Abstract
More than 2 decades ago, the discovery of osteoprotegerin (OPG) as inhibitor of the receptor of activator of nuclear factor Kb (RANK) ligand (RANKL) revolutionized our understanding of bone biology and oncology. Besides acting as decoy receptor for RANKL, OPG acts as decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). OPG, RANKL, and TRAIL are ubiquitously expressed, stimulating per se pivotal signaling cascades implicated in cancer. In the context of cancer cell–bone cell interactions, cancer cells skew the OPG/RANKL/RANK (RANKL cognate receptor) balance towards bone destruction and tumor growth through favoring the RANKL/RANK interface, circumventing OPG. Numerous preclinical and clinical studies demonstrate the dual role of OPG in cancer: antitumor and tumor-promoting. OPG potentially conveys an antitumor signal through inhibiting the tumor-promoting RANKL signaling—both the osteoclast-dependent and the osteoclast-independent—and the tumor-promoting TRAIL signaling. On the other hand, the presumed tumor-promoting functions of OPG are: (i) abrogation of TRAIL-induced apoptosis of cancer cells; (ii) abrogation of RANKL-induced antitumor immunity; and (iii) stimulation of oncogenic and prometastatic signaling cascades downstream of the interaction of OPG with diverse proteins. The present review dissects the role of OPG in bone oncology. It presents the available preclinical and clinical data sustaining the dual role of OPG in cancer and focuses on the imbalanced RANKL/RANK/OPG interplay in the landmark “vicious cycle” of skeletal metastatic disease, osteosarcoma, and multiple myeloma. Finally, current challenges and future perspectives in exploiting OPG signaling in bone oncology therapeutics are discussed.

Similar content being viewed by others
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
D’Amico L, Roato I (2015) The impact of immune system in regulating bone metastasis formation by osteotropic tumors. J Immunol Res. https://doi.org/10.1155/2015/143526
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319. https://doi.org/10.1016/S0092-8674(00)80209-3
Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T et al (1997) Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 234:137–142. https://doi.org/10.1006/bbrc.1997.6603
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N et al (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337. https://doi.org/10.1210/endo.139.3.5837
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176. https://doi.org/10.1016/S0092-8674(00)81569-X
The American Society for Bone and Mineral Research President’s Committee on Nomenclature (2000) Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. J Bone Miner Res 15:2293–2296. https://doi.org/10.1359/jbmr.2000.15.12.2293
Emery JG, McDonnel P, Burke MB, Deen KC, Lyn S, Silverman C et al (1998) Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem 273:14363–14367. https://doi.org/10.1074/jbc.273.23.14363
Baud’huin M, Duplomb L, Teletchea S, Lamoureux F, Ruiz-Velasco C, Maillasson M et al (2013) Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev 24:401–409. https://doi.org/10.1016/j.cytogfr.2013.06.001
Mundy GR, Raisz LG, Cooper RA, Schechter GP, Salmon SE (1974) Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med 291:1041–1046. https://doi.org/10.1056/NEJM197411142912001
Galasko C (1976) Mechanisms of bone destruction in the development of skeletal metastases. Nature 263:507–508
Martin TJ (2013) Historically significant events in the discovery of RANK/RANKL/OPG. World J Orthoped 4:186–197. https://doi.org/10.5312/wjo.v4.i4.186
Chambers TJ (1980) The cellular basis of bone resorption. Clin Orthop Relat Res 151:283–293
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s. https://doi.org/10.1158/1078-0432.CCR-06-0931
Dai X, Ma W, He X, Jha RK (2011) Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Med Sci Monit 17:177–190. https://doi.org/10.12659/MSM.881893
van Oosterwijk JG, Anninga JK, Gelderblom H, Cleton-Jansen AM, Bovée JV (2013) Update on targets and novel treatment options for high-grade osteosarcoma and chondrosarcoma. Hematol Oncol Clin North Am 27:1021–1048. https://doi.org/10.1016/j.hoc.2013.07.012
Paget S (1989) The distrubution of secondary growths in cancer of the breast, 1889. Cancer Metastasis Rev 8:98–101
Yamaguchi K, Kinosaki M, Goto M, Kobayashi F, Tsuda E, Morinaga T et al (1998) Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem 273:5117–5123. https://doi.org/10.1074/jbc.273.9.5117
Walsh MC, Choi Y (2014) Biology of the RANKL–RANK–OPG system in immunity, bone, and beyond. Front Immunol 5:511. https://doi.org/10.3389/fimmu.2014.00511
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER et al (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179. https://doi.org/10.1038/36593
Wong B, Josien R, Lee SY, Sauter B, Li HL, Steinman RM et al (1997) TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186:2075–2080
Ιkeda T, Kasai M, Utsuyama M, Hirokawa K (2001) Determination of three isoforms of the Receptor activator of nuclear factor-kappa B ligand and their differential expression in bone and thymus. Endocrinology 142:1419–1426. https://doi.org/10.1210/endo.142.4.8070
Boyce BF, Xing L (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 9:S1. https://doi.org/10.1186/ar2165
Cheng ML, Fong L (2013) Effects of RANKL-targeted therapy in immunity and cancer. Front Oncol 3:329. https://doi.org/10.3389/fonc.2013.00329
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673–682
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271:12687–12690. https://doi.org/10.1074/jbc.271.22.12687
Trivedi R, Mishra DP (2015) Trailing TRAIL resistance: novel targets for TRAIL sensitization in cancer cells. Front Oncol 5:69. https://doi.org/10.3389/fonc.2015.00069
Janssen EM, Droi NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD et al (2005) CD4 + T-cell help controls CD8 + T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434:88–93. https://doi.org/10.1038/nature03337
Wang R, Li JC (2015) TRAIL suppresses human breast cancer cell migration via MADD/CXCR1. Asian Pac J Cancer Prev 16:2751–2756
Holen I, Shipman CM (2006) Role of osteoprotegerin (OPG) in cancer. Clin Sci 110:279–291. https://doi.org/10.1042/CS20050175
Tsukamoto S, Ishikawa T, Iida S, Ishiguro M, Mogushi K, Mizushima H et al (2011) Clinical significance of osteoprotegerin expression in human colorectal cancer. Clin Cancer Res 17:2444–4450. https://doi.org/10.1158/1078-0432.CCR-10-2884
Treskova I, Topolcan O, Windrichova J, Simanek V, Slouka D, Treska V, Kucera R (2018) OPG, OPN, EGF and VEGF levels at individual breslow score stages in malignant melanoma. Anticancer Res 38:4907–4911. https://doi.org/10.21873/anticanres.12806
Vik A, Brodin EE, Mathiesen EB, Brox J, Jørgensen L, Njølstad I et al (2015) Serum osteoprotegerin and future risk of cancer and cancer-related mortality in the general population: the Tromso study. Eur J Epidemiol 30:219–230. https://doi.org/10.1007/s10654-014-9975-3
Park HS, Lee A, Cha BJ, Bae JS, Song BJ, Jung SS (2014) Expression of receptor activator of nuclear factor kappa-B as a poor prognostic marker in breast cancer. J Surg Oncol 110:807–812. https://doi.org/10.1002/jso.23737
Goswami S, Sharma-Walia N (2015) Osteoprotegerin secreted by inflammatory and invasive breast cancer cells induces aneuploidy, cell proliferation and angiogenesis. BMC Cancer 15:935. https://doi.org/10.1186/s12885-015-1837-1
Goswami S, Sharma-Walia N (2016) Osteoprotegerin rich tumor microenvironment: implications in breast cancer. Oncotarget 7:42777–42791. https://doi.org/10.18632/oncotarget.8658
Weichhaus M, Segaran P, Renaud A, Geerts D, Connelly L (2014) Osteoprotegerin expression in triple-negative breast cancer cells promotes metastasis. Cancer Med 3:1112–1125. https://doi.org/10.1002/cam4.277
Heymann MF, Riet A, Le Goff B, Battaglia S, Paineau J, Heymann D (2008) OPG, RANK and RANK ligand expression in thyroid lesions. Regul Pept 148:46–53. https://doi.org/10.1016/j.regpep.2008.02.004
Holen I, Cross SS, Neville-Webbe HL, Cross NA, Balasubramanian SP, Croucher PI et al (2005) Osteoprotegerin OPG expression by breast cancer cells in vitro and breast tumors in vivo—a role in tumor cell survival? Breast Cancer Res Treat 92:207–215. https://doi.org/10.1007/s10549-005-2419-8
Benslimane-Ahmim Z, Pereira J, Lokajczyk A, Dizier B, Galy-Fauroux I, Fischer AM et al (2017) Osteoprotegerin regulates cancer cell migration through SDF-1/CXCR12 axis and promotes tumour development by increasing neovascularization. Cancer Lett 395:11–19. https://doi.org/10.1016/j.canlet.2017.02.032
Goswami S, Sharma-Walia N (2016) Crosstalk between osteoprotegerin (OPG), fatty acid synthase (FASN) and cycloxygenase-2 (COX-2) in breast cancer: implications in carcinogenesis. Oncotarget 7:58953–58974. https://doi.org/10.18632/oncotarget.9835
Chung ST, Geerts D, Roseman K, Renaud A (2017) Connelly L (2017) Osteoprotegerin mediates tumor-promoting effects of Interleukin-1 beta in breast cancer cells. Mol Cancer 16:27. https://doi.org/10.1186/s12943-017-0606-y
Cross SS, Yang Z, Brown NJ, Balasubramanian SP, Evans CA, Woodward JK et al (2006) Osteoprotegerin (OPG)—a potential new role in the regulation of endothelial cell phenotype and tumour angiogenesis? Int J Cancer 118:1901–1908. https://doi.org/10.1186/1476-4598-8-49
Benslimane-Ahmim Z, Poirier F, Delomenie C, Lokajczyk A, Grelac F, Galy-Fauroux I et al (2013) Mechanistic study of the proangiogenic effect of osteoprotegerin. Angiogenesis 16:575–593. https://doi.org/10.1007/s10456-013-9337-x
Mc Conigle JS, Giachelli CM, Scatena M (2009) Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis 12:35–46
Kobayashi-Sakamoto M, Isogai E, Holen I (2010) Osteoprotegerin induces cytoskeletal reorganization and activates FAK, Src, and ERK signaling in endothelial cells. Eur J Haematol 85:26–35. https://doi.org/10.1111/j.1600-0609.2010.01446.x
Zannettino AC, Holding CA, Diamond P, Atkins GJ, Kostakis P, Farrugia A et al (2005) Osteoprotegerin (OPG) is localized to the Weibel-Palade bodies of human vascular endothelial cells and is physically associated with von Willebrand factor. J Cell Physiol 204:714–723. https://doi.org/10.1002/jcp.20354
Scatena M, Giachell C (2002) The alpha(v)beta3 integrin, NF-kappaB, osteoprotegerin endothelial cell survival pathway. Potential role in angiogenesis. Trends Cardiovasc Med 12:83–88. https://doi.org/10.1016/S1050-1738(01)00151-7
Reid PE, Brown NJ, Holen I (2009) Breast cancer cells stimulate osteoprotegerin (OPG) production by endothelial cells through direct cell contact. Mol Cancer 8:49. https://doi.org/10.1186/1476-4598-8-49
Malyankar UM, Scatena M, Suchland KL, Yun TJ, Vlark EA, Giachelli CM (2000) Osteoprotegerin in an αvβ5-induced NF-k B-dependent survival factor for endothelial cells. J Biol Chem 275:20959–20962. https://doi.org/10.1074/jbc.C000290200
Benslimane-Ahmim Z, Heymann D, Dizier B, Lokajczyk A, Brion R, Laurendeau I et al (2011) Osteoprotegerin, a new actor in vasculogenesis, stimulates endothelial colony-forming cells properties. J Thromb Haemost 9:834–843. https://doi.org/10.1111/j.1538-7836.2011.04207.x
Renema N, Navet B, Heymann MF, Lezot F, Heymann D (2016) RANK–RANKL signalling in cancer. Biosci Rep 36:e00366. https://doi.org/10.1042/BSR20160150
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee H et al (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468:98–102. https://doi.org/10.1038/nature09387
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R et al (2010) RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468:103–107. https://doi.org/10.1038/nature09495
Casimiro S, Mohammad KS, Pires R, Tato-Costa J, Alho I, Teixeira R et al (2013) RANKL/RANK/MMP-1 molecular triad contributes to the metastatic phenotype of breast and prostate cancer cells in vitro. PLoS ONE 8:e63153. https://doi.org/10.1371/journal.pone.0063153
Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL et al (2007) Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 446:690–694. https://doi.org/10.1038/nature05656
Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C et al (2008) Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res 18:858–870. https://doi.org/10.1038/cr.2008.84
Chen LM, Kuo CH, Lai TY, Lin YM, Su CC, Hsu HH et al (2011) RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J Cell Biochem 112:933–941. https://doi.org/10.1002/jcb.23009
Yamada T, Tsuda M, Takahashi T, Totsuka Y, Shindoh M, Ohba Y (2011) RANKL expression specifically observed in vivo promotes epithelial mesenchymal transition and tumor progression. Am J Pathol 178:2845–2856. https://doi.org/10.1016/j.ajpath.2011.02.003
Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM et al (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470:548–553. https://doi.org/10.1038/nature09707
Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM (2011) RANKL/RANK -beyond bones. J Mol Med 89:647–656. https://doi.org/10.1007/s00109-011-0749-z
Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G et al (2016) LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22:539–546. https://doi.org/10.1038/nm.4076
Bhatia P, Sanders MM, Hansen MF (2005) Expression of receptor activator of nuclear factor-kappaB is inversely correlated with metastatic phenotype in breast carcinoma. Clin Cancer Res 11:162–165
Owen S, Ye L, Sanders AJ, Mason MD, Jiang WG (2013) Expression profile of receptor activator of nuclear-κB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancer. Anticancer Res 33:199–206
Papanastasiou AD, Sirinian C, Kalofonos HP (2012) Identification of novel human receptor activator of nuclear factor-kB isoforms generated through alternative splicing: implications in breast cancer cell survival and migration. Breast Cancer Res 14:R112. https://doi.org/10.1186/bcr3234
Picarda G, Lamoureux F, Geffroy L, Delepine P, Montier T, Laud K et al (2010) Preclinical evidence that use of TRAIL in Ewing’s sarcoma and osteosarcoma therapy inhibits tumor growth, prevents osteolysis, and increases animal survival. Clin Cancer Res 16:2363–2374. https://doi.org/10.1158/1078-0432.CCR-09-1779
Liu JX, Zhang ZC, Shao ZW, Pu FF, Wang BC, Zhang YK et al (2017) TRAIL-R1 as a novel surface marker for circulating giant cell tumor of bone. Oncotarget 8:50724–50730. https://doi.org/10.18632/oncotarget.17042
Weichhaus M, Chung STM, Connelly L (2015) Osteoprotegerin in breast cancer: beyond bone remodeling. Mol Cancer 14:117. https://doi.org/10.1186/s12943-015-0390-5
Esposito M, Kang Y (2014) Targeting tumor-stromal interactions in bone metastasis. Pharmacol Ther 141:222–323. https://doi.org/10.1016/j.pharmthera.2013.10.006
Mishra A, Shiozawa Y, Pienta KJ, Taichman RS (2011) Homing of cancer cells to the bone. Cancer Microenviron 4:221–235. https://doi.org/10.1007/s12307-011-0083-6
Sleeman JP, Christofori G, Fodde R, Collard JG, Berx G, Decraene C et al (2012) Concepts of metastasis in flux: the stromal progression model. Semin Cancer Biol 22:174–186. https://doi.org/10.1016/j.semcancer.2012.02.007
Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM (2012) Bone marrow microenvironment in multiple myeloma progression. J Biomed Biotechnol. https://doi.org/10.1155/2012/157496
Luo G, He Y, Yu X (2018) Bone marrow adipocyte: an intimate partner with tumor cells in bone metastasis. Front Endocrinol (Lausanne) 9:339. https://doi.org/10.3389/fendo.2018.00339
Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B et al (2015) Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6:7808
Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM (2014) Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513:100–104
Miki T, Yano S, Hanibuchi M, Kanematsu T, Muguruma H, Sone S (2004) Parathyroid hormone-related protein (PTHrP) is responsible for production of bone metastasis, but not visceral metastasis, by human small cell lung cancer SBC-5 cells in natural killer cell-depleted SCID mice. Int J Cancer 108:511
Buenrostro D, Mulcrone PL, Owens P, Sterling JA (2016) The bone microenvironment: a fertile soil for tumor growth. Curr Osteoporos Rep 14:151–158. https://doi.org/10.1007/s11914-016-0315-2
Wu MY, Li CJ, Yiang GT, Cheng YL, Tsai AP, Hou YT et al (2018) Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem 46:1423–1438. https://doi.org/10.1159/000489184
Xiang L, Gilkes DM (2019) The contribution of the immune system in bone metastasis pathogenesis. Int J Mol Sci 20:E999. https://doi.org/10.3390/ijms20040999
Salvador F, Llorente A, Gomis RR (2019) From latency to overt bone metastasis in breast cancer: potential for treatment and prevention. J Pathol 249:6–18. https://doi.org/10.1002/path.5292
Graham N, Qian BZ (2018) Mesenchymal stromal cells: emerging roles in bone metastasis. Int J Mol Sci 19:E1121. https://doi.org/10.3390/ijms19041121
Rucci N, Teti A (2010) Osteomimicry: how tumor cells try to deceive the bone. Front Biosc (Schol Ed) 2:907–915. https://doi.org/10.2741/110
Rucci N, Teti A (2018) Osteomimicry: how the seed grows in the soil. Calcif Tissue Int 102:131–140. https://doi.org/10.1007/s00223-017-0365-1
Tan CC, Li GX, Tan LD, Du X, Li XQ, He R et al (2016) Breast cancer cells obtain an osteomimetic feature via epithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction. Oncotarget 7:79688–79705. https://doi.org/10.18632/oncotarget.12939
Chu GC, Chung LW (2014) RANK-mediated signaling network and cancer metastasis. Cancer Metastasis Rev 33:497–509. https://doi.org/10.1007/s10555-013-9488-7
Sosa MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14:611–622. https://doi.org/10.1038/nrc3793
Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A et al (2012) RANK induces epithelial–mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res 72:2879–2888
Liu Y, Wang J, Ni T, Wang L, Wang Y, Sun X (2016) CCL20 mediates RANK/RANKL-induced epithelial–mesenchymaltransition in endometrial cancer cells. Oncotarget 7:25328–25339. https://doi.org/10.18632/oncotarget.8291
Tsubaki M, Komai M, Fujimoto S, Itoh T, Imano M, Sakamoto K et al (2013) Activation of NF-κB by the RANKL/RANK system up-regulates snail and twist expressions and induces epithelial-to-mesenchymal transition in mammary tumor cell lines. J Exp Clin Cancer Res 32:62. https://doi.org/10.1186/1756-9966-32-62
Xiang L, Gilkes DM (2019) The contribution of the immune system in bone metastasis pathogenesis. Int J Mol Sci 20:999. https://doi.org/10.3390/ijms20040999
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I et al (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440:692–696
Takeshita S, Fumoto T, Naoe Y, Ikeda K (2014) Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem 289:16699–16710
Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V (2017) Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab 25:661–672
Dougall WC (2012) Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18:326–335. https://doi.org/10.1158/1078-0432.CCR-10-2507
Mountzios G, Dimopoulos MA, Bamias A, Papadopoulos G, Kastritis E, Syrigos K et al (2007) Abnormal bone remodeling process is due to an imbalance in the receptor activator of nuclear factor–κB ligand (RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors metastatic to the skeleton. Acta Oncol 46:221–229. https://doi.org/10.1080/02841860600635870
Shemanko CS, Cong Y, Forsyt A (2016) What is breast in the bone? Int J Mol Sci 17:E1764. https://doi.org/10.3390/ijms17101764
Molyneux SD, Di Grappa MA, Beristain AG, McKee TD, Wai DH, Paderova J et al (2010) Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest 120:3310–3325
Navet B, Ando K, Vargas-Franco JW, Brion R, Amiaud J, Mori K et al (2018) The intrinsic and extrinsic implications of RANKL/RANK signaling in osteosarcoma: from tumor initiation to lung metastases. Cancers (Basel) 10:E398. https://doi.org/10.3390/cancers10110398
Mori K, Le Goff B, Berreur M, Riet A, Moreau A, Blanchar F et al (2007) Human osteosarcoma cells express functional receptor activator of nuclear factor-kappa B. J Pathol 211:555–562. https://doi.org/10.1002/path.2140
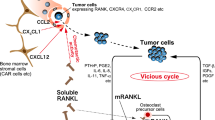
Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V et al (2007) Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res 67:7308–7318. https://doi.org/10.1158/0008-5472.CAN-06-4130
Wittrant Y, Lamoureux F, Mori K, Riet A, Kamijo A, Heymann D, Redini F (2006) RANKL directly induces bone morphogenetic protein-2 expression in RANK-expressing POS-1 osteosarcoma cells. Int J Oncol 28:261–269
Beristain AG, Narala SR, Di Grappa MA, Khokha R (2012) Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci 125:943–955. https://doi.org/10.1242/jcs.094029
Chen Y, Di Grappa MA, Molyneux SD, McKee TD, Waterhouse P, Penninger JM, Khokha R (2015) RANKL blockade prevents and treats aggressive osteosarcomas. Sci Transl Med 7:31. https://doi.org/10.1126/scitranslmed.aad0295
Cowan AJ, Allen C, Barac A et al (2018) Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol 4:1221–1227. https://doi.org/10.1001/jamaoncol.2018.2128
Raje NS, Bhatta S, Terpos E (2019) Role of the RANK/RANKL pathway in multiple myeloma. Clin Cancer Res 25:12–20. https://doi.org/10.1158/1078-0432.CCR-18-1537
Vallet S, Filzmoser JM, Pecherstorfer M, Podar K (2018) Myeloma bone disease: update on pathogenesis and novel treatment strategies. Pharmaceutics 10:E202. https://doi.org/10.3390/pharmaceutics10040202
Goranova-Marinova V, Goranov S, Pavlov P, Tzvetkova T (2007) Serum levels of OPG, RANKL and RANKL/OPG ratio in newly-diagnosed patients with multiple myeloma. Clin Correl Haematol 92:1000–1001
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J et al (2003) A Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102:1064–1069
Sfiridaki K, Pappa CA, Tsirakis G, Kanellou P, Kaparou M, Stratinaki M et al (2011) Angiogenesis-related cytokines, RANKL, and osteoprotegerin in multiple myeloma patients in relation to clinical features and response to treatment. Mediators Inflamm 2011:867576. https://doi.org/10.1155/2011/86757
Spanoudakis E, Papoutselis M, Terpos E, Dimopoulos MA, Tsatalas C, Margaritis D et al (2016) Overexpression of RANKL by invariant NKT cells enriched in the bone marrow of patients with multiple myeloma. Blood Cancer J 6:e500. https://doi.org/10.1038/bcj.2016.108
Pitari MR, Rossi M, Amodio N, Botta C, Morelli E, Federico C et al (2015) Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget 6:27343–27358. https://doi.org/10.18632/oncotarget.4398
Liu T, Qin AP, Liao B, Shao HG, Guo LJ, Xie GQ, Yang L, Jiang TJ (2014) A novel microRNA regulates osteoclast differentiation via targeting protein inhibitor of activated STAT3 (PIAS3). Bone 67:156–165
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Searched for Xgeva. Accessed in May 2019
Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lace DL et al (1999) A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1β, TNF-α, PTH, PTHrP, and 1,25(OH)2D3. J Bone Miner Res 14:1478–1485. https://doi.org/10.1359/jbmr.1999.14.9.1478
de Groot AF, Appelman-Dijkstra NM, van der Burg SH, Kroep JR (2018) The anti-tumor effect of RANKL inhibition in malignant solid tumors—a systematic review. Cancer Treat Rev 62:18–28. https://doi.org/10.1016/j.ctrv.2017.10.010
Capparelli C, Kostenuik PJ, Morony S, Starnes C, Weimann B, Van G et al (2000) Osteoprotegerin prevents and reverses hypercalcemia in a murine model of humoral hypercalcemia of malignancy. Cancer Res 60:783–787
Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR (2001) Kostenuik PJ (2001) Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61:4432–4436
Yonou H, Kanomata N, Goya M, Kamij T, Yokose T, Hasebe T et al (2003) Osteoprotegerin/osteoclastogenesis inhibitory factor decreases human prostate cancer burden in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Cancer Res 63:2096–2102
Luger NM, Honore P, Sabino MA, Schwei MJ, Rogers SD, Mach DB et al (2001) Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res 61:4038–4047
Castellano D, Sepulveda JM, García-Escobar I, Rodriguez-Antolín A, Sundlöv A, Cortes-Funes H (2011) The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist 16:136–145. https://doi.org/10.1634/theoncologist.2010-0154
Ottewell PD, Wang N, Brown HK, Fowles CA, Croucher PI, Eaton CL, Holen I (2015) OPG-Fc inhibits ovariectomy-induced growth of disseminated breast cancer cells in bone. Int J Cancer 137:968–977. https://doi.org/10.1002/ijc.29439
Miller RE, Jones JC, Tometsko M, Blake ML, Dougall WC (2014) RANKL inhibition blocks osteolytic lesions and reduces skeletal tumor burden in models of non-small-cell lung cancer bone metastases. J Thorac Oncol 9:345–354
Cody JJ, Rivera AA, Lyons GR, Yang SW, Wang M, Sarver DB et al (2010) Arming a replicating adenovirus with osteoprotegerin reduces the tumor burden in a murine model of osteolytic bone metastases of breast cancer. Cancer Gene Ther 17:893–905. https://doi.org/10.1038/cgt.2010.47
Canon J, Bryant R, Roudier M, Branstetter DG, Dougall WC (2012) RANKL inhibition combined with tamoxifen treatment increases anti-tumor efficacy and prevents tumor-induced bone destruction in an estrogen receptor-positive breast cancer bone metastasis model. Breast Cancer Res Treat 135:771–780. https://doi.org/10.1007/s10549-012-2222-2
Zinonos I, Luo K, Labrinidis A, Liapis V, Hay S, Panagopoulos V et al (2014) Pharmacologic inhibition of bone resorption prevents cancer-induced osteolysis but enhances soft tissue metastasis in a mouse model of osteolytic breast cancer. Int J Oncol 45:532–540. https://doi.org/10.3892/ijo.2014.2468
Lamoureux F, Picarda G, Garrigue-Antar L, Baudhuin M, Trichet V, Vidal A et al (2009) Glycosaminoglycans as potential regulators of osteoprotegerin therapeutic activity in osteosarcoma. Cancer Res 69:526–536. https://doi.org/10.1158/0008-5472.CAN-08-2648
Body JJ, Greipp P, Coleman RE, Facon T, Geur F, Fermand JP et al (2003) A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 97:887–892. https://doi.org/10.1002/cncr.11138
Qiao B, Shui W, Cai L, Guo S, Jiang D (2015) Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma. Drug Design Dev Ther 9:969–976. https://doi.org/10.2147/DDDT.S77116
Higgs JT, Jarboe JS, Lee JH, Chand D, Lee CM, Deivanayagam C et al (2015) Variants of osteoprotegerin lacking TRAIL binding for therapeutic bone remodeling in osteolytic malignancies. Mol Cancer Res 13:819–827. https://doi.org/10.1158/1541-7786.MCR-14-0492
Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V et al (2011) Anticancer efficacy of Apo2L/TRAIL is retained in the presence of high and biologically active concentrations of osteoprotegerin in vivo. J Bone Miner Res 26:630–643. https://doi.org/10.1002/jbmr.244
Yu X, Kong W, Zheng K (2013) Expression of osteoprotegerin and osteoprotegerin ligand in giant cell tumor of bone and its clinical significance. Oncol Lett 5:1133–1139. https://doi.org/10.3892/ol.2013.1199
Yamagishi T, Kawashima H, Ogose A, Ariizumi T, Sasaki T, Hatano H et al (2016) Receptor-activator of nuclear KappaB ligand expression as a new therapeutic target in primary bone tumors. PLoS ONE 11:e0154680. https://doi.org/10.1371/journal.pone.0154680
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deligiorgi, M.V., Panayiotidis, M.I., Griniatsos, J. et al. Harnessing the versatile role of OPG in bone oncology: counterbalancing RANKL and TRAIL signaling and beyond. Clin Exp Metastasis 37, 13–30 (2020). https://doi.org/10.1007/s10585-019-09997-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-019-09997-8