Abstract
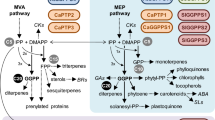
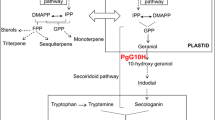
Geranylgeranyl-diphosphate synthases (GGDPS) catalyze branch point enzymatic reactions producing isoprenoid-derived products which are necessary for plant growth and responses to a wide range of biotic and abiotic stresses. In our study, full length geranylgeranyl-diphosphate synthase 1 (PgGGDPS1) and 2 (PgGGDPS2) cDNA were isolated and characterized from the flower of Panax ginseng and 4-year old P. ginseng cv. Gumpoong. The cDNA had open reading frame of 1032 and 1116 bp with a deduced amino acid sequence of 343 and 371 residues for GGDPS1 and GGDPS2, respectively. The calculated molecular mass of GGDPS1 and GGDPS2 were approximately 37.66 and 40.21 kDa with a predicated isoelectric point of 5.32 and 6.23 and predicted localization of plastid. A GenBank Blast X search revealed that the deduced amino acid of PgGGDPS1 shared a high degree of homology with GGDPS from Panax notoginseng. The transcription pattern of GGDPS genes was different at various developmental stages. Both GGDPS genes were highly expressed in aerial parts of the plant, especially in rapidly growing tissues such as 4-year old flower and stem tissues. Transcript level of PgGGDPS1 was differentially induced in ginseng not only during Pseudomonas syringae pv tomato infection but also after exposure to abiotic stresses. Our results suggested that the induction of GGDPS genes specifically PgGGDPS1 by drought stress may affect chlorophyll levels, intracellular GA content and accumulation of carotenoids as the precursor for higher production of ABA and possibly stomatal closure as the barrier for water loss.





Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- CLD:
-
chain length determination
- EST:
-
expressed sequence tags
- GA3:
-
gibberellin
- GGDP:
-
geranylgeranyl diphosphate
- GGDPS:
-
geranylgeranyl-diphosphate synthase
- GGR:
-
geranylgeranyl reductase
- MJ:
-
methyl jasmonates
- RT-PCR:
-
reverse transcriptase-polymerase chain reaction
- SA:
-
salicylic acid
- YE:
-
yeast extract
References
Aitken, S. M., Attucci, S., Ibrahim, R. K., & Gulick, P. J. (1995). A cDNA encoding geranylgeranyl pyrophosphate synthase from whitelupin. Plant Physiology, 108, 837–838.
Aliyev, R. Т., Coskuncelebi, K., Beyazoglu, O., & Hacieva, S. I. (2000). Alterations in the genome of wheat seedlings grown under drought stress and the effect of gibberellic acid on these alterations. Review Biology, 93, 183–189.
Ament, K., Van Schie, C. C., Bouwmeester, H. J., Haring, M. A., & Schuurink, R. C. (2006). Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of −4, 8, 12-trimethyltrideca-1, 3, 7, 11-tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta, 224, 1197–1208.
Artimo, P., Jonnalagedda, M., Arnold, K., Baratin, D., Csardi, G., de Castro, E., Duvaud, S., Flegel, V., Fortier, A., Gasteiger, E., et al. (2012). ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research, 40, W597–W603.
Badillo, A., Steppuhn, J., Deruere, J., Camara, B., & Kuntz, M. (1995). Structure of a functional geranylgeranyl pyrophosphate synthase gene from Capsicum annuum. Plant Molecular Biology, 27, 425–428.
Bantignies, B., Liboz, T., & Ambid, C. (1995). Nucleotide sequence of a Catharanthus roseus geranylgeranyl pyrophosphate synthase gene. Plant Physiology, 110, 336.
Bartley, G. E., & Scolnik, P. A. (1995). Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell, 7, 1027–1038.
Chen, A. J., Kroon, P. A., & Poulter, C. D. (1994). Isoprenyl diphosphate synthases protein-sequence comparisons, a phylogenetic tree and redictions of secondary structure. Protein Science, 3, 600–607.
El-Meleigy, E. A., Hassanein, R. A., & Abdel Kader, D. Z. (1999). Improvement of drought tolerance in Araches hypogaea L. plant by some growth substances 1. Growth and productivity. Bulletin Faculty Science Association University, 28, 159–185.
Emanuelson, O., Nielsen, H., Brunak, S., & von Heijne, G. (2000). Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology, 300, 1005–1016.
Engprasert, S., Taura, F., Kawamukai, M., & Shoyama, Y. (2004). Molecular cloning and functional expression of geranylgeranyl pyrophosphate synthase from Coleus forskohlii Briq. BMC Plant Biology, 4, 18.
Ge, X. C., & Wu, J. Y. (2004). Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Science, 168, 487–491.
Gray, J. C. (1987). Control of isoprenoid biosynthesis in higher plants. Advances in Botanical Research, 14, 25–91.
Hedden, P., & Kamiya, Y. (1997). Gibberellin biosynthesis: enzymes, genes and their regulation. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 431–460.
Hefner, J., Ketchum, F. E. B., & Croteau, R. (1998). Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Archives of Biochemistry and Biophysics, 360, 62–74.
Hua, W., Song, J., Li, C., & Wang, Z. (2012). Molecular cloning and characterization of the promoter of SmGGPPs and its expression pattern in Salvia miltiorrhiza. Molecular Biology Reports, 39, 5775–5783.
Kai, G., Liao, P., Zhang, T., Zhou, W., Wang, J., Xu, H., Liu, Y., & Zhang, L. (2010). Characterization, expression profiling, and functional identification of a gene encoding geranylgeranyl diphosphate synthase from Salvia miltiorrhiza. Biotechnology and Bioprocess Engineering, 15, 236–245.
Kelen, M., Çubuk Demìralay, E., Demìralay, E., Sen, S., & özkan, G. (2004). Separation of Abscisic acid, Indole-3-Acetic acid, Gibberellic acid in 99 R (Vitisberlandierix Vitisrupestris) and rose oil (Rosa damascene Mill.) by Reversed Phase Liquid Chromatography. Turkish Journal of Chemistry, 28, 603–610.
Kim, Y. J., Jang, M. G., Noh, H. Y., Lee, H. J., Sukweenadhi, J., Kim, J. H., Kim, S. Y., Kwon, W. S., & Yang, D. C. (2014). Molecular characterization of two glutathione peroxidase genes of Panax ginseng and their expression analysis against environmental stresses. Gene, 535, 33–41.
Kirk, J. T. O., & Allen, R. L. (1965). Dependence of chloroplast pigment synthesis on protein synthesis: Effect of actidione. Biochemical Biophysical Research Communications, 21, 523–530.
Kleinig, H. (1989). The role of plastids in isoprenoid biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology, 40, 39–59.
Kuntz, M., Romer, S., Suire, C., Hugueney, P., Weil, J. H., Schantz, R., & Camara, B. (1992). Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from Capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant Journal, 2, 25–34.
Laferriere, A., & Beyer, P. (1991). Purification of geranylgeranyl diphosphate synthase from Sinapis alba etioplasts. Biochimica et Biophysica Acta, 1077, 167–172.
Lee, C. H., & Kim, J. H. (2014). A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research, 38, 161–166.
Liang, P. H., Ko, T. P., & Wang, A. H. J. (2002). Structure, mechanism and function of prenyltransferases. European Journal of Biochemistry, 269, 3339–3354.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–82.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta CT) method. Methods, 25, 402–408.
Morris, P. C., Kumar, A., Bowles, D. J., & Cuming, A. C. (1990). Osmotic stress and abscisic acid regulate the expression of the Em gene of wheat. European Journal of Biochemistry, 190, 625–630.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assay with tobacco tissue cultures. Plant Physiology, 15, 473–499.
Oh, S. K., Kim, I. J., Shin, D. H., Yang, J., Kang, H., & Han, K. H. (2000). Cloning, characterization, and heterologous expression of a functional geranylgeranyl pyrophosphate synthase from sunflower (Helianthus annuus L.). Journal of Plant Physiology, 157, 535–542.
Okada, K., Saito, T., Nakagawa, T., Kawamukai, M., & Kamiya, Y. (2000). Five geranylgeranyldiphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiology, 122, 1045–1056.
Scolnik, P. A., & Bartley, G. E. (1994). Nucleotide sequence of an Arabidopsis cDNA for geranylgeranyl pyrophosphate synthase. Plant Physiology, 104, 1469–1470.
Scolnik, P. A., & Bartley, G. E. (1995). Nucleotide sequence of a putative geranylgeranyl pyrophosphate synthase (GenBank accession no. L40577) from Arabidopsis (PGR 95–018). Plant Physiology, 108, 1343.
Scolnik, P. A., & Bartley, G. E. (1996). Two more members of Arabidopsis geranylgeranyl pyrophosphate synthase family (PGR 96–014). Plant Physiology, 110, 1435.
Sitthithaworn, W., Kojima, N., Viroonchatapan, E., Suh, D. Y., Iwanami, N., Hayashi, T., Noji, M., Saito, K., Niwa, Y., & Sankawa, U. (2001) Geranylgeranyl diphosphate synthase from Scoparia dulcis and Croton sublyratus. Plastid localization and conversion to a farnesyl diphosphate synthase by mutagenesis. Chem Pharm Bull, 49, 197–292.
Song, M. Y., Kim, B. S., & Kim, H. (2014). Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. Journal of Ginseng Research, 38, 106–115.
Tanimoto, E. (1991). Gibberellin requirement for the normal growth of roots. In N. Takahashi, B. O. Phinney, & J. MacMillan (Eds.), Gibberellins (pp. 229–240). New York: Springer.
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J., & Zhu, J. K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant Journal, 45, 523–539.
Wang, K., & Ohnuma, S. I. (1999). Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution. Trends Biochemical Science, 24, 445–451.
Zhu, X. F., Suzuki, K., Okada, K., Tanaka, K., Nakagawa, T., Kawamukai, M., & Matsuda, H. (1997a). Cloning and functional expression of a novel geranylgeranyl pyrophosphate synthase gene from Arabidopsis thaliana in Escherichia coli. Plant Cell Physiology, 38, 357–361.
Zhu, X. F., Suzuki, K., Saito, T., Okada, K., Tanaka, K., Nakagawa, T., Matsuda, H., & Kawamukai, M. (1997b). Geranylgeranyl pyrophosphatesynthase encoded by the newly isolated gene GGPS6 from Arabidopsis thaliana is localized in mitochondria. Plant Molecular Biology, 35, 331–341.
Acknowledgments
This research was supported by iPET (312064-03-1-HD040), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimi, S., Kim, YJ., Devi, B.S.R. et al. Isolation and characterization of Panax ginseng geranylgeranyl-diphosphate synthase genes responding to drought stress. Eur J Plant Pathol 142, 747–758 (2015). https://doi.org/10.1007/s10658-015-0648-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0648-1