Ecology and Evolution, 8, 453.
Xue, Y., Chen, H., Yang, J. R., Liu, M., Huang, B., & Yang, J. (2018). Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. ISME Journal, 12, 2263–2277.
You, L., Tong, X., Te, S. H., Tran, N. H., Sukarji, N. H., He, Y., & Gin, K. Y. H. (2022). Multi-class secondary metabolites in cyanobacterial blooms from a tropical water body: Distribution patterns and real-time prediction. Water Research, 212, 118129.
Yu, Y., Zhang, Q., Liu, G., Deng, Y., Kang, J., Zhang, F., Lu, T., Sun, L., & Qian, H. (2021). Proteomic analysis of zebrafish brain damage induced by Microcystis aeruginosa bloom. Science of the Total Environment, 795, 148865.
Zamyadi, A., MacLeod, S. L., Fan, Y., McQuaid, N., Dorner, S., Sauvé, S., & Prévost, M. (2012). Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Research, 46(5), 1511–1523.
Zhang, Y., Husk, B. R., Duy, S. V., Dinh, Q. T., Sanchez, J. S., Sauvé, S., & Whalen, J. K. (2021). Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec Canada. Chemosphere, 274, 129781.
Zhao, Y., Xue, Q., Su, X., Xie, L., Yan, Y., & Steinman, A. D. (2015). Microcystin-LR induced thyroid dysfunction and metabolic disorders in mice. Toxicol, 328, 135–141.
Zhao, K., Wang, L., You, Q., Pan, Y., Liu, T., Zhou, Y., Zhang, J., Pang, W., & Wang, Q. (2021). Influence of cyanobacterial blooms and environmental variation on zooplankton and eukaryotic phytoplankton in a large, shallow, eutrophic lake in China. Science of the Total Environment, 773, 145421.
Zhou, Y., Wang, L., Zhou, Y., & Mao, X.-Z. (2020). Eutrophication control strategies for highly anthropogenic influenced coastal waters. Science of the Total Environment, 705, 135760.
Zhu, X., Dao, G., Tao, Y., Zhan, X., & Hu, H. (2021). A review on control of harmful algal blooms by plant-derived allelochemicals. Journal of Hazardous Materials, 401, 123403.
Funding
Open access funding provided by University of Pretoria. JOU gratefully acknowledges the financial support from the National Research Foundation, South Africa (Grant no: 138445).
Author information
Authors and Affiliations
Contributions
AI and JOU wrote the manuscript. AI AJK, KMM, ZPK, and JOU reviewed it, and all authors approved the final version.
Corresponding author
Ethics declarations
Ethical Approval
No humans or animals were actively involved in the study.
Consent to Participate
There were no human participants in the study.
Consent to Publish
All authors supported the publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Igwaran, A., Kayode, A.J., Moloantoa, K.M. et al. Cyanobacteria Harmful Algae Blooms: Causes, Impacts, and Risk Management. Water Air Soil Pollut 235, 71 (2024). https://doi.org/10.1007/s11270-023-06782-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06782-y