Abstract
Although several studies have examined the functional diversity of freshwater macroinvertebrates, the variety of methodologies combined with the absence of a synthetic review make our understanding of this field incomplete. Therefore, we reviewed the current methodology for assessing functional diversity in freshwater macroinvertebrate research. Our review showed that most papers quantified functional diversity using biological traits, among which feeding habits were the most common traits probably due to the assumed links between feeding and ecosystem functions. A large number of diversity measures have been applied for quantifying functional diversity of freshwater macroinvertebrate assemblages, among which Rao’s quadratic entropy looks like the most frequent. In most papers, functional diversity was positively related to taxon richness, and functional redundancy was a key concept in explaining this correlation. Most studies detected strong influence of the environmental factors as well as human impact on functional diversity. Finally, our review revealed that functional diversity research is biased towards European running waters and is hindered by yet insufficient information on the autecology of macroinvertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In response to the growing threat of biodiversity loss in virtually all ecosystems, much effort has been devoted to exploring and predicting the consequences of anthropogenic disturbance in ecological communities. In spite of the increasing number of studies, it is still unclear how biological diversity and ecosystem functioning are governed under natural conditions and how they are impacted by human activity (Reiss et al., 2009). Increasing number of experiments have shown that biodiversity enhances, rather than simply responds to ecosystem functions (Balvanera et al., 2006; Cardinale et al., 2009).
Community ecologists and conservation biologists have quantified several facets of diversity simultaneously within species assemblages (Devictor et al., 2010). Of these, taxonomic diversity is the most commonly considered, but it does not fully represent phylogenetic and functional differences among species. Phylogenetic diversity incorporates differences in the evolutionary history of species. Finally, functional diversity relies on those components of biodiversity that influence how an ecosystem operates or functions (Tilman et al., 1997). Consequently, it is the facet of diversity that provides the link between ecosystem functioning and biodiversity (Petchey & Gaston, 2006). As such, Cadotte et al. (2011) and Gagic et al. (2015) reviewed functional diversity measures to bring to the fore emerging ecological patterns and provide clues about ecosystems management and decision-making. Their literature reviews indicate that functional diversity is one of the best predictors of ecosystem function.
According to our current understanding, the effect of biodiversity on ecosystem functioning is manifested through traits, where a trait is “a well-defined, measurable property of organisms, usually measured at individual level and used comparatively across species” (McGill et al., 2006). More generally, a set of traits determines where a species can live (see Lavorel et al., 1997). Ideally, these properties would include process rates (i.e. resource consumption rates), or should reflect specific abilities of organisms (e.g. the existence of specific digestive enzymes). In most cases, however, such information is not available and only surrogates of species functions are applied. For instance, specific leaf area (the ratio of leaf area to leaf mass) is a good surrogate of net photosynthetic rate (Violle et al., 2007), or specific mouthpart characters of aquatic insects might predict feeding specific food items (Cummins, 1974). Thanks to the terminological clarification made by plant ecologists, traits reflecting the effects of organisms on ecosystem functions are collectively called as ‘effect traits’ (Violle et al., 2007) and functional diversity should quantify the variability or diversity of these effect traits (Fig. 1).
Plant ecologists also suggested that the performance and the existence of species in a given environment depend on ‘response traits’ (Violle et al., 2007). Linking this idea to the habitat templet theory of Southwood (1977) suggests that environmental variables can be considered as filters, which constrain organisms. As a result, the response traits are properties of organisms, which allow them coping with different environmental conditions (see e.g. Poff, 1997). Within this theoretical framework, the response of functional diversity to environmental variables and to human impact is an indirect and rather complex mechanism (Fig. 1).
Macroinvertebrates (i.e. invertebrate animals >0.25 mm in length, Rosenberg & Resh, 1993) play an important role in freshwater ecosystems by feeding on algae, coarse detritus or fine particulate organic matter (i.e. contributing to carbon and nitrogen cycles), by engineering (Mermillod-Blondin, 2011; Statzner, 2012) and by providing food for higher trophic levels, such as fish (Covich et al., 1999). However, our knowledge on the way functional diversity of macroinvertebrate assemblages influences patterns and processes in freshwater ecosystems needs to be broadened. Small-scale experimental studies have suggested that the species richness of macroinvertebrates drives ecosystem functioning (Cardinale et al., 2002; Frainer et al., 2014), whereas recent reviews have drawn a more complex picture based on field studies (Lecerf & Richardson, 2010; Vaughn, 2010; Dolédec & Bonada, 2013). In particular, the link between ecosystem functioning and biodiversity depends on a cascade effect among species’ dominance and identity, the existence of positive interactions among species, sequence of species loss, species traits and the environmental context (e.g. Vaughn, 2010). Vaughn (2010) also argued that successful prediction of linkages between biodiversity and ecosystem functioning requires a multitude of empirical approaches. Finally, studies looking at the biodiversity ecosystem-functioning (B-EF) relationship have usually been assessed at the local scale, and therefore the results do not apply directly at a regional scale (Dolédec & Bonada, 2013).
Our review focuses on macroinvertebrate assemblages of freshwater habitats, while those of marine ecosystems or brackish waters are beyond our scope. Although studies regarding these realms overlap to some extent, freshwater and marine sciences have developed more or less independently. Note that we have only considered entire assemblages and their biodiversity, rather than individual or infra-individual levels of organization (see Literature survey).
Although “functional diversity” and related terms are frequently used in studies of freshwater macroinvertebrate assemblages, the terminology used and the concept itself may lack consistency and mathematical clarity, which can cause confusions (see Schmera et al., 2014, 2015). In addition, our present knowledge is based on separate case studies appearing as independent snapshots. However, disentangling how functional diversity of freshwater macroinvertebrate assemblages is governed under natural conditions, while potentially also influenced by human stressors, remains challenging (Fig. 1, see also Dolédec & Statzner, 2010). As former reviews in freshwater ecology have mostly dealt with trait–environment relationships (Bonada et al., 2006; Heino et al., 2013), the present paper focuses on (i) how functional diversity is conceptualized and quantified, (ii) to what extent taxonomic and functional diversity are correlated, (iii) how functional diversity responds to environmental variables and human impact and finally (iv) whether functional diversity drives ecosystem functions and, if so, which ones.
Literature survey
On 28th April 2015, we performed a literature search in ISI Science Citation Index Expanded database from 1900 to 2014 with the following combination of relevant keywords: (“functional diversity” OR “functional richness” OR “functional evenness” OR “functional divergence” or “functional regularity” OR “functional complementarity” OR “functional specialization” OR “functional dispersion” OR “functional redundancy”) AND (“invertebrat*” OR “macroinvertebrat*”). This search resulted in 297 records. Then, after examining the abstracts, only papers related to freshwater assemblages and ecosystems were retained (i.e. studies dealing with marine, lagoon or estuary ecosystems and the functional diversity of enzymes were disregarded), which reduced the number of papers to 90. Finally, each paper was read carefully to check its relevance to macroinvertebrate biodiversity and community ecology in freshwater realms. Twelve papers were irrelevant. Thus, we ended up with 78 relevant papers of which 6 were reviews (all of these papers are cited in the reference list). The earliest of the 78 papers was published in 2000.
How functional diversity is conceptualized and measured?
Recent reviews clearly show that functional diversity has been given a wide variety of conceptual and methodological definitions (Petchey et al., 2004; Botta-Dukát, 2005; Mason et al., 2005; Mouillot et al., 2005; Ricotta, 2005; Petchey & Gaston, 2006; Ricotta & Moretti, 2008; Villéger et al., 2008; Poos et al., 2009; Laliberté & Legendre, 2010; Mouchet et al., 2010; Schleuter et al., 2010). Here, our aim is not to compile yet another overview on the strong and weak points of the different concepts and measures—it has been made by the authors cited above. Instead, we attempt to explain the complex nature of the various terms, which have appeared in association with studies on functional diversity of freshwater macroinvertebrate assemblages. In doing this, we treat the terms concept and measure separately. The term concept will be used in a broad sense referring to general ideas and the term measure as a mathematical expression for quantifying a biological property (for example, conceptualized as functional diversity). In this section, we detail key concepts having primary importance in functional diversity research, namely functional diversity, functional richness, functional evenness, functional divergence, functional redundancy, functional complementarity and functional contribution (Table 1), while measures will be discussed later.
Concepts used in macroinvertebrate studies
Mason et al. (2005) suggested that functional diversity comprises three primary components: functional richness, functional evenness and functional divergence. Although the recent literature uses these terms both as concepts (i.e. different conceptual aspects of functional diversity) as well as measures (when the mathematical expression provided by the developers are followed), in this section we adhere to the former meaning. Functional richness is defined as the amount of trait space occupied by the species, functional evenness relates to the distribution of abundances in the trait space, whereas functional divergence expresses how the abundance distribution maximizes difference in the trait space (Mason et al., 2005; Mouillot et al., 2005).
The concept of functional redundancy is based on observations that some species perform similar roles in communities and ecosystems, and may therefore be substituted with no or very little impact on ecosystem processes (Rosenfeld, 2002; Dolédec & Bonada, 2013). For example, Bêche & Statzner (2009) and Statzner et al. (2004) demonstrated that strong habitat filters prevailing in streams of the USA and Europe promoted functional redundancy in invertebrate communities. Hence, functional redundancy has strong implication in biological conservation because it may compensate for losses of ecosystem functioning after a decline in species richness. Nevertheless, functional redundancy also has some limitations. By examining fish faunas of tropical reefs, Mouillot et al. (2014) observed high functional redundancy combined with high functional vulnerability: some unique combinations of functional traits were represented by a single species. If two species have different roles, then these species complement each other and the concept describing their functional difference is known as functional complementarity (Petchey, 2003). Finally, functional contribution is the functional value (or contribution) of species to the functional diversity of communities (Schmera et al., 2009b).
From our literature survey, functional diversity was the most frequently used term (59 hits out of 78 records) followed by functional redundancy (17), functional richness (11) and functional divergence (5). Rarely occurring concepts were functional evenness (3), functional complementarity (3) and functional contribution (1). That is, freshwater ecologists have considered several concepts of functional diversity in a rather unbalanced manner. The high frequency of the terms like functional redundancy and richness suggests that freshwater macroinvertebrate research is focused on how species can replace each other regarding ecosystem functions (functional redundancy) and on the proportion of the unique functions (functional richness). This is an obvious indication that functional diversity research is usually oriented towards applied ecology and conservation biology.
Do we use mathematically defined terms?
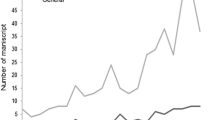
The 78 papers were read carefully to check whether functional diversity or related terms have received mathematical definitions. More than half of the papers (40) used mathematical definitions, whereas a high proportion of the papers (38) did not (e.g. Charvet et al., 2000; Statzner et al., 2005; Dolédec & Statzner, 2008; Brouard et al., 2012). Papers without mathematical definitions include reviews, because reviews generally focused on new findings rather than methodology (e.g. Heino et al., 2013). In other papers without mathematical definitions, functional diversity was interpreted as functional differences among taxa without precise mathematical definition (e.g. Mermillod-Blondin et al., 2002), or functional diversity was used only as a keyword (e.g. Mermillod-Blondin et al., 2004). Noticeably, the number of papers studying functional diversity, as well as that of papers using mathematical terms has increased since 2000 (Fig. 2). In our view, this is a clear indication that functional diversity is treated in our field both as a concept and a measure. We do not have any objection against this, but researchers should keep in mind the dual meaning, and clearly separate concept from measure and recall that some measures have been shown to perform better than others in terms of biodiversity–ecosystem function relationship (Flynn et al., 2011).
Which traits should be used to characterize functional diversity?
Traits in freshwater macroinvertebrate research include a wide variety of features, where trait is a variable that is characterized by one element of a set of distinguishable states (Schmera et al., 2015). Gathering information on the functional roles of freshwater macroinvertebrates is a challenging task, and apart from the availability of information, there is no consensus on which traits (or features) of macroinvertebrate taxa should be used to calculate functional diversity. In this section, we first review the most frequently used groups of traits and, second, we overview the development of functional diversity research in the last 15 years.
The first conceptual model on the functional role of freshwater macroinvertebrates is attributable to Cummins (1974). He studied streams from a functional perspective and described the processing of particulate and dissolved organic matter. Macroinvertebrates were central to his model, which distinguished between species feeding on coarse or fine particulate organic matter, grazing on periphyton and capturing live prey. From a theoretical point of view, this model provided the basis for subsequent and successful river ecosystem concepts (e.g. Vannote et al., 1980).
Twenty years later after the original proposal by Cummins (1974), the research team studying the Upper Rhône River (hereafter referred to as the “Rhône group”; see Chevenet et al. 1994; Dolédec & Statzner, 1994; Statzner et al., 1994; Usseglio-Polatera, 1994) pioneered the use of species traits to characterize entire floodplain communities and to examine trait–environmental variability relationships. The team’s major objective was to use long-term research made on the Upper Rhône for testing the habitat templet concept of Southwood (1977). This concept, adapted to river systems by Townsend & Hildew (1994), suggests that the temporal disturbance and spatial heterogeneity of the river habitats provide the frame for characteristic species traits to evolve. Within their framework, biological traits included maximal body size, potential number of descendants per reproductive cycle, potential number of reproductive cycles per individual, reproductive technique, parental care, distance travelled with or against the current, attachment to soil or substrate, body form and flexibility, resistant life stages, potential for regeneration, food types, feeding habits and respiration. On the other hand, habitat requirements described reproductive period, tolerance to variation in humidity and variability in habitat use. In parallel to this work, Usseglio-Polatera et al. (2000) and Tachet et al. (2010) developed a trait database for European invertebrate genera including “biological traits” such as maximum body size, life span, number of reproduction cycles per year, types of aquatic stages, reproduction technique, dispersal, resistance forms, respiration mode, locomotion and substrate relation (hereafter referred to locomotion), food types and feeding habits. They also provided “ecological traits” or habitat requirements including lateral and longitudinal distribution, altitudinal and substrate preferences, current velocity, trophic status, salinity and temperature preferences, saprobic values and tolerance to low pH. We should emphasize, however, that these traits were suggested for improving the mechanistic understanding of species–environment relationships and not for quantifying functional diversity per se. In addition, the assignment whether a trait is biological or ecological largely depends on the authors. For example, Mondy & Usseglio-Polatera (2014) recently considered biological traits such as maximal body size, life span, number of reproductive cycles per year, types of aquatic stages, reproduction technique, dispersal, resistance forms, respiration mode, locomotion and substrate relation (hereafter referred to locomotion), food types and feeding habits as Eltonian traits, whereas ecological traits such as lateral and longitudinal distribution, altitudinal and substrate preferences, current velocity, trophic status, salinity and temperature preferences and saprobic values and tolerance to low pH were termed Grinnellian traits. Here, we follow Usseglio-Polatera et al. (2000) terminology and use these terms as biological and ecological traits.
In quantifying functional diversity, we found that most papers (28) used only biological traits (including only a subset of biological traits), whereas a small proportion of the papers (8) used both biological and ecological traits. Ecological traits included the above set of Grinnellian traits (Usseglio-Polatera et al., 2000; Colas et al., 2013); rheophily and thermal preference (Poff et al., 2006; Milner et al., 2011; Brown & Milner, 2012); rheophily alone (Colzani et al., 2013); temperature, pH, trophic status, longitudinal distribution, microhabitat and current velocity preferences (Martinez et al., 2013) and substrate preferences (Vaz et al., 2014). Although we do not state that the conclusions drawn from these studies are incorrect, we argue following Verberk et al. (2013) that ecological traits describing habitat preferences of macroinvertebrates should not be used for assessing functional diversity because ecological traits should be regarded as response traits (Violle et al., 2007), and response traits are not directly linked to ecosystem functions (Fig. 1, but see indirect effects in Frainer et al., 2014). We should note, however, that the idea of effect and response traits was developed in the context of plant ecology, and the categorization of traits strongly depends on the actual situation. In the case of freshwater invertebrates, the clear separation of response and effect traits is rather challenging in practice. Authors studying the functional diversity of freshwater macroinvertebrate assemblages used all biological traits (Bady et al., 2005; Peru & Dolédec, 2010), but mostly traits related to feeding habits (Nhiwatiwa et al., 2009; Schmera et al., 2009a, b; Kadoya et al., 2011; Podani et al., 2013), size and feeding habits (Pavoine & Dolédec, 2005) and feeding habits, locomotion and substrate relation (Heino, 2005, 2008; Heino et al., 2008). The frequent occurrence of macroinvertebrate feeding habits is not surprising since they are, as already indicated, directly related to important ecosystem functions in freshwater ecosystems. Regarding the other side of the coin and without questioning the importance of feeding habits in quantifying functional diversity, we should also emphasize that other biological traits may provide useful information on ecosystem functioning (e.g. body size predicts a very large proportion of variability of any process rate; see e.g. Lecerf & Richardson, 2011; or several traits are involved in export of prey animals to terrestrial environments through insect emergence). Therefore, we argue that macroinvertebrate researchers should not restrict themselves to studying only feeding habits-related resource consumption, but recommend the examination of other functions provided by macroinvertebrates in freshwater and terrestrial ecosystems. We also suggest that (1) further autecological studies are needed for quantifying additional and more expressive effect traits of macroinvertebrates, and (2) functional and trait diversity should clearly be separated: the first is an ecosystem function-related term using only effect traits, while the second has no such restrictions.
Measuring functional diversity
The various ways for measuring functional diversity in the papers selected from our literature survey include 14 measures, mainly delivered from terrestrial ecology. Here, we present the chronological appearance of these measures in order to demonstrate the development of this field.
Shannon diversity, richness and evenness
Usseglio-Polatera et al. (2000) applied the Shannon diversity index commonly used to quantify taxonomic diversity (Magurran, 2004), to macroinvertebrate groups defined from the traits of taxa (mostly genera). Several forthcoming papers used the Shannon formula for quantifying functional diversity of freshwater macroinvertebrates (Haybach et al., 2004; Devin et al., 2005; Heino, 2005; Bazzanti et al., 2009). In addition to Shannon diversity, Heino (2008) and Göthe et al. (2014) considered functional richness and functional evenness.
Rao’s quadratic entropy
Following Champely & Chessel (2002), Pavoine & Dolédec (2005) used Rao’s quadratic entropy (Rao, 1982) for measuring functional diversity of freshwater macroinvertebrate assemblages. Bady et al. (2005) demonstrated that functional diversity accumulation curves saturated faster than species richness accumulation curves, a first illustration of the potential functional redundancy within freshwater invertebrate assemblages (see also Bêche & Statzner, 2009). Rao’s quadratic entropy incorporates the pairwise distances of taxa weighted by their relative abundances and appears to be a weighted version of Simpson diversity index. Consequently, the measure is the expected distance between two randomly selected individuals (Ricotta, 2005). According to Mason et al. (2005), Rao’s quadratic entropy quantifies the divergence aspect of functional diversity (see also Brown & Milner, 2012). Pavoine & Dolédec (2005) argued that Rao’s quadratic entropy has an obvious advantage over usual diversity indices because, in addition to incorporating differences in traits, it takes into account the abundance differences between species. There is no doubt that Rao’s quadratic entropy has become the most frequently used measure of functional diversity by occurring in 19 of 40 papers that defined functional diversity mathematically (e.g. Peru & Dolédec, 2010; Vandewalle et al., 2010; Colas et al., 2011; Gallardo et al., 2011; Buendia et al., 2013; Graeber et al., 2013; Paillex et al., 2013; Reynaga & Dos Santos, 2013). In 2014, almost all reviewed papers used Rao’s quadratic entropy for measuring functional diversity of freshwater macroinvertebrates (e.g. Boersma et al., 2014; Feld et al., 2014; Kovalenko et al., 2014; Lange et al., 2014; Vaz et al., 2014).
Number of unique combinations of trait states
Functional diversity measures discussed until now consider traits as independent variables. However, Poff et al. (2006) argued that individual traits of freshwater macroinvertebrate taxa are inter-dependent due to phylogenetic (evolutionary) constraints. To handle this inter-dependency of traits, they measured functional diversity as the number of unique combinations of trait states (Poff et al., 2006). This measure counts the entities (individuals or taxa) differing in (the combination of) trait states. Consequently, the unit of this diversity measure is the pattern (combination) of trait states. This concept was used under the name “unique trait combinations” in Schmera et al. (2012) and Schmera et al. (2013). In agreement with the original proposal (Poff et al., 2006) and following the recommendation of Schmera et al. (2015), this measure should be called “number of unique combinations of trait states”.
Number of trait states
Bêche & Resh (2007) and Bêche & Statzner (2009) proposed to count the number of trait categories as a measure of functional diversity. This measure was also used as “number of traits present” (see Milner et al., 2011) or should be called as “number of trait states” following the terminology of Schmera et al. (2015). This measure disregards evolutionary constraints but focuses on traits assumed to be subject to selection. The same concept was used when trait states were represented by functional group identities (Gallardo et al., 2009, 2014; Nhiwatiwa et al., 2009). In this respect, functional feeding group identities, and in fact the functional feeding groups, are integral parts of the trait-based analyses.
Simpson diversity
Bêche & Resh (2007) applied the Simpson diversity function (Magurran, 2004) to the frequency-distribution of abundance-weighted categories for each trait, and the average measure was termed as trait diversity, whereas Gallardo et al. (2009) calculated the same index for abundance-weighted functional groups of macroinvertebrates. If applied to functional diversity, it is less sensitive to rare functional groups than Shannon diversity (Ricotta & Szeidl, 2006).
Average pairwise distance and MFAD
Bêche & Resh (2007) also used the average pairwise trait-distance between taxa after Heemsbergen et al. (2004), who used this measure for the first time. It is easy to see that this measure is insensitive to the abundance of the taxa.
There are other measures disregarding abundance. For instance, studying functional attribute diversity (FAD) measured through the sum of pairwise dissimilarities of traits among taxa (Walker et al., 1999), Schmera et al. (2009a) recognized that the measure was extremely sensitive to the number of species and increased upon addition of a new species if it was functionally identical to another one already present in the community. As a remedy, Schmera et al. (2009a) developed a new measure termed as “modified FAD” (or MFAD). According to Colas et al. (2013), MFAD quantifies functional divergence. Until now, MFAD has been used only in a few papers examining freshwater macroinvertebrate communities (Schmera et al., 2009b; Colas et al., 2013).
Dendrogram-based measure
Petchey & Gaston (2002) derived the measurement of functional diversity using dendrograms from phylogenetic diversity research. The dendrogram-based measure includes the following three steps: (1) calculating the functional trait dissimilarity matrix of taxa, (2) obtaining the dendrogram from this dissimilarity matrix by cluster analysis, and (3) quantifying functional diversity of the community as the total branch length of the dendrogram. Although the original idea provoked intensive debate on how dendrograms should be used for quantifying functional diversity (Podani & Schmera, 2006, 2007; Petchey & Gaston, 2007, 2009), several papers have used them for quantifying functional diversity of freshwater assemblages (Vidakovic & Palijan, 2010; Kadoya et al., 2011; Patrick & Swan, 2011; Brown & Milner, 2012; Colzani et al., 2013; Martinez et al., 2013). According to Mason et al. (2005), dendrograms quantify the functional richness aspect of functional diversity.
Functional divergence and dispersion
Quantifying the functional diversity of aquatic insects in the Atlantic Forest (Brazil) with dendrograms, Colzani et al. (2013) considered functional divergence and functional dispersion. The authors defined functional divergence as an aspect of functional diversity that enumerates the degree to which an abundance distribution maximizes divergence (or differences) in functional characters within the community (Mason et al., 2005; Mouillot et al., 2005). They further defined functional dispersion as the spread of species within the functional space (see Laliberté & Legendre, 2010). Both measures have rarely been used in quantifying the functional diversity of freshwater macroinvertebrate assemblages (e.g. Frainer et al., 2014).
Combinatorial functional diversity
Recently, Podani et al. (2013) argued that not only unique trait states could quantify functional diversity (see also Poff et al., 2006), but functional diversity can also rely upon the frequency distribution of trait combinations. The methodological framework proposed by the authors is based on information theory and includes several terms like combinatorial functional diversity, combinatorial functional evenness, combinatorial functional richness, functional associatum as well as functional heterogeneity (Podani et al., 2013). Other authors in freshwater science, probably due to a relative new publication and methodology, have not yet used this combinatorial functional diversity framework.
Convex hull
Boersma et al. (2014) recently examined invertebrate assemblages of pools in arid-land streams and quantified functional richness by the volume of a convex hull (i.e. the smallest polyhedron that encloses the points representing species in the functional trait space). This measure was proposed by Villéger et al. (2008) and criticized by Podani (2009) because zero or near zero convex hull volumes might be obtained independently on how wide the individual trait ranges are.
Our review shows that of the 14 measures, Rao’s quadratic entropy is the most frequently used measure for quantifying functional diversity of freshwater macroinvertebrates (Fig. 3). This suggests that, in most cases, functional diversity is interpreted as the expected distance between two randomly selected individuals. In addition to this clear interpretation, the success of this measure could be explained by the facts that this measure was the first to incorporate traits of freshwater macroinvertebrates into the direct measurement of functional diversity (Shannon diversity is based on groups of taxa where traits are used indirectly for producing groups) and that this measure was frequently used by members of the “Rhône group”, who have attained a leading role in trait-based research. Further frequently used measures are the Shannon diversity, dendrogram-based measures and the number of trait states (Fig. 3).
How functional diversity is related to taxonomic diversity?
Most of the surveyed studies showed positive relationship between taxon diversity and functional diversity (Haybach et al., 2004; Bêche & Resh, 2007; Heino, 2008; Bazzanti et al. 2009; Bêche & Statzner, 2009; Vandewalle et al., 2010; Gallardo et al., 2011; Feld et al., 2014), while others revealed a positive and saturating relationship (Bady et al., 2005; Bêche & Statzner, 2009). Finally, a single paper stated that functional diversity fluctuates fairly independently from taxonomic diversity (Reynaga & Dos Santos, 2013). This trend follows Cadotte et al. (2011) who showed that the positive relationship between species richness and functional diversity is not supported in every case. They argued that functional redundancy, type of traits used for quantifying functional diversity, type of functional diversity measures and the strength of environmental filters can all influence the relationship between taxon diversity and functional diversity.
Functional redundancy is a key factor in explaining the taxon richness–functional diversity relationship. A high number of functionally unique species leads to a linear relationship between taxonomic and functional diversity, whereas a high number of redundant species causes a saturating relationship. Poff et al. (2006) recognized that only a limited number of unique trait states are represented in stream macroinvertebrate assemblages having high functional redundancy (Bêche & Resh, 2007; Bêche & Statzner, 2009; Brown & Milner, 2012). In agreement with this finding, Boersma et al. (2014) demonstrated experimentally that due to high functional redundancy, drying did not affect functional richness or functional diversity of stream macroinvertebrates. Mueller et al. (2013) also argued that functional diversity measured through trait (state-) richness shows limited variability.
Three independent studies using different measures proved that the functional diversity of freshwater macroinvertebrates presents relatively high stability and converges to the maximum faster than species richness in the function of sampling effort (Bady et al., 2005; Schmera et al., 2009a; Peru & Dolédec, 2010). These findings can be explained by the functional redundancy within the community.
Heino et al. (2008) and Gallardo et al. (2011) examined the relationship among different diversity measures (including functional ones) for freshwater macroinvertebrate assemblages. They found that even if functional diversity correlated with some, but not all, biodiversity measures, correlations were not high enough to guarantee that any biodiversity measure could replace functional diversity. They concluded that each index quantifies a unique aspect of biodiversity, and the use of multiple measures might describe the multi-faceted aspect of biodiversity more appropriately, a feature in accordance with findings in terrestrial ecosystems (see e.g. Mouchet et al., 2010).
On the role of taxonomic resolution and autecological knowledge in shaping functional diversity research
Our literature survey revealed that only three papers used species-level identification to assess functional diversity, focusing on one (Schmera et al., 2009a, b) or two insect orders (Pavoine & Dolédec, 2005), while the majority of the papers applied mixed taxonomic resolutions, with most individuals being identified at genus or family levels. The appropriate taxonomic resolution to be used in macroinvertebrate studies, a broad zoological group, has been the subject of many papers (see e.g. Lenat & Resh, 2001). One explanation is that, in most taxonomic groups, species are rather difficult to identify at early life stages. In addition, larvae of less known groups of freshwater macroinvertebrates are also difficult to identify at the species level (e.g. Chironomidae). There are considerable advances in autecological information on macroinvertebrates identified in Europe at species level (Schmedtje & Colling, 1996; Moog, 2002; Hering et al., 2004; Furse et al., 2006; Schmidt-Koiber & Hering, 2015). However, autecological information is often lacking for many species due, for example, to their small abundance and thus being less studied (see Supplementary Table 1 for some trait databases). As autecological information is measurable at individual level, we do not see any theoretical objection against the use of taxonomical resolution higher than species level. In a few studies, macroinvertebrates identified at genus level accurately described the variation of biological traits in the assemblages (Dolédec et al., 2000; Gayraud et al., 2003; but see Waringer et al., 2013). Thus, genus level seems to be a good compromise between sufficient taxonomic resolution and available biological trait information. However, studies are still silent on how taxonomical resolution influences functional diversity measures. We hence suggest that the effects of taxonomic resolution on variation in different functional diversity measures will be examined in future studies.
Most studies examining the functional diversity of freshwater macroinvertebrates are based on identification over the species level. A recent study by Waringer et al. (2013) showed that contrasting characters of species are disregarded when species are aggregated to genera or families (see also Tachet et al., 2010), and thus handling genera and especially families as homogeneous units may prevent from the real functional diversity value of communities. Using this example, we by no means state that identification at higher ranks is useless or the findings based on these data are misleading, but emphasize that more detailed autecological knowledge at the species level would strengthen our understanding on functional diversity of freshwater macroinvertebrate assemblages. Most importantly, the biological trait information is biased towards the most abundant species, which are easy to collect and study, whereas knowledge is often lacking for rare species (Dolédec & Statzner, 2010).
Our literature survey on the functional diversity of freshwater macroinvertebrates is biased towards Europe and North America, while South America, Africa, Asia, Australia and Oceania are underrepresented (Fig. 4). This global unevenness should encourage research in the latter continents. Regarding habitat types, research is also biased, because streams (including rivers) are overrepresented (36 of 40 papers), while ponds (2) and lakes (2) are exemplified by much fewer papers. All of these suggest that most of our knowledge is originated from the study of European and North American streams, and this bias might influence our synthesis.
How functional diversity responds to environmental variables and to human impacts?
Most, but not all, studies suggest that functional diversity of macroinvertebrate assemblages responds to different environmental factors (Table 2). The most likely explanation for this dependence is that individual species are sensitive to environmental filtering (Townsend & Hildew, 1994), through their response traits, and thus environmental filtering has an indirect effect on response traits, and this change modifies functional diversity (Fig. 1). Interestingly, we did not find any study that examines how phylogenetic constrains influence the link between response and effect traits. In contrast, in terrestrial ecology, Cadotte et al. (2011) demonstrated that by comprising trait variability across taxonomic levels, phylogenetic diversity could be a better predictor of ecosystem function than species diversity. Finally, we should extend Fig. 1 by the note that abiotic factors might also have direct impact on ecosystem functions (i.e. temperature mediates multiple processes, Truchy et al., 2015).
Results regarding the sensitivity of functional diversity of macroinvertebrates to human impact are contradictory (Table 3). Most of the studies have shown that functional diversity is sensitive to human impact. Despite the globally similar response of functional diversity and species richness to human impacts, our review has detected differences as well. These differences may stem from variation in anthropogenic pressure, the selected functional diversity measures, taxonomic resolution and taxon pools over the study regions as well as from the existence of functional redundancy. For instance, Devin et al. (2005) found that invasive species replaced some native macroinvertebrate taxa in the Moselle River, and thus taxon richness did not change. In contrast, functional diversity of macroinvertebrates significantly increased due to functional redundancy (taxon loss did not result in functional loss) and due to the new functions provided by the invasive taxa.
How functional diversity drives ecosystem functions and which ones?
As the definition of functional diversity promises a link between biodiversity and ecosystem functions, we examined which ecosystem functions were examined and how functional diversity of macroinvertebrates drives these functions. Interestingly, none of the papers using a mathematically defined functional diversity measure specified or quantified any ecological functions.
This finding is surprising, because experimental evidences suggest that the diversity of suspension feeders influences the filtering of suspended particulate material from water (Cardinale et al., 2002) and because the effects of detritivore diversity on leaf litter processing has become a flagship of stream ecology research (Jonsson & Malmqvist, 2000, 2003; Gessner et al., 2010; Lecerf & Richardson, 2010; Frainer et al., 2014; Frainer & McKie, 2015).
The most likely explanation is that the authors using the term “functional diversity” were not interested in concrete ecological functions, and focused only on aspects of diversity that influence ecosystem functions in general. For understanding this, we should also keep in mind that macroinvertebrates play many different functions in freshwater habitats. Consequently, if a taxon list is not restricted to a single functional group (i.e. detritivores), then functional diversity cannot be connected to a single ecosystem function (i.e. detritus processing) only. This indicates missing information on how functional diversity of entire macroinvertebrate assemblages can be linked to ecosystem functions. Consequently, functional diversity measured by several traits should be interpreted as a general indicator.
On the other hand, studies examining macroinvertebrate diversity—macroinvertebrate ecosystem function relationships have never used the term “functional diversity”, only modelled biodiversity through changes in species richness. This indicates that studying biodiversity through species richness might be more straightforward than through a multi-faceted term like functional diversity.
Recommendations for future research
Incorporate phylogenetic relatedness of the taxa in quantifying functional diversity
Although some functional diversity measures assume the non-independence of traits, a direct incorporation of phylogenetic relatedness of taxa into the measurement of functional diversity is still missing.
Use the same methodology
Unfortunately, the limited number of replicated studies did not allow us to draw solid and statistically sound conclusions. In addition, the wide variety of functional diversity measures with different mathematical properties prevented us from conducting a meta-analysis, which may weaken our conclusions. However, although the use of a single functional diversity measure would support drawing general conclusions, it would at the same time severely restrict the examination of the complex nature of functional diversity. For supporting comparative analyses, we suggest to use already used indices for quantifying functional diversity of freshwater macroinvertebrates.
Study autecology of macroinvertebrates
Our review showed that autecological information on many species is often lacking. This missing knowledge clearly hampers the development of functional diversity research. This also suggests that further autecological studies are needed.
Study the relationship between taxonomic resolution and functional diversity
Our review showed that the knowledge on the effect of taxonomic resolution on functional diversity is still in infancy. As most of the studies use mixed taxonomic resolutions, our present state of knowledge might strongly be influenced by the relationship between taxonomic resolution and functional diversity. Examining this relationship would result in a stronger support of our present state of knowledge.
Study functional diversity of freshwater macroinvertebrates in underrepresented continents and habitats
We found that functional diversity research is biased towards European and North American streams and further studies are required in less examined continents (Africa, Asia, Australia and Oceania) and habitats (ponds, lakes).
Narrow the gap between functional diversity research and research on biodiversity—ecosystem functioning
We found that functional diversity research does not meet with ecosystem functions. This suggests that knowledge on the impact of functional diversity on ecosystem processes is apparent, and functional diversity of entire macroinvertebrate assemblages can be regarded as general indicator. Thus, in the future, effort should be made to uncover the relationship between functional diversity and ecosystem functions.
Conclusions
Although theory suggests a direct link between functional diversity and ecosystem functions, the latter are rarely addressed in freshwater macroinvertebrate research. Consequently, freshwater ecologists quantify functional diversity as a general indicator, and little is known about the true functions of macroinvertebrates in freshwaters. We found that functional diversity of macroinvertebrates is sensitive to different environmental variables as well as to the different kinds of anthropogenic impact. Finally, several factors hinder drawing general conclusions from the existing studies, including bias towards certain geographic regions and certain measures of functional diversity.
References
Bady, P., S. Dolédec, C. Fesl, S. Gayraud, M. Bacchi & F. Schöll, 2005. Use of invertebrate traits for the biomonitoring of European large rivers: The effects of sampling effort on genus richness and functional diversity. Freshwater Biology 50: 159–173.
Balvanera, P., A. B. Pfisterer, N. Buchmann, J. S. He, T. Nakashizuka, D. Raffaelli & B. Schmid, 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology Letters 9: 1146–1156.
Bazzanti, M., V. D. Bella & F. Grezzi, 2009. Functional characteristics of macroinvertebrate communities in Mediterranean ponds (Central Italy): influence of water permanence and mesohabitat type. Annales de Limnologie – International. Journal of Limnology 45: 29–39.
Bêche, L. A. & V. H. Resh, 2007. Biological traits of benthic macroinvertebrates in California Mediterranean-climate streams: long-term annual variability and trait diversity patterns. Fundamental and Applied Limnology 169: 1–23.
Bêche, L. A. & B. Statzner, 2009. Richness gradients of stream invertebrates across the USA: taxonomy- and trait-based approaches. Biodiversity & Conservation 18: 3909–3930.
Boersma, K. S., M. T. Bogan, B. A. Henrichs & D. A. Lytle, 2014. Invertebrate assemblages of pools in arid-land streams have high functional redundancy and are resistant to severe drying. Freshwater Biology 59: 491–501.
Bonada, N., N. Prat, V. H. Resh & B. Statzner, 2006. Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annual Review of Entomology 51: 495–523.
Botta-Dukát, Z., 2005. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. Journal of Vegetation Science 16(5): 533–540.
Brown, L. E. & A. M. Milner, 2012. Rapid loss of glacial ice reveals stream community assembly process. Global Change Biology 18: 2195–2204.
Brouard, O., R. Cereghino, B. Corbara, C. Leroy, L. Pelozuelo, A. Dejan & J. F. Carrias, 2012. Understorey environments influence functional diversity in tank-bromeliad ecosystems. Freshwater Biology 57: 815–823.
Buendia, C., C. N. Gibbins, D. Verica, R. J. Batalla & A. Douglas, 2013. Detecting the structural and functional impacts of fine sediment on stream invertebrates. Ecological Indicators 25: 184–196.
Cadotte, M. W., K. Carscadden & N. Mirotchnick, 2011. Beyond species:functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology 48: 1079–1087.
Cardinale, B. J., M. A. Palmer & S. L. Collins, 2002. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415: 426–429.
Cardinale, B. J., D. M. Bennett, G. E. Nelson & K. Gross, 2009. Does productivity drive diversity or vice versa? A test of the multivariate productivity-diversity hypothesis in streams. Ecology 90: 1227–1241.
Champely, S. & D. Chessel, 2002. Measuring biological diversity using Euclidean metrics. Environmental Ecological Statistics 9: 167–177.
Charvet, S., B. Statzner, P. Usseglio-Polatera & B. Dumont, 2000. Traits of benthic macroinvertebrates in semi-natural French streams: an initial application to biomonitoring in Europe. Freshwater Biology 43: 277–296.
Chevenet, F., S. Dolédec & D. Chessel, 1994. A fuzzy coding approach for the analysis of long-term ecological data. Freshwater Biology 31: 295–309.
Colas, F., V. Archaimbault & S. Devin, 2011. Scale-dependency of macroinvertebrate communities: responses to contaminated sediment within run-of-river dams. Science of Total Environment 409: 1336–1343.
Colas, F., V. Archaimbault, J.-F. Férard, J. Bouquerel, M.-C. Roger & S. Devin, 2013. Benthic indicators of sediment quality associated with run-of-river reservoirs. Hydrobiologia 703: 149–164.
Colzani, E., T. Siqueira, M. T. Suriano & F. O. Roque, 2013. Response of aquatic insects functional diversity to landscape changes in Atlantic Forest. Biotropica 45: 343–350.
Covich, A. P., M. A. Palmer & T. A. Crowl, 1999. The role of benthic invertebrate species in freshwater ecosystems. BioScience 49: 119–127.
Cummins, K. W., 1974. Structure and function of stream ecosystems. BioScience 24: 631–641.
Devictor, V., D. Mouillot, C. Meynard, F. Jiguet, W. Thuiller & N. Mouquet, 2010. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecology Letters 13: 1030–1040.
Devin, S., J. N. Beisel, P. Usseglio-Polatera & J. C. Moreteau, 2005. Changes in functional diversity in an invaded freshwater ecosystem: the Moselle River. Hydrobiologia 542: 113–120.
Dolédec, S. & N. Bonada, 2013. So What? Implications of loss of biodiversity for ecosystem functioning. In Sabater, S. & A. Elosegi (eds), River Conservation Challenges and Opportunities. Fundacion BBVA, Madrid: 169–192.
Dolédec, S. & B. Statzner, 1994. Theoretical habitat templets, species traits, and species richness: 548 plant and animal species in the Upper Rhône River and its floodplain. Freshwater Biology 31: 523–538.
Dolédec, S. & B. Statzner, 2008. Invertebrate traits for the biomonitoring of large European rivers: an assessment of specific types of human impact. Freshwater Biology 53: 617–634.
Dolédec, S. & B. Statzner, 2010. Response of freshwater biota to human disturbances: contribution of J-NABS to the developments in ecological integrity assessments. Journal of the North American Benthological Society 29: 286–311.
Dolédec, S., M. J. Olivier & B. Statzner, 2000. Accurate description of the abundance of taxa and their biological traits in stream invertebrate communities? Effect of taxonomic and spatial resolution. Archiv für Hydrobiologie 148: 25–43.
Feld, C. K., F. de Bello & S. Dolédec, 2014. Biodiversity of traits and species both show weak response to hydromorphological alteration in lowland river macroinvertebrates. Freshwater Biology 59: 233–248.
Flynn, D. F. B., N. Mirotchnick, M. Jain, M. I. Palmer & S. Naeem, 2011. Functional and phylogenetic diversity as predictors of biodiversity-ecosystem function relationships. Ecology 92: 1573–1581.
Frainer, A. & B. G. McKie, 2015. Shifts in the diversity and composition of consumer traits constrain the effects of land use on stream ecosystem functioning. Advances in Ecological Research 52: 169–200.
Frainer, A., B. G. McKie & B. Malmqvist, 2014. When does diversity matter? Species functional diversity and ecosystem functioning across habitat and seasons in a field experiment. Journal of Animal Ecology 83: 460–469.
Furse, M., D. Hering, O. Moog, P. F. M. Verdonschot, R. K. Johnson, K. Brabec, K. Gritzalis, A. Buffagni, P. Pinto, N. Frieberg, J. Murray-Bligh, J. Kokes, R. Albert, P. Usseglio-Polatera, P. Haase, R. Sweeting, R. Bis, K. Szoszkiewicz, H. Soszka, G. Springe, F. Sporka & I. Krno, 2006. The STAR project: context, objectives and approaches. Hydrobiologia 566: 3–29.
Gagic, V., I. Bartomeus, T. Jonsson, A. Taylor, C. Winqvist, C. Fischer, E. M. Slade, I. Steffan-Dewenter, M. Emmerson, S. G. Potts, T. Tscharnke, W. Weisser & R. Bommarco, 2015. Functional identity and diversity predict ecosystem functioning better than species-based indices. Proceedings of the Royal Society B 282: 2014–2620.
Gallardo, B., S. Gascon, A. Cabezas, M. Gonzalez, M. Garcia & F. A. Comin, 2009. Relationship between invertebrate traits and lateral environmental gradients in a Mediterranean river-floodplain. Fundamental and Applied Limnology 173: 281–292.
Gallardo, B., S. Gascon, X. Quintana & F. A. Comin, 2011. How to choose a biodiversity indicator – redundancy and complementarity of biodiversity metrics in freshwater ecosystems. Ecological Indicators 11: 1177–1184.
Gallardo, B., S. Dolédec, A. Paillex, D. B. Arscott, F. Sheldon, F. Zilli, S. Mérigoux, E. Castella & F. A. Comín, 2014. Response of benthic macroinvertebrates to gradients in hydrological connectivity: a comparison of temperate, subtropical, Mediterranean and semiarid river floodplains. Freshwater Biology 59: 630–648.
Gayraud, S., B. Statzner, P. Bady, A. Haybach, F. Schöll, P. Usseglio-Polatera & M. Bacchi, 2003. Invertebrate traits for the biomonitoring of large European rivers: an initial assessment of alternative metrics. Freshwater Biology 48: 2045–2064.
Gessner, M. O., C. M. Swan, C. K. Dang, B. McKie, R. D. Bargett, D. H. Wall & S. Hattenschwiler, 2010. Diversity meets decomposition. Trends in Ecology and Evolution 25: 372–380.
Göthe, E., L. Sandin, C. R. Allen & D. G. Angeler, 2014. Quantifying spatial scaling patterns and their local and regional correlates in headwater streams: implications for resilience. Ecology and Society 19: 15.
Graeber, D., M. T. Pusch, S. Lorenz & M. Brauns, 2013. Cascading effects of flow reduction on the benthic invertebrate community in a lowland river. Hydrobiologia 717: 147–159.
Haybach, A., F. Schöll, B. König & F. Kohmann, 2004. Use of biological traits for interpreting functional relationships in large rivers. Limnologica 34: 451–459.
Heemsbergen, D. A., M. P. Berg, M. Loreau, J. R. van Hal, J. H. Faber & H. A. Verhoef, 2004. Biodiversity effects on soil processes explained by intraspecific functional dissimilarity. Science 306: 1019.
Heino, J., 2005. Functional diversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshwater Biology 50: 1578–1587.
Heino, J., 2008. Patterns of functional biodiversity and function-environment relationships in lake littoral macroinvertebrates. Limnology and Oceanography 53: 1446–1455.
Heino, J., H. Mykrä & J. Kotanen, 2008. Weak relationship between landscape characteristics and multiple facets of stream macroinvertebrate biodiversity in a boreal drainage basin. Landscape Ecology 23: 417–426.
Heino, J., D. Schmera & T. Erős, 2013. A macroecological perspective of trait patterns in stream communities. Freshwater Biology 58: 1539–1555.
Hering, D., O. Moog, L. Sandin & P. Verdonschot, 2004. Overview and application of the AQEM assessment system. Hydrobiologia 516: 1–20.
Jonsson, M. & B. Malmqvist, 2000. Ecosystem process rate increases with animal species richness: evidence from leaf-eating, aquatic insects. Oikos 89: 519–523.
Jonsson, M. & B. Malmqvist, 2003. Mechanisms behind positive diversity effects on ecosystem functioning: testing the facilitation and interference hypotheses. Oecologia 134: 554–559.
Kadoya, T., M. Akasaka, T. Aoki & N. Takamura, 2011. A proposal of framework to obtain integrated biodiversity indicator for agricultural ponds incorporating the simultaneous effects of multiple pressures. Ecological Indicators 11: 1396–1402.
Kovalenko, K. A., V. J. Brady, J. J. H. Ciborowski, S. Ilyuskin & L. B. Johnson, 2014. Functional changes in littoral macroinvertebrate communities in response to watershed-level anthropogenic stress. Plos One 9: e101499.
Laliberté, E. & P. Legendre, 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91: 299–305.
Lange, K., C. R. Townsend & C. D. Matthaei, 2014. Can biological traits of stream invertebrates help disentangle the effects of multiple stressors in an agricultural catchment? Freshwater Biology 59: 2431–2446.
Lavorel, S., S. McIntyre, J. Landsberg & T. D. A. Forbes, 1997. Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends in Ecology & Evolution 12: 474–478.
Lecerf, A. & J. S. Richardson, 2010. Biodiversity-ecosystem function research: insight gained from streams. River Research and Applications 26: 45–54.
Lecerf, A. & J. S. Richardson, 2011. Assessing the functional importance of large-bodied invertebrates in experimental headwater streams. Oikos 120: 950–960.
Lenat, D. R. & V. H. Resh, 2001. Taxonomy and stream ecology – The benefits of genus- and species-level identifications. Journal of the North American Benthological Society 20: 287–298.
Magurran, A. E., 2004. Measuring Biological Diversity. Blackwell Science Ltd, Oxford.
Martinez, A., A. Larranaga, A. Basarungen, J. Perez, C. Medoza-Lera & J. Pozo, 2013. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711: 31–42.
Mason, N. W. H., D. Mouillot, W. G. Lee & J. B. Wilson, 2005. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111: 112–118.
McGill, B. J., B. J. Enquist, E. Weiher & M. Westoby, 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21: 178–185.
Mermillod-Blondin, F., 2011. The functional significance of bioturbation and biodeposition on biogeochemical processes at the water-sediment interface in freshwater and marine ecosystems. Journal of the North American Benthological Society 30: 770–778.
Mermillod-Blondin, F., M. Gerino, M. C. des Chatelliers & V. Degrange, 2002. Functional diversity among 3 detritivorous hyporheic invertebrates: an experimental study in microcosms. Journal of the North American Benthological Society 21: 132–149.
Mermillod-Blondin, F., J.-P. Gaudet, M. Gerino, G. Desrosiers, J. Jose & M. C. des Chatelliers, 2004. Relative influence of bioturbation and predation on organic matter processing in river sediments: a microcosm experiment. Freshwater Biology 49(7):895–912.
Milner, A. M., A. L. Robertson, L. E. Brown, S. H. Sonderland, M. McDermott & A. J. Veal, 2011. Evolution of a stream ecosystem in recently deglaciated terrain. Ecology 92: 1924–1935.
Mondy, C. P. & P. Usseglio-Polatera, 2014. Using fuzzy-coded traits to elucidate the non-random role of anthropogenic stress in the functional homogenization of invertebrate assemblages. Freshwater Biology 59: 584–600.
Moog, O., 2002. Fauna Aquatica Austriaca. A Comprehensive Species Inventory of Austrian Aquatic Organisms with Ecological Notes. Wasserwirtschaftkataster, Bundesministerium für Land- und Fortwirtschaft, Umwelt und Wasserwirtschaft, Vienna.
Mouillot, D., N. W. H. Norman, O. Dumay & J. B. Wilson, 2005. Functional regularity: a neglected aspect of functional diversity. Oecologia 142: 353–359.
Mouchet, M. A., S. Villéger, N. W. H. Mason & D. Mouillot, 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Functional Ecology 24: 867–876.
Mouillot, D., S. Villéger, V. Parravicini, M. Kulbicki, J. E. Arias-Gozalez, M. Bender, P. Chabanet, S. R. Floeter, A. Friedlander, L. Vigliola & D. R. Bellwood, 2014. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proceedings of the National Academy of Sciences of the United States of America 111: 13757–13762.
Mueller, M., J. Pander & J. Geist, 2013. Taxonomic sufficiency in freshwater ecosystems: effects of taxonomic resolution, functional traits, and data transformation. Freshwater Science 32: 762–778.
Nhiwatiwa, T., T. D. Bie, B. Vervaeke, M. Barson, M. Stevens, M. P. M. Vanhove & L. Brendonck, 2009. Invertebrate communities in dry-season pools of a large subtropical river: patterns and processes. Hydrobiologia 630: 169–186.
Patrick, C. H. & C. M. Swan, 2011. Reconstructing the assembly of stream-insect metacommunity. Journal of the North American Benthological Society 30: 259–272.
Paillex, A., S. Dolédec, E. Castella, S. Mérigoux & D. C. Aldridge, 2013. Functional diversity in a large river floodplain: anticipating the response of native and alien macroinvertebrates to the restoration of hydrological connectivity. Journal of Applied Ecology 50: 97–106.
Pavoine, S. & S. Dolédec, 2005. The apportionment of quadratic entropy: a useful alternative for partitioning diversity in ecological data. Environmental and Ecological Statistics 12: 125–138.
Peru, N. & S. Dolédec, 2010. From compositional to functional biodiversity metrics in bioassessment: a case study using stream macroinvertebrate communities. Ecological Indicators 10: 1025–1036.
Petchey, O. L., 2003. Integrating methods that investigate how complementarity influences ecosystem functioning. Oikos 101(2): 323–330.
Petchey, O. L. & K. K. Gaston, 2002. Functional diversity (FD), species richness and community composition. Ecology Letters 5: 402–411.
Petchey, O. L. & K. J. Gaston, 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9: 741–758.
Petchey, O. L. & K. J. Gaston, 2007. Dendrograms and measuring functional diversity. Oikos 116: 1422–1426.
Petchey, O. L. & K. J. Gaston, 2009. Dendrograms and measures of functional diversity: a second installment. Oikos 118: 1118–1120.
Petchey, O. L., A. Hector & K. J. Gaston, 2004. How different measures of functional diversity perform? Ecology 85: 847–857.
Podani, J., 2009. Convex hulls, habitat filtering, and functional diversity: mathematical elegance versus ecological interpretability. Community Ecology 10: 244–250.
Podani, J. & D. Schmera, 2006. On dendrogram-based measures of functional diversity. Oikos 115: 179–185.
Podani, J. & D. Schmera, 2007. How should a dendrogram-based measure of functional diversity function? A rejoinder to Petchey and Gaston. Oikos 116: 1427–1430.
Podani, J., C. Ricotta, J. G. Pausas & D. Schmera, 2013. Combinatorial functional diversity: an information theoretical approach. Community Ecology 14: 180–188.
Poff, N. L., 1997. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16: 391–409.
Poff, N. L., J. D. Olden, N. K. M. Vieira, D. S. Finn, M. P. Simmons & B. C. Kondratieff, 2006. Functional trait niches of North American lotic insects: trait-based ecological applications in light of phylogenetic relationships. Journal of the North American Benthological Society 25: 730–755.
Poos, M. S., S. C. Walker & D. A. Jackson, 2009. Functional-diversity indices can be driven by methodological choices and species richness. Ecology 90: 341–347.
Rao, C. R., 1982. Diversity and dissimilarity coefficients: a unified approach. Theoretical Population Biology 21: 24–43.
Reiss, J., J. R. Bridle, J. M. Montoya & G. Woodward, 2009. Emerging horizons in biodiversity and ecosystem functioning research. Trends in Ecology and Evolution 24: 505–514.
Reynaga, M. C. & D. A. Dos Santos, 2013. Contrasting taxonomical and functional responses of stream invertebrates across space and time in a Neotropical basin. Fundamental and Applied Limnology 183: 121–133.
Ricotta, C., 2005. A note on functional diversity measures. Basic and Applied Ecology 6: 479–486.
Ricotta, C. & M. Moretti, 2008. Quantifying functional diversity with graph-theoretical measures: advantages and pitfalls. Community Ecology 9: 11–16.
Ricotta, C. & L. Szeidl, 2006. Towards a unifying approach to diversity measures: bridging the gap between the Shannon entropy and Rao’s quadratic index. Theoretical Population Biology 70: 237–243.
Rosenberg, D. M. & V. H. Resh, 1993. Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, New York.
Rosenfeld, J. S., 2002. Functional redundancy in ecology and conservation. Oikos 98: 156–162.
Schleuter, D., M. Daufrene, F. Massol & C. Argillier, 2010. A user’s guide to functional diversity indices. Ecological Monographs 80: 469–484.
Schmedtje, U. & M. Colling, 1996. Ökologische Typisierung der aquatischen Makrofauna. Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft 4/96.
Schmera, D., B. Baur & T. Erős, 2012. Does functional redundancy of communities provide insurance against human disturbances? An analysis using regional-scale stream invertebrate data. Hydrobiologia 693: 183–194.
Schmera, D., T. Erős & J. Heino, 2013. Habitat filtering determines spatial variation of macroinvertebrate community traits in northern headwater streams. Community Ecology 14: 77–88.
Schmera, D., T. Erős & J. Podani, 2009a. A measure for assessing functional diversity in ecological communities. Aquatic Ecology 43: 157–163.
Schmera, D., J. Podani & T. Erős, 2009b. Measuring the contribution of community members to functional diversity. Oikos 118: 961–971.
Schmera, D., J. Podani, T. Erős & J. Heino, 2014. Combining taxon-by-trait and taxon-by-site matrices for analysing trait patterns of macroinvertebrate communities: a rejoinder to Monaghan & Soares (2014). Freshwater Biology 59: 1551–1557.
Schmera, D., J. Podani, J. Heino, T. Erős & N. L. R. Poff, 2015. A proposed unified terminology of species traits in stream ecology. Freshwater Science 34: 823–830.
Schmidt-Koiber, A. & D. Hering, 2015. www.freshwaterecology.info – an online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecological Indicators 53: 271–282.
Southwood, T. R. E., 1977. Habitat, the templet for ecological strategies. Journal of Animal Ecology 46: 337–365.
Statzner, B., V. H. Resh & S. Dolédec, 1994. Ecology of the Upper Rhône River: a test of habitat templet theories. Freshwater Biology 31: 253–554.
Statzner, B., 2012. Geomorphological implications of engineering bed sediments by lotic animals. Geomorphology 157: 49–65.
Statzner, B., S. Dolédec & B. Hugueny, 2004. Biological trait composition of European stream invertebrate communities: assessing the effects of various trait filter types. Ecography 27(4): 470–488.
Statzner, B., P. Bady, S. Dolédec & F. Schöll, 2005. Invertebrate traits for the biomonitoring of large European rivers: an initial assessment of the trait patterns in least impacted river reaches. Freshwater Biology 50: 2136–2161.
Tachet, H., P. Richoux, M. Bournaud & P. Usseglio-Polatera, 2010. Invertébrés d’eau douce. CNRS éditions, Paris.
Tilman, D., J. Knops, D. Wedin, P. B. Reich, M. Ritchie & E. Sieman, 1997. The influence of functional diversity and composition on ecosystem processes. Science 277: 1300–1302.
Townsend, C. R. & A. G. Hildew, 1994. Species traits in relation to a habitat templet for river systems. Freshwater Biology 31: 265–275.
Truchy, A., D.-G. Angeler, R. A. Sponseller, R. K. Johnson & B. G. McKie, 2015. Linking biodiversity, ecosystem functioning and services, and ecological resilience: towards an integrative framework for improved management. Advances in Ecological Research 53: 55–96.
Usseglio-Polatera, P., 1994. Theoretical habitat templets, species traits, and species richness: aquatic insects in the Upper Rhône River and its floodplain. Freshwater Biology 31: 417–437.
Usseglio-Polatera, P., M. Bournaud, P. Richoux & H. Tachet, 2000. Biological and ecological traits of benthic freshwater macroinvertebrates: relationship and definition of groups with similar traits. Freshwater Biology 43: 175–205.
Vandewalle, M., F. de Bello, M. P. Berg, T. Bolger, S. Dolédec, F. Dubs, C. K. Feld, R. Harrington, P. A. Harrison, S. Lavorel, P. M. da Silva, M. Moretti, J. Niemela, P. Santos, T. Sattler, J. P. Sousa, M. T. Sykes, A. J. Vanbergen & B. A. Woodcock, 2010. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodiversity and Conservation 19: 2921–2947.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Vaughn, C. C., 2010. Biodiversity losses and ecosystem function in freshwaters: emerging conclusions and research directions. BioScience 60: 25–35.
Vaz, P. G., S. Dias, P. Pinto, E. C. Mereten, C. T. Robinson, D. R. Warren & F. C. Rego, 2014. Effects of burn status and conditioning on colonization of wood by stream macroinvertebrates. Freshwater Science 33: 832–846.
Verberk, W. C. E. P., C. G. E. van Noordwijk & A. G. Hildrew, 2013. Delivering on a promise: integrating species traits to transform descriptive community ecology into a predictive science. Freshwater Science 32: 531–547.
Vidakovic, J. & G. Palijan, 2010. Development and functional structure of epiphytic nematode communities on the submerged macrophyte Ceratophyllum demersum in Lake Sakadas, Kopacki Rit, Croatia. Nematology 12: 289–302.
Villéger, S., N. W. M. Mason & D. Mouillot, 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89: 2290–2301.
Violle, C., M.-L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel & E. Garnier, 2007. Let the concept of trait be functional!. Oikos 116(5): 882–892.
Walker, B., A. Kinzig & J. Langride, 1999. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2: 95–113.
Waringer, J., W. Graf & H. Malicky, 2013. Problems associated with extrapolating ecological traits to higher-than-species level exemplified in the description of the larvae of Potamophylax haidukorum Malicky, 1999, Potamophylax winneguthi (Klapálek, 1902) and Melamphophylax austriacus Malicky, 1990. Limnologica 43: 441–450.
Acknowledgments
This research was supported by the Hungarian Scientific Research Fund (OTKA K104279) and the Academy of Finland. The last author benefited from funds of the European Community’s Seventh Framework Program, Grant Agreement No. 603629-ENV-2013-6.2.1-Globaqua.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Judit Padisák
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schmera, D., Heino, J., Podani, J. et al. Functional diversity: a review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 787, 27–44 (2017). https://doi.org/10.1007/s10750-016-2974-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2974-5