Abstract
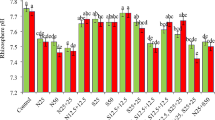
Gluconacetobacter diazotrophicus PAL5 and Azospirillum brasilense REC3 are plant growth promoting bacteria. They are able to produce hydroxamate and catechol type siderophores, respectively, when iron is not available, chelating this metal to facilitate its absorption. Iron is required by plants and is involved in physiological processes as part of many important compounds. The aim of this work was to evaluate the two siderophores producing bacteria in their contribution to iron nutrition for strawberry plants through the growth index, leaf and root area, greenness index, total soluble phenolic compounds and total iron content. Strawberry plants were grown hydroponically with a 16-h photoperiod in Hoagland nutrient solution, modified in iron sources, and inoculated with each bacterium. At day 60, the highest values of growth index, root area, greenness index, and iron content, were obtained for treatments with reduced iron, and the lowest values in treatments without iron addition. Values in treatments with oxidized iron and inoculated with bacteria were similar to those obtained with reduced iron and uninoculated plants. At day 30, phenolic compounds were higher in treatments without iron addition and uninoculated, while they decreased when plants were inoculated. For treatments with reduced iron, phenolic compounds content was low and increased when plants were inoculated. In conclusion, the siderophores produced by G. diazotrophicus PAL5 and A. brasilense REC3 can contribute to the iron nutrition of hydroponically grown strawberry plants. The participation of the hydroxamates was better than that of the catechols in the provision of iron to the plants.







Similar content being viewed by others
References
Abadía J, Abadía A (1993) Iron and plant pigments. In: Barton LL, Hemming BC (eds) Iron chelation in plants and soil microorganisms. Academic Press, New York, pp 327–343
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Bashan Y, de Bashan LE (2005) Plant growth-promoting. In: Hillel D (ed) Encyclopedia of soils in the environment, 1st edn. Elsevier, Oxford, pp 103–115
Bashan Y, de Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. In: Sparks DL (ed) Advances in agronomy. Elsevier, San Diego, pp 77–136
Bashan Y, Holguin G (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228
Bellenger JP, Wichard T, Kustka AB, Kraepiel AML (2008) Uptake of molybdenum and vanadium by a nitrogen-fixing soil bacterium using siderophores. Nat Geosci 1:243
Bienfait HF (1988) Mechanisms in Fe-efficiency reactions of higher plants. J Plant Nutr 11(6–11):605–629. https://doi.org/10.1038/ngeo161
Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ (2009a) New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environ Microbiol 11(5):1079–1091. https://doi.org/10.1111/j.1462-2920.2008.01838.x
Braud A, Jézéquel K, Bazot S, Lebeau T (2009b) Enhanced phytoextraction of an agricultural Cr-and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74(2):280–286. https://doi.org/10.1016/j.chemosphere.2008.09.013
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20(1):33–40. https://doi.org/10.1016/j.tplants.2014.07.005
Budzikiewicz H (2010) Microbial siderophores. In: Kinghorn A, Falk H, Kobayashi J (eds) Fortschritte der Chemie organischer Naturstoffe (Progress in the chemistry of organic natural products). Springer, Vienna, pp 1–75
Bussler W, Epstein E (1972) Mineral nutrition of plants: principles and perspectives. J Plant Nutr Soil Sci 132:158–159. https://doi.org/10.1002/jpln.19721320211
Carley HE, Watson RD (1966) A new gravimetric method for estimating root-surface areas. Soil Sci 102(5):289–291
Cavalcante VA, Döbereiner J (1988) A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 108(1):23–31. https://doi.org/10.1007/BF02370096
Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329(1–2):1–25. https://doi.org/10.1007/s11104-009-0266-9
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25(11):1919–1928. https://doi.org/10.1007/s11274-009-0090-7
Chu BC, Garcia-Herrero A, Johanson TH, Krewulak KD, Lau CK, Peacock RS, Vogel HJ (2010) Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 23:601–611. https://doi.org/10.1007/s10534-010-9361-x
Colombo C, Palumbo G, He JZ, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments 14(3):538–548. https://doi.org/10.1007/s11368-013-0814-z
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol R 66(2):223–249. https://doi.org/10.1128/MMBR.66.2.223-249.2002
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54(1):183–206
Delaporte-Quintana P, Grillo-Puertas M, Lovaisa NC, Teixeira KR, Rapisarda VA, Pedraza RO (2017) Contribution of Gluconacetobacter diazotrophicus to phosphorus nutrition in strawberry plants. Plant Soil 419(1–2):335–347
de Santiago A, García-López AM, Quintero JM, Avilés M, Delgado A (2013) Effect of Trichoderma asperellum strain T34 and glucose addition on iron nutrition in cucumber grown on calcareous soils. Soil Biol Biochem 57:598–605. https://doi.org/10.1016/j.soilbio.2012.06.02020
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2015) InfoStat versión 2015. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. https://www.infostat.com.ar. Accessed 26 April 2019
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32(12):1682–1694. https://doi.org/10.1111/j.1365-3040.2009.02028.x
El-Baz FK, Mohamed AA, Aboul-Enein AM, Salama ZA (2004) Alteration in root exudates level during Fe-deficiency in two cucumber cultivars. Int J Agric Biol 6:45–48
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. In: A manual for the West Asia and North Africa region, pp 170–176
Fuentes-Ramírez L, Jimenez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J (1993) Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil 154(2):145–150. https://doi.org/10.1007/BF00012519
Gamalero E, Glick BR (2011) Mechanisms used by plant growth-promoting bacteria. Bacteria in agrobiology: plant nutrient management. Springer, Berlin, Heidelberg, pp 17–46
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. https://doi.org/10.6064/2012/963401
Grillo-Puertas M, Delaporte-Quintana P, Pedraza RO, Rapisarda VA (2018) Intracellular polyphosphate levels in Gluconacetobacter diazotrophicus affect tolerance to abiotic stressors and biofilm formation. Microbes Environ 33(4):440–445
Guerrero-Molina MF, Lovaisa NC, Salazar SM, Martínez-Zamora MG, Díaz-Ricci JC, Pedraza RO (2015) Physiological, structural and molecular traits activated in strawberry plants after inoculation with the plant growth-promoting bacterium Azospirillum brasilense REC 3. Plant Biol 17(3):766–773. https://doi.org/10.1111/plb.12270
Gupta C, Durbey R, Maheshwari D (2002) Plant growth enhancement and suppression of Macrophomina phaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol Fert Soils 35(6):399–405
Hancock JF (1999) Strawberries. CABI Publishing, New York, p 237
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216(4):541–551. https://doi.org/10.1007/s00425-002-0920-4
Hochmuth G (2011) Iron (Fe) nutrition of plants. Technical report, IFAS Extension Service, University of Florida, pp 1–7
Hopkins WG (1999) Introduction to plant physiology, 2nd edn. Wiley, New York, pp 61–76
Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144(1):278–285
Kirschbaum DS, Borquez AM (2006) Nutrición mineral de la frutilla (Fragaria x ananassa Duch.). III simpósio nacional de morango. II encontro sobre pequenas frutas e frutas nativas do Mercosur. Palestras 3:118–127
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286(5776):885
Kumar BD (1999) Fusarial wilt suppression and crop improvement through two rhizobacterial strains in chick pea growing in soils infested with Fusarium oxysporum f. sp. ciceris. Biol Fert Soils 29(1):87–91
Logeshwaran P, Thangaraju M, Rajasundari K (2009) Hydroxamate siderophores of endophytic bacteria Gluconacetobacter diazotrophicus isolated from sugarcane roots. Aust J Basic Appl Sci 3:3564–3567
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol R 71(3):413–451
Mimmo T, Del Buono D, Terzano R, Tomasi N, Vigani G, Crecchio C, Cesco S (2014) Rhizospheric organic compounds in the soil–microorganism–plant system: their role in iron availability. Eur J Soil Sci 65(5):629–642
Neilands JB (1973) Microbial iron transport compounds (siderochromes). In: Eichorn GL (ed) Inorganic biochemistry, vol 1. Elsevier Scientific Publishing Co., Amsterdam, pp 167–202
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45):26723–26726
Pedraza RO (2016) Acetic acid bacteria as plant growth promoters. In: Matsushita K, Toyama H, Tonouchi N, Okamoto-Kainuma A (eds) Acetic acid bacteria. Springer, Tokyo, pp 101–120
Pedraza RO, Ramírez-Mata A, Xiqui ML, Baca BE (2004) Aromatic amino acid aminotransferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol Lett 233(1):15–21
Pedraza RO, Motok J, Tortora ML, Salazar SM, Díaz-Ricci JC (2007) Natural occurrence of Azospirillum brasilense in strawberry plants. Plant Soil 295(1–2):169–178
Pedraza RO, Motok J, Salazar SM, Ragout AL, Mentel MI, Tortora ML, Díaz-Ricci JC (2010) Growth-promotion of strawberry plants inoculated with Azospirillum brasilense. World J Microbiol Biotechnol 26(2):265–272
Pestana M, Correia PJ, Saavedra T, Gama F, Abadía A, de Varennes A (2012) Development and recovery of iron deficiency by iron resupply to roots or leaves of strawberry plants. Plant Physiol Biochem 53:1–5
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. Rev Biol Fert Soils 51(4):403–415
Pii Y, Borruso L, Brusetti L, Crecchio C, Cesco S, Mimmo T (2016) The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol Biochem 99:39–48
Reis VM, Teixeira KRdS (2015) Nitrogen fixing bacteria in the family Acetobacteraceae and their role in agriculture. J Basic Microbiol 55(8):931–949
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397(6721):694
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80(1):175–180
RStudio Team (2015) Integrated development for R. RStudio, Inc. Boston, MA. https://www.rstudio.com. Accessed 26 April 2019
Saxena B, Modi M, Modi VV (1986) Isolation and characterization of siderophores from Azospirillum lipoferum D-2. Microbiology 132(8):2219–2224
Sayyed RZ, Chincholkar SB (2009) Siderophore-producing Alcaligenes feacalis exhibited more biocontrol potential Vis-à-Vis chemical fungicide. Curr Microbiol 58(1):47–51
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671
Shah S, Karkhanis V, Desai A (1992) Isolation and characterization of siderophore, with antimicrobial activity, from Azospirillum lipoferum M. Curr Microbiol 25(6):347–351
Siebner-Freibach H, Hadar Y, Chen Y (2003) Siderophores sorbed on Ca-montmorillonite as an iron source for plants. Plant Soil 251(1):115–124
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituents. J Sci Food Agric 10(1):63–68
Tapia-Hernandez A, Mascarua-Esparza MA, Caballero-Mellado J (1990) Production of bacteriocins and siderophore-like activity by Azospirillum brasilense. Microbios 64(259):73–83
Tortora ML, Díaz-Ricci JC, Pedraza RO (2011) Azospirillum brasilense siderophores with antifungal activity against Colletotrichum acutatum. Arch Microbiol 193(4):275–286
Tortora ML, Díaz-Ricci JC, Pedraza RO (2012) Protection of strawberry plants (Fragaria ananassa Duch.) against anthracnose disease induced by Azospirillum brasilense. Plant Soil 356(1–2):279–290
Trejo-Téllez LI, Gómez-Merino FC (2014) Nutrient management in strawberry: effects on yield, quality and plant health. In: Malone N (ed) Strawberries. Nova Science Publishers, Hauppauge, pp 239–267
Ulrich A, Mostafa MAE, Allen WW, Davis PA (1980) Strawberry deficiency symptoms: a visual and plant analysis guide to fertilization. University of California Agriculture Sci Publication, pp 30–31
Valentinuzzi F, Pii Y, Vigani G, Lehmann M, Cesco S, Mimmo T (2015) Phosphorus and iron deficiencies induce a metabolic reprogramming and affect the exudation traits of the woody plant Fragaria× ananassa. J Exp Bot 66(20):6483–6495
Verma VC, Singh SK, Prakash S (2011) Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss J Basic Microbiol 51(5):550–556
Walker EL, Connolly EL (2008) Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol 11(5):530–535
Zhao Q, Shen Q, Ran W, Xiao T, Xu D, Xu Y (2011) Inoculation of soil by Bacillus subtillis Y-IVI improves plant growth and colonization of the rhizosphere and interior tissues of muskmelon (Cucumis melo L.). Biol Fert Soils 47(5):507–514
Acknowledgements
This paper is dedicated to the memory of Prof. Yoav Bashan. We thank Dr. Roque Interdonato for his help in the determination of phenolic compounds. This work was supported by Secretaría de Ciencia, Arte y Tecnología, Universidad Nacional de Tucumán (Program A621). PDQ is fellow of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delaporte-Quintana, P., Lovaisa, N.C., Rapisarda, V.A. et al. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Regul 91, 185–199 (2020). https://doi.org/10.1007/s10725-020-00598-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-020-00598-0