Abstract
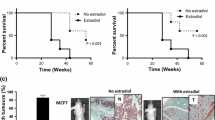
Tumor cells in bone can induce the activation of osteoclasts, which mediate bone resorption and release of growth factors and calcium from the bone matrix, resulting in a cycle of tumor growth and bone breakdown. Targeting the bone microenvironment by the inhibition of RANKL, an essential mediator of osteoclast function, not only prevents tumor-induced osteolysis but also decreases skeletal tumor burden in preclinical models. The inhibition of skeletal tumor progression after the inhibition of osteoclasts is via interruption of the “vicious cycle” of tumor/bone interactions. The majority of breast cancer patients at risk for bone metastases harbor estrogen receptor-positive (ER+) tumors. We developed a mouse model for ER+ breast cancer bone metastasis and evaluated the effect of RANKL inhibition on tumor-induced osteolysis and skeletal tumor growth both alone and in combination with tamoxifen. Luciferase-labeled MCF-7 cells (MCF-7Luc) formed metastatic foci in the hind limbs following intracardiac injection and caused mixed osteolytic/osteoblastic lesions. RANKL inhibition by OPG-Fc treatment blocked osteoclast activity and prevented tumor-induced osteolysis, as well as caused a marked decrease in skeletal tumor burden. Tamoxifen as a single agent reduced MCF-7Luc tumor growth in the hind limbs. In a combination experiment, OPG-Fc plus tamoxifen resulted in significantly greater tumor growth inhibition than either single agent alone. Histologic analysis revealed a decrease in the proliferation of tumor cells by both single agents, which was enhanced in the combination treatment. Upon treatment with OPG-Fc alone or in combination with tamoxifen, there was a complete absence of osteolytic lesions, demonstrating the ability of RANKL inhibition to prevent skeletal related morbidity in an ER+ model. The combination approach of targeting osteoclasts and the bone microenvironment by RANKL inhibition and the tumor directly via hormonal therapy may provide additional benefit to reducing skeletal tumor progression in ER+ breast cancer patients.







Similar content being viewed by others
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80(8 Suppl):1588–1594
James JJ, Evans AJ, Pinder SE, Gutteridge E, Cheung KL, Chan S, Robertson JF (2003) Bone metastases from breast carcinoma: histopathological–radiological correlations and prognostic features. Br J Cancer 89(4):660–665. doi:10.1038/sj.bjc.6601198
Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J (2009) Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16(1):67–78. doi:10.1016/j.ccr.2009.05.017
Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23(22):4925–4935
Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE (2005) Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 97(1):59–69
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2(8):584–593
Roodman GD (2004) Mechanisms of bone metastasis. N Engl J Med 350:1655–1664
Blair JM, Zhou H, Seibel MJ, Dunstan CR (2006) Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol 3(1):41–49
Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ (2001) Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61(11):4432–4436
Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ, Dougall WC (2008) Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 25(2):119–129. doi:10.1007/s10585-007-9127-1
Canon J, Bryant R, Roudier M, Osgood T, Jones J, Miller R, Coxon A, Radinsky R, Dougall WC (2010) Inhibition of RANKL increases the anti-tumor effect of the EGFR inhibitor panitumumab in a murine model of bone metastasis. Bone 46(6):1613–1619. doi:10.1016/j.bone.2010.03.001
Miller RE, Roudier M, Jones J, Armstrong A, Canon J, Dougall WC (2008) RANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther 7(7):2160–2169
Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET (2001) Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest 107(10):1235–1244
Tannehill-Gregg SH, Levine AL, Nadella MV, Iguchi H, Rosol TJ (2006) The effect of zoledronic acid and osteoprotegerin on growth of human lung cancer in the tibias of nude mice. Clin Exp Metastasis 23:19–31
Visvanathan K, Chlebowski RT, Hurley P, Col NF, Ropka M, Collyar D, Morrow M, Runowicz C, Pritchard KI, Hagerty K, Arun B, Garber J, Vogel VG, Wade JL, Brown P, Cuzick J, Kramer BS, Lippman SM (2009) American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol 27(19):3235–3258. doi:10.1200/JCO.2008.20.5179
Finch AJ, Soucek L, Junttila MR, Swigart LB, Evan GI (2009) Acute overexpression of Myc in intestinal epithelium recapitulates some but not all the changes elicited by Wnt/beta-catenin pathway activation. Mol Cell Biol 29(19):5306–5315. doi:10.1128/MCB.01745-08
Kim TH, Shivdasani RA (2011) Genetic evidence that intestinal Notch functions vary regionally and operate through a common mechanism of Math1 repression. J Biol Chem 286(13):11427–11433
Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN (2003) Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat 78(1):105–118
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC (2010) RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468(7320):103–107. doi:10.1038/nature09495
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, Pasparakis M, Khokha R, Ormandy CJ, Widschwendter M, Schett G, Penninger JM (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468(7320):98–102
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 Pt 2):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
Holland PM, Miller R, Jones J, Douangpanya H, Piasecki J, Roudier M, Dougall WC (2010) Combined therapy with the RANKL inhibitor RANK-Fc and rhApo2L/TRAIL/dulanermin reduces bone lesions and skeletal tumor burden in a model of breast cancer skeletal metastasis. Cancer Biol Ther 9(7):539–550. doi:11266 [pii]
Ignatoski KM, Escara-Wilke JF, Dai JL, Lui A, Dougall W, Daignault S, Yao Z, Zhang J, Day ML, Sargent EE, Keller ET (2008) RANKL inhibition is an effective adjuvant for docetaxel in a prostate cancer bone metastases model. Prostate 68(8):820–829
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28(35):5132–5139. doi:10.1200/JCO.2010.29.7101
Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damiao R, Tammela TL, Egerdie B, Van Poppel H, Chin J, Morote J, Gomez-Veiga F, Borkowski T, Ye Z, Kupic A, Dansey R, Goessl C (2011) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. doi:10.1016/S0140-6736(11)61226-9
Acknowledgments
We thank Angela Coxon for critical review of the manuscript and Geoff Smith for editorial assistance.
Conflict of interest
All authors are employees of Amgen, Inc. and have received stock/stock options from Amgen, Inc.
Ethical standards
All experiments conducted herein comply with the laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2012_2222_MOESM1_ESM.pdf
Supplemental Fig. 1. OPG-Fc treatment did not affect the subcutaneous growth of MCF-7Luc cells. Tumor cells were implanted into the flank and treatment with either PBS or OPG-Fc (3 mg/kg, 3×/week) began on day 36 post tumor implantation. Data represent mean tumor volume ± SEM for each group (N = 10/group). (PDF 4 kb)
Rights and permissions
About this article
Cite this article
Canon, J., Bryant, R., Roudier, M. et al. RANKL inhibition combined with tamoxifen treatment increases anti-tumor efficacy and prevents tumor-induced bone destruction in an estrogen receptor-positive breast cancer bone metastasis model. Breast Cancer Res Treat 135, 771–780 (2012). https://doi.org/10.1007/s10549-012-2222-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2222-2