Abstract
Cellulose has been intensively investigated for biomedical applications, because of its excellent properties, like biodegradability, biocompatibility, abundant availability of renewable resources and cost-effectiveness. Among all sources of cellulose, marine macroalgae or seaweeds, are acquiring tremendous attention, due to their high availability around the world. Moreover, the atypical proliferation of some exotic macroalgae species represents a serious problem to the ecosystems, since their accumulation threatens native oceanic species and resources worldwide. Several studies already reported the successfully extraction of cellulose and its derivatives from brown, green and red macroalgae. The extracted cellulose properties vary according to the type of algae, their maturity and the used extraction methods. This review will cover the main methods used to extract cellulose from algae, focusing on more sustainable ones, as well as its further processing into the various cellulose derivatives. Electrospun nanofibers have revealed great potential for biomedical applications, such as delivery of therapeutic agents, tissue engineering, wound dressings and enzyme immobilization. Pure cellulose presents some drawbacks, such as limited solubility in organic solvents and its inability to fuse due to inter and intra-molecular hydrogen bonding. To overcome these limitations, cellulose derivatives, which includes microcrystalline cellulose, cellulose nanocrystals, cellulose nanofibers, cellulose acetate and carboxymethyl cellulose, have been extensively studied to generate electrospun fibers. Therefore, this review aims to explore the marine seaweeds as a promising source of cellulose and its derivatives, the extraction methodologies of these compounds, as well as to demonstrate the potential of nanofibers developed by electrospinning with algae-based cellulose for biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymers obtained from natural sources represent many ecological advantages over synthetic ones, because they are renewable, readily available, relatively easy to isolate, biocompatible and non-toxic. Their use could reduce the large amount of non-biodegradable waste produced daily by some industries. Among them, cellulose, a biodegradable and biocompatible polymer, has been intensively investigated for biomedical applications, because of its chemical, optical, mechanical, and rheological properties. Some of the applications of cellulose-based materials are osseointegration, hemodialysis and biosensors, smart textile fibers, tissue engineering scaffolds, wound dressing and many others (Oprea and Voicu 2020; Surendran and Sherje 2022). While presenting a high potential for biomedical applications, cellulose also presents some drawbacks, such as moisture sensitivity, insolubility in water and in most common organic solvents, and low resistance against microbial attacks. Therefore, the exploration of its derivatives can be a promising approach to overcome these limitations (Seddiqi et al. 2021).
There are several derivatives that can be obtained from cellulose, such as microcrystalline cellulose (MCC), cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs). Furthermore, cellulose can be chemically modified by replacing its hydroxyl groups with functional groups, which generally includes etherification and esterification, to address less favorable properties or to develop new desired characteristics. Cellulose acetate (CA), cellulose nitrate, cellulose sulfate, methyl cellulose and carboxymethyl cellulose (CMC) are some examples of chemically modified cellulose (Seddiqi et al. 2021). Table 1 summarizes the cellulose derivatives.
Cellulose can be obtained from different sources, including plants, bacteria and algae. Among all the available resources, marine macroalgae or seaweeds, are becoming a promising renewable source of biopolymers, including cellulose, due to its high abundance and availability (Bombin et al. 2020). Seaweeds are multicellular organisms that feed on atmospheric and dissolved carbon dioxide (CO2) in water, light, and nutrients and are the basis of most of the food chains in aquatic ecosystem (Pardilhó et al. 2022). Besides presenting a high content of diverse polysaccharides, they are also a natural source of polyphenols, vitamins and peptides. Furthermore, seaweed simple growth requirements, rapid proliferation rates and fewer resources inputs for their cultivation (require no land and fertilizers, pesticides, and freshwater) makes them an attractive alternative compared to the terrestrial biomass (Youssouf et al. 2017; Baghel et al. 2021; Bar-Shai et al. 2021).
In fact, marine algal biomass is widely available around the world, especially in the coastal zone. In some areas, the atypical proliferation of some macroalgae species represents a serious problem to the ecosystems, since their accumulation may threaten native oceanic species and resources worldwide. Moreover, accumulation of unusually high amounts of biomass, leads to negative environmental impacts, mainly due to the release of greenhouse gases through decomposition, as well as health concerns associated with the emergence of disease vectors. Currently, algae waste is mostly sent to landfills or left abandoned on beaches due to the lack of appropriate management protocols for this residual biomass (Pardilhó et al. 2021, 2022). Blooms of some invasive algae species have led to a significant negative impact on the landscape and pose a serious danger to the aquatic ecosystem, by causing seaweed blooms, clogging nets, clogging waterways, and altering nutrient regimes in areas near fisheries, aquaculture systems, and desalination facilities. Recycling this algae waste should be a priority, instead of its disposal, being a new opportunity to use this algae biomass to produce high value-added products, following the circular economy principles (Pardilhó et al. 2022; Pereira et al. 2021).
Seaweed biomass has also been extensively harvested from the wild for the extraction of phycocolloids, such as alginates, carrageenan and agar, used in the biomedical and in the food industries. The extraction of these compounds from algae biomass generates large amount of solid waste, which currently has no value, and which is discharged into the environment after the removal of the phycocolloids. However, this solid waste can be used in its entirety to extract cellulose and its derivatives, promoting the circular economy and sustainability (Ilyas and Atikah 2021).
For those reasons, marine macroalgae are ideal sources of different biomaterials with great potential to be used for the development of systems for tissue regeneration, drug delivery, wound healing applications, and others. Valorization and exploitation of this marine biomass waste is a promising approach to obtain several natural materials with outstanding properties for biomedical applications, in line with circular economy (Pinteus et al. 2018). Cellulose is generally extracted from different algae sources by a combination of a bleaching, alkali treatments, and acid hydrolysis (Samiee et al. 2019; Torres and De-la-Torre 2022). However, there is a need to look for more sustainable ways to improve the process’ efficiency, time and costs, along with the sustainable use of resources and materials. Implementing green chemistry in extraction processes will allow to address these concerns, aiming to achieve a faster extraction rate, a reduction in processing steps and a reduction of the use of hazardous solvents, which can be toxic to human health and the environment (Singh et al. 2021).
Several remarkable applications of algae-based cellulose have been developed in the form of electrospun nanofibers, nanoparticles, hydrogels, and aerogels. Recently, electrospinning technique has been acquiring increasing attention in the biomedical sector. Electrospinning is the most frequently and efficient technique used to produce ultrafine polymeric fibers (Iliou et al. 2022). In fact, this technique stands out for its high versatility, cost-effectiveness, simplicity of use, accuracy, and for the possibility to easily tune the composition and the mechanical characteristics of nanofiber-based membranes, which can be processed with several strategies to improve their biological properties. Electrospinning allows the production of fibers with controllable diameters ranging from micrometers (µm) to nanometers (nm), which demonstrate several advantages to be used in biomedical applications, for example, as wound dressing systems. In fact, electrospun nanofibers exhibit similar microstructure to extracellular matrix (ECM), promoting cell adhesion, proliferation, migration and differentiation, which will lead to the regeneration of injured skin. Furthermore, the ability to incorporate bioactive compounds, either by adsorption onto nanofibers surface or encapsulation into nanofibers matrix, allows the development of new functionalities, including antimicrobial activity (Costa et al. 2022; Ribeiro et al. 2021a, b; Ribeiro et al. 2021a, b; Samadian et al. 2020).
Therefore, this review aims to explore the great potential of algae as a natural and renewable source of cellulose and its derivatives and to show their posterior use in electrospun nanofibers for biomedical applications. Moreover, this review also aims to bring an updated overview of the main used extracted procedures of cellulose and its derivatives from algae, focusing on the green methodologies. The combination of cellulose-based nanofibers using cellulose extracted from seaweeds can be a very attractive approach to develop new scaffolds for biomedical applications.
Algae as a potential source of cellulose and its derivatives
Marine algae are one of the most ancient inhabitants of the planet. These are photosynthetic organisms with complex and peculiar taxonomy. Currently, they are classified in two main types of algae, macroalgae and microalgae, being macroalgae the focus of this review. Macroalgae, also referred as seaweeds, are large and multicellular photosynthetic aquatic organisms. They inhabit the coastal zone at depths with sufficient light to conduct photosynthesis (Andryukov et al. 2020; Wan et al. 2022). According to their pigmentation, they can be divided into three groups: Chlorophyta (green), Rhodophyta (red), and Ochrophyta-Phaeophyceae (brown) (Otero et al. 2023). Over millions of years of existence in the marine ecosystem, these organisms have acquired the ability to develop effective antibiotic protection mechanisms against pathogenic microorganisms, and numerous strategies for survival in extreme abiotic environmental conditions. For these reasons, many of these organisms have a unique chemical structure that others do not possess (Leandro et al. 2019; Shannon and Abu-Ghannam 2016).
As previously mentioned, invasive algae species often lead to negative consequences for the new host ecosystem causing environmental and health concerns. Thus, the recycling of these algae and their appropriate utilization will have a high positive impact in circular economy, as well as the environment, by removing it from the ecosystems and using it as a natural resource, that supposedly has no longer any function, to develop high value products for different industries (Yu et al. 2021; Pardilhó et al. 2022). Some examples of algae species considered invasive are: Cladophorales, Ulva, Chaetomorph (green macroalgaes) (Yu et al. 2021), Sargassum sp., Rugulopteryx okamurae (brown macroalgae), (Robledo et al. 2021; Roca et al. 2022; Yan et al. 2021) and Dasysiphonia japonica (red macroalgae) (Young et al. 2022).
Marine algae are rich sources of bioactive compounds with anticancer, anticoagulant, antioxidant, anti-inflammatory, antimicrobial properties and cell proliferative effects. As a result, their compounds have been widely studied and used in the pharmaceutical, cosmetic, and food industries (Iliou et al. 2022; Wang et al. 2017). Polysaccharides are the main components of macroalgae, which includes agar, alginates, carrageenans, ulvans, cellulose, fucoidans, laminarin, and floridean starch (Birgersson et al. 2023; Jönsson et al. 2020; Otero et al. 2023). Cellulose and other polysaccharides are presented in seaweed cell walls. Figure 1 describes the cell walls of the three types of algae. Figure 1A shows the cell wall structure of the green algae, which consists of four families of polysaccharides, ulvan and cellulose as main constituents, and certain hemicellulose constituents, such as β-1,4-D-glucuronan and β-1,4-D-xyloglucan as minor amounts. Figure 1B shows the cell wall of red algae, which are constituted by microfibrils of cellulose, glucomannan and hemicellulose material, consisting of sulfated glucan and xylogalactans. Finally, Fig. 1C shows the structure of brown algae cell wall, which contains sulfated polysaccharides, cellulose microfibrils, alginate (Baghel et al. 2021).
Representative cell wall composition of A green, B red and C brown seaweeds. Image retrieved with permission from (Baghel et al. 2021)
Cellulose extracted from various sources may contain unique and distinct properties in terms of dimensions, morphology, aspect ratio, crystallinity and physicochemical properties. Cellulose has been successfully extracted from green, brown and red macroalgae. Several studies investigated the cellulose content of different species of algae from different groups. It was concluded that in 19 species of green seaweeds studied, the percentage of cellulose found was in average of 9.67%, ranging from 1.5 to 34% on dry weight (DW) of biomass. In 15 species of brown seaweeds, the cellulose content ranged from 2.2 to 10.2% on a DW basis, with an average of 7.88%. Finally, in 47 species of red seaweeds, the cellulose content ranged from 0.85 to 18% DW with an average of 4.75%. Therefore, the cellulose content reported for seaweed biomass ranges between 0.85 and 34% (Baghel et al. 2021).
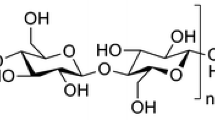
Cellulose, the most abundant polysaccharide on earth, presents biocompatibility, biodegradability, renewability and non-toxicity, which make this polymer and its derivatives very attractive for various biomedical applications. This polymer is composed of repeating d-glucose units (Fig. 2), linked together by β 1 → 4 glycosidic bonds (Birgersson et al. 2023; Seddiqi et al. 2021; French 2017). The cellulose chains are joined together by hydrogen bonds and Van der Waals forces, constituting the primary elements of the supramolecular structure, named elementary nanofibrils, with a high degree of crystallinity and purity, that self-assemble in the form of microfibrils with alternating amorphous and crystalline regions. The strong inter and intra molecular bonds and the linearity of the cellulose molecules are responsible for its crystallinity. Moreover, the degree of crystallinity of this semi-crystalline polymer depends on its origin, extraction method, and pretreatment (Oprea and Voicu 2020; Seddiqi et al. 2021).
Repeating unit (glucose residues) of cellulose. Created with ChemDraw (French 2017)
Cellulose exists in four different allomorphs, including cellulose I, II, III, and IV. Cellulose I is divided into the Iα and Iβ sub-allomorphs, with the Iα form found in algal and extra-cellular bacterial cellulose and the Iβ form found in trees, other higher plants, and tunicates. Both cellulose I forms have parallel chains in their crystallites (Nishiyama et al. 2002, 2003). Cellulose II (Langan et al. 2001) can be obtained from cellulose I, by regeneration (dissolution and recrystallization) or mercerization (treatment with aqueous NaOH). It is a more stable form of cellulose, and presents an antiparallel chain arrangement. The difference in crystallinity of cellulose I and II leads to distinct properties. Cellulose III can be obtained by treatment of cellulose I or II with amines (Wada et al. 2004, 2009). Furthermore, cellulose III can be transformed into cellulose IV with glycerol at high temperatures (Nunes 2017; Seddiqi et al. 2021; Surendran and Sherje 2022). Figure 3 shows the phase transition between various crystalline allomorphs of cellulose (Seddiqi et al. 2021).
Schematic diagram of the phase transition between various crystalline allomorphs of cellulose (cellulose I, II, III, and IV). Image retrieved under the terms of the CC-BY license from (Seddiqi et al. 2021)
The poor processability of cellulose due to the limited solubility in water and many organic solvents, resulting from the strong intra-molecular and inter-molecular hydrogen bonds that are present between the individual chains, limits its use. In this way, the exploration of cellulose derivatives has been the focus of several research studies, considering their better dissolution ability (Hosny et al. 2022). The difference between the various cellulose derivatives is related to the shape, size and degree of crystallinity of their particles (Shokri and Adibkia 2013). Nanocellulose, including CNCs and CNFs, can be extracted by chemical or mechanical treatments. In addition, cellulose can be also chemically modified, through its hydroxyl groups, mainly the primary alcohol group, originating different cellulose derivatives. The main advantage of this modification is to broaden its application and to improve its compatibility with other materials (Gomri et al. 2022). Two of the main groups of cellulose derivatives are cellulose esters and ethers with different physicochemical and mechanical properties. Various types of cellulose esters, which are generally water insoluble polymers with good film forming characteristics, have been used in commercial products or in pharmaceutical investigations, such as CA. On the other hand, cellulose ethers are high molecular weight compounds produced by replacing the hydrogen atoms of hydroxyl groups of cellulose with alkyl or substituted alkyl groups. Their properties can be determined by their molecular weights, chemical structure and distribution of the substituent groups, degree of substitution and molar substitution. One of these examples, is the CMC (Shokri and Adibkia 2013). In the following section, the extraction methodologies of the different cellulose derivatives will be explored.
Extraction methodologies of cellulose and its derivatives from algae
Cellulose and its derivatives have been successfully extracted from green (El-Sheekh et al. 2023; Wahlström et al. 2020), brown (Doh and Whiteside 2020) and red (El et al. 2018) macroalgae. Several macroalgae have been characterized with high cellulose content, such as Ceramium (18.5%), Chaetomorpha (36.5–41%), Chondria (16.4%), Cladophora (20–45%), Corallina (15.2%), Fucus (13.5%), Gelidiella (11.3–13.6%), Gracilaria (10.5%), Griffithsia (22%), Halidrys (14%), Hypnea (11.4%), Laminaria (1.1–20%), Rhizoclonium (38.6%) and Ulva (1.8–19%) (Aswathi Mohan et al. 2022). The physical and chemical properties of the extracted cellulose can vary according to the sources, the maturity of the biomass, and extraction methods used, as well as pre and post treatments, although the molecular structure of cellulose from different sources is identical (Hamouda and Abdel-Hamid 2022; Trache et al. 2020; Wahlström et al. 2020).
The extraction of cellulose by fractionation techniques is an essential step, since cellulosic fibers do not occur as an isolated component, and the other non-cellulosic components may negatively affect the cellulose modification and dissolution processes (Araújo et al. 2020). The extraction of cellulose can involve a washing pre-treatment of the material in order to remove dirt and other impurities. Subsequently, the size reduction of raw materials using mechanical processes is performed in order to expand the raw material and chemical contact surface area (Samiee et al. 2019). Afterwards, the most common and effective step-treatment is the combination of bleaching and alkaline pre-treatments, followed by dilute acid hydrolysis. The bleaching step aims to remove pigments, in order to obtain highly purified, whiteness extracted cellulose. In this step, sodium chlorite (NaClO2) and hydrogen peroxide (H2O2) are mainly used, however, chlorite-free H2O2 is preferred because it is considered a more environmentally friendly bleaching agent. The alkaline treatment uses mainly sodium hydroxide (NaOH) to remove the hemicellulose and lignin. Finally, the acid hydrolysis is used to cause the cleavage of glycosidic bonds through acid penetration into the cellulose fibers, being the most used process. In this step, strong mineral acids, like hydrochloric acid (HCl) and sulfuric acid (H2SO4), are commonly employed (Pardilhó et al. 2021; Singh et al. 2017; Tarchoun et al. 2019; Wang et al. 2020; Zanchetta et al. 2021). Finally, several post-treatments can be applied, such as solvent elimination, neutralization, washing, centrifugation, drying, among others (Trache et al. 2020; Wahlström et al. 2020).
However, these processes generate toxic and hazardous wastes, require the use of high temperatures, time and energy, which leads to negative impact on the environment (Flórez-Fernández et al. 2019; Trache et al. 2020; Zanchetta et al. 2021). In alkaline step, due to the strong alkaline nature of NaOH, massive amount of acid/water is required to neutralize/dilute the effluent. Moreover, acid hydrolysis, with the mineral acids has many disadvantages, like difficult economic recovery of acid, the requirement of a large amount of alkali to neutralize the effluent along with the generation of high amount of salt and low thermal stability of the extracted cellulose (Sankhla et al. 2021). Thus, green and sustainable methods have recently been explored for the extraction of cellulose and other compounds from algae, which can reduce the application of harmful solvents (Flórez-Fernández et al. 2019; Singh et al. 2021). Therefore, seeking for a sustainable and economic approaches able to improve the efficiency and the easiness of the processes, and at the same time to reduce their environmental impact is of extremely importance (Trache et al. 2020). In this context, the use of less toxic solvents and the application of less energy-intensive processes can be a suitable alternative to achieve higher sustainability of the processes (Morais et al. 2020). The production of cellulose from land-based biomass requires strong delignification processes to break the lignin. The use of macroalgae as source of cellulose and its derivatives can simplify the purification process of cellulose, since most macroalgae are lignin free or contain low lignin content, allowing the use of less severe extraction conditions. This can result in more environmentally friendly processes (He et al. 2018; Wahlström et al. 2020; Zanchetta et al. 2021). The extraction processes of the different cellulose derivatives from algae and their characteristics will be explored in the following subsections.
Extraction of microcrystalline cellulose (MCC) from macroalgae
MCC is a purified form of partially depolymerized cellulose with a diameter in micron scale (Seddiqi et al. 2021). MCC possesses unique mechanical and physicochemical properties, such as renewability, biodegradability, biocompatibility, non-toxicity, high mechanical properties, low density, large surface area, and good hygroscopicity. During the extraction process of MCC, the length of the cellulosic polymer chains decreases, and the amorphous regions are removed, while the crystalline ones remain intact, leaving the MCC with a high degree of crystallinity, ranging from 55 to 80% (Baruah et al. 2020). Depending on the cellulose source and processing conditions, such as the reaction time and temperature, and drying conditions, the degree of crystallinity of MCC varies, which can affect its functionality (Samiee et al. 2019). MCC is used in different industries due to its unique mechanical and physicochemical properties, such as stiffness and mechanical strength. For this reason, MCC is commonly used as a green reinforcing agent, allowing the development of biodegradable biocomposites. They can also be applied in cosmetic (thickeners, binders), pharmaceutical compounds (as binders or adsorbents), food (stabilizers, anti-caking agents, fat substitutes and emulsifiers), among others (Tarchoun et al. 2019; Thielemans et al. 2022).
MCC can be extracted and purified from cellulose using different methodologies, including enzymatic hydrolysis, steam explosion, and acid hydrolysis, the latter being the most used one. Thus, MCC is generally isolated using three treatments: alkaline treatment, oxidative bleaching and acid hydrolysis (Samiee et al. 2019; Tarchoun et al. 2019).
Acid hydrolysis and mechanical treatment can also be combined. For example, He et al. reported the extraction of MCC from Saccharina japonica algae by combining chemical and mechanical treatments. For the extraction of cellulose, the algae were subjected to successive washing, bleaching, alkali and finally acid treatments to remove proteins, minerals and other impurities. To decrease the degree of polymerization (DP) and maximally remove the amorphous regions to obtain MCC, the extracted cellulose was then hydrolyzed with HCl, followed by ball-milling and sieving. In this case, the mechanical treatment promoted a further decrease of the DP in a moderate manner. The extracted cellulose presented a DP of 695, and a highly crystalline structure (CrI of 90.9%), while MCC exhibited a DP of 235 and a CrI of 88.6% (He et al. 2018).
Extraction of nanocellulose from macroalgae
Nanocellulose is referred to cellulosic material with at least one dimension in the nanometer range. Nanocellulose mainly includes three subcategories: (i) CNCs, (ii) CNFs and (iii) bacterial nanocellulose (BNC). The main differences between CNCs and CNFs lie in their dimensions, crystallinity and shape (Fig. 4). CNCs consist of cylindrical, elongated, less flexible, and rod like shape, with higher crystallinity and relatively smaller sizes (2–15 nm wide, 100–500 nm long), while CNFs refer to an entangled network structure with flexible, longer and wide nanofibers (20–50 nm wide, 500–1500 nm long) with lower crystallinity. Nevertheless, their dimensions as well as other properties are dependent on the cellulose source, extraction method and processing conditions (pre and post treatments) (Tayeb et al. 2018; Teixeira et al. 2020; Trache et al. 2020; Ross et al. 2021; Zanchetta et al. 2021). CNCs and CNFs exhibit characteristics such as biocompatibility, biodegradability, low cytotoxicity, active functionalization groups, large surface area and high mechanical strength, making them very attractive for biomedical applications, including for drug delivery systems, wound dressings and medical implants. Additionally, they can act as fillers, allowing for instance the production of bio-based electrospun nanofibers with improved performance (Ribeiro et al. 2021a, b; Ribeiro et al. 2021a, b; Yu et al. 2021). On the other hand, BNC, refers to nanostructured cellulose produced by bacteria (Trache et al. 2020).
TEM images of a CNCs and b CNFs. Image retrieved under the terms of the CC-BY license from (Tayeb et al. 2018)
Cellulose nanocrystals (CNCs)
The production of CNCs involves breaking down larger units (cm) into smaller units (nm), where the amorphous nature of the cellulose is separated from the fibers in order to collect only the crystalline component. Various approaches are available for CNC extraction, such as acid hydrolysis, ionic liquid method, TEMPO ((2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl) oxidation method and enzymatic hydrolysis, being the strong acid hydrolysis (generally around 60% H2SO4) the most common used (Mali and Sherje 2022; Pradeep et al. 2022). Afterwards, CNCs can be purified and dispersed (by mechanical treatments) (El et al. 2018; Salminen et al. 2017).
Achaby et al. reported the successful extraction of CNCs from red algae waste, using an alkali and bleaching treatments followed by acid hydrolysis with 64 wt% H2SO4 at 50 °C (Fig. 5). In this case, the influence of the hydrolysis time (30, 40 and 80 min) on the properties of the extracted CNCs were evaluated. The obtained CNCs presented a needle-like shape, which was not affected by the duration of hydrolysis. Nevertheless, increasing the hydrolysis time resulted in large amount of negatively charged sulfate groups on the surface of CNCs; reduction of the extraction yield (13.76, 12.9 and 11.18%, respectively, with respect to the starting raw materials); reduction of CNC diameters (9.1, 7.6 and 5.2 nm, respectively) and lengths (315.7, 294.5 and 285.4 nm, respectively); increase of CNC aspect ratio (35, 42 and 57, respectively); reduction of CNC thermal stability; and increase of the CrI (81, 83 and 87%, respectively) (El et al. 2018).
Schematic representation of CNC extraction at different hydrolysis times and digital images of the obtained products. Image retrieved under the terms of the CC-BY license from (El et al. 2018)
Liu et al. also used acid hydrolysis to extract CNCs from kelp (Laminaria japonica) waste. Several processing conditions were also evaluated, and the optimized one was achieved using 51 wt% H2SO4 at 30 °C for 70 min, which allowed the extraction of CNCs with rod shape, an extraction yield of 52.3%, and a CrI of 69.4% (Liu et al. 2017).
Besides the characteristics of CNCs being affected by process conditions, they could also vary according to the source. In this way, the properties of the CNCs extracted from three different groups of algae (red, brown and green) were studied. Isolating CNCs was accomplished with a four-step process: de-polymerization, bleaching, acid hydrolysis (51% H2SO4, at 45 °C for 30 min), and mechanical dispersion (ultrasonication). All the extracted CNCs showed rod shaped. The results showed that brown algae achieved the highest yield (26.1 and 25.8% for kombu and sargassum, respectively), followed by the green algae (16.3% for sea lettuce) and the red algae (13.3 and 17.4% for dulse and nori, respectively). The obtained CNCs presented an aspect ratio as about 2–15, which is in accordance with the typical aspect ratio of CNCs that varies from 1 to 100. Moreover, brown seaweed group had higher aspect ratio than other two groups. Regarding the crystallinity, the CNCs extracted from kombu exhibited the highest CrI (98.89%), while the one extracted from sea lettuce presented the lowest one (CrI 66.97%). CNCs from sargassum, dulse, and nori, showed CrI of 83.06, 84.91 and 87.54%, respectively, demonstrating that all samples showed enough CrI to act as a filler, promoting an increase of the mechanical and barrier properties of polymer films (Doh et al. 2020).
In the attempt to use a greener extraction method, Singh et al. extracted CNCs from a red seaweed (Gelidiella aceroso) using a microwave-assisted alkali treatment. In the traditional alkali method, the solution should be heated for 2–4 h to remove lignin and hemicellulose. However, in this process, microwave radiation can be used as an alternative energy source, due to its ability to rapidly generate heat, and thus, reducing the process time. The bleaching and acid hydrolysis using ultrasonication were performed, and the extracted CNCs presented rod-like shape, a CrI of 60%, an extraction yield of 30%, an average diameter of 32 nm ranging from 14 to 50 nm, and an average size of 408 nm ranging from 305–512 nm. Therefore, this method revealed to be a more efficient and greener approach for the isolation of CNCs from seaweed {Formatting Citation}. The same methodology was used to extract CNCs from red seaweed biomass, which were used to improve the mechanical, barrier and thermal properties of poly(vinyl alcohol) (PVA) films (Singh et al. 2018).
Cellulose nanofibrils (CNFs)
CNFs are produced by disintegration of cellulose fibers into particles with diameters in nanoscale, and contains both crystalline and amorphous regions, being the crystalline regions responsible for its strength, while the amorphous domains provide a certain flexibility (Teixeira et al. 2020; Wahlström et al. 2020). The extraction of CNFs from algae involves (i) separating cellulose from other impurities, (ii) pre-treatment of the cellulose (mechanical, biological, or chemical), to improve the defibrillation of the cellulose and (iii) mechanical disintegration of the fibers where cellulose bundles are completely delaminated to individual nanofibrils (Zanchetta et al. 2021). Regarding the mechanical pre-treatments, the most commonly used are high-pressure homogenization, microfluidization, and grinding. However, these processes require high energy consumption, so biological or chemical pre-treatments are usually used, prior to mechanical disintegration. These pre-treatments could also improve the defibrillation of the cellulose allowing the production of well-separated nanofibrils with a proper crystallinity. Typically, the enzymatic hydrolysis method uses two enzymes, laccase, for the degradation of lignin and hemicellulose, and cellulase for the hydrolysis of extra cellulose compounds. The combination of enzymatic hydrolysis together with high pressure shearing and homogenization can lead to controlled fibrillation of CNF (Mitbumrung et al. 2022; Surendran and Sherje 2022; Xiang et al. 2016). TEMPO-mediated oxidation, etherification, periodate and chlorite oxidation, ionic liquids, deep eutectic solvents, and phosphorylation are some examples of chemical pre-treatments. These give rise to a higher degree of fibrillation, bringing them closer to the ideal characteristics, and also lead to a significant reduction in energy consumption (Jonasson et al. 2020). Finally, the mechanical process aims to achieve a total fibrillation to individual cellulose fibers, being the most used high-pressure homogenization (homogenizer, microfluidizer), ultra-fine friction grinding and refining (Zanchetta et al. 2021).
Xiang et al. conducted a study in which cellulose was extracted from the green macroalgae Cladophora glomerata and subjected to microfluidization, with or without enzymatic hydrolysis pre-treatment to produce CNF. After the bleaching, alkali treatment and acid hydrolysis, the extracted cellulose passed through the microfluidizer 10 or 20 passes. It was found that increasing microfluidization times produced CNF with reduced crystallinity and with smaller and more uniform sizes. The pre-treatment was also evaluated, and the CNF produced by enzymatic hydrolysis followed by microfluidization presented lower crystallinity, in comparison to CNF produced only with mechanical treatment. Moreover, the enzymatic pre-treatment did not have evident effect on the diameter distribution of CNF, which can be due to the fact that CNF produced by only microfluidization already presented the microfibril levels. Finally, CNF produced from macroalgae showed higher crystallinity, lower diameters and higher thermal stability compared to CNF produced from bleached eucalyptus pulp (BEP), showing the potential of algae source for the obtention of nanocellulose (Xiang et al. 2016).
One the other hand, Wahlstrom et al. reported a successful extraction of CNF from green macroalgae Ulva lactuca (Ulva fenestrata), without any pre-treatment prior the mechanical homogenization process. Cellulose was extracted and then disintegrated into lignin-free CNF using a mechanical process. The obtained CNF showed characteristic peaks for the cellulose I allomorph and a CrI of 48%, confirming that the nanofibrils were semicrystalline. Characterization of the CNF morphology showed nanofibrils similar to those found in lignocellulose. The absence of lignin in Ulva lactuca macroalgae, make this source even more attractive compared with land-based biomass, since delignification is energy consuming and the final cellulose fraction may contain residual chemicals from the lignin removing process (Wahlström et al. 2020). In another study, CNF was also successfully extracted from Laminaria Hyperborea brown seaweed using high-pressure homogenization, without the need for mechanical, biological or chemical pre-treatments. Only a single pass through the 200 µm and 100 µm interaction chambers of the high-pressure homogenizer was required. The produced CNFs were found to have a good degree of crystallinity, high storage modulus, high water retention values, good morphological properties, high aspect ratio and good thermal properties (Onyianta et al. 2020).
Extraction of cellulose from macroalgae and its modification to cellulose acetate (CA)
CA, the acetate ester form of cellulose, has environmentally friendly properties, namely, biodegradability, wettability, and renewability coupled with excellent processibility. Furthermore, it is biocompatible and presents excellent mechanical strength (Zamel and Khan 2021; Janmohammadi et al. 2023). Compared to the poor solubility of cellulose, CA is soluble in some organic solvents, being the solubility dependent on the degree of the substitution of acetate groups, which make this polymer very advantageous for different applications. Among cellulose derivatives, CA stands out as it has been widely applied for the production of electrospun nanofibers, showing great potential to be used in drug delivery applications as well as in wound healing systems (Khoshnevisan et al. 2018, 2019). Figure 6 shows the chemical structure of CA.
Chemical structure of CA. Image adapted under the terms of the CC-BY license from (Teramoto 2015)
CA is usually produced by the acetylation of hydroxyl groups of cellulose with acetic anhydride, acetic acid (solvent), and sulfuric acid (catalyst) (Seddiqi et al. 2021). However, more sustainable routes to perform the acetylation are being explored, namely the replacement of sulfuric and acetic acids, by environmentally friendly reagents, such as iodine as catalyst in the presence of acetic anhydride, phosphotungstic acid as catalyst and ionic liquid at room temperature. Although the application of these reagents represents potential environmental benefits, they still need more research and better environmental impact assessments using standardized and reliable tools (Araújo et al. 2020).
Up to date, only studies with the most common techniques are reported, and the green ones are still under investigation. Madub et al. reported the extraction of cellulose from green seaweeds of the Ulva genus followed by its chemically modification to obtain CA, using acetic anhydride and acetic acid. The yield of the acetylated product was about 78%, the % acetylation was 44.6, and the degree of substitution (DS) was 3.00, which translates into an average of 3 hydroxyl groups being acetylated per disaccharide unit (Madub et al. 2021).
Extraction of cellulose from macroalgae and its modification to carboxymethyl cellulose (CMC)
CMC is an anionic, water-soluble derivative of cellulose, prepared by adding carboxymethyl groups (-CH2-COOH) to some of the hydroxyl groups of the cellulose chain (Rahman et al. 2021). The molecular weight, DS, and the distribution of carboxymethyl substituents along the polymer chain will affect the properties of CMC (Seddiqi et al. 2021). Recently, CMC-based dressings have received some attention due to their physicochemical properties, namely for being flexible, able to absorb exudate, to promote angiogenesis and autolytic debridement. In addition, CMC is not toxic to humans, and it can maintain a moist environment at the wound surrounding areas, which will help in the growth of ECM and re-epithelialization (El-Newehy et al. 2016; Kanikireddy et al. 2020). Nowadays, CMC is the most widely used cellulose ether with applications in several industries (Jia et al. 2016). Figure 7 shows the chemical structure of CMC.
Chemical structure of CMC. Image adapted under the terms of the CC-BY license from (Teramoto 2015)
Lakshmi et al. extracted cellulose from green seaweed Ulva fasciata, followed by its modification into CMC. For the preparation of CMC, the extracted cellulose, was mixed with NaOH solution and isopropanol, followed by chloroacetic acid. The DS of CMC was 0.51, and this parameter plays an important role in the water solubility of CMC, since its hydro affinity increases with increasing DS. CMC was used to produce films, which showed to be more transparent (70%) than the films containing commercial CMC (60%). Moreover, CMC films with silver (Ag) nanoparticles and CMC films with leaf extracts demonstrated antibacterial activity against Escherichia coli (E. coli). Furthermore, the biodegradable nature of algae CMC film was also confirmed. Thus, it was concluded that CMC algae films exhibit potential applications in several industries (Lakshmi et al. 2017).
The studies exploring the extraction methodologies of cellulose and its derivatives as well as their properties are summarized in Table 2.
Cellulose-based electrospun nanofibers for biomedical applications
The combination of electrospinning technique with cellulose-based materials allows the production of ultrafine nanofibrous membranes with highly porous structure, biocompatibility, biodegradability, hydrophilicity, low density, thermostability, flexibility and easy chemical modification. However, the production of cellulose nanofibers by electrospinning remains a challenge, due to its poor solubility in commonly used solvents. Thus, for some applications, cellulose derivatives, particularly CA, have been widely used, due to their improved solubility in many organic and inorganic solvents (Teixeira et al. 2020; Zhang et al. 2021). Despite the potential of algae as a renewable and valuable source of cellulose, the production of electrospun nanofibers using seaweed extracted cellulose and its derivatives is still poor explored. In the following section, a briefly explanation of the electrospinning technique as well as the advantages of using these membranes in different biomedical applications will be presented. Moreover, the potential of cellulose-based electrospun nanofibers for the same applications will be also addressed, considering cellulose derived from algae and other sources.
Electrospinning technique
Electrospinning is an electro-hydrodynamic technique used to produce thin fibers with diameters ranging from µm to nm. A variety of materials (e.g., polymers, ceramics and metals) can be used to produce these fibers, and they can present different morphologies, patterns and functionalities. Electrospun micro/nanofibers demonstrate unique properties, such as large surface area, high encapsulation efficiency, high porosity, physical structure that mimics the ECM, thus supporting cell adhesion, proliferation, migration and differentiation, flexibility, possibility of loading and release a variety of drugs, vast possibilities for surface functionalization and wide selection of the matrix materials. All these characteristics make electrospinning a very attractive technique for different applications, including in biomedical field (Costa et al. 2022).
The electrospinning device consists of a high-voltage power supply, a spinneret connected to a syringe pump, and a metal collector (Fig. 8). This technique is simple to use, straightforward, versatile and cost-effective, with the possibility of large-scale production. Initially, a polymer solution is loaded into a syringe, and the solution is passed to the tip of the needle, to form a pendant droplet. Upon electrification, the electrostatic repulsion among the surface charges deforms the droplet into a conical shape, known as Taylor cone. When the repulsive electric forces overcome the surface tension of polymeric droplet, a charged liquid jet is ejected from the tip of Taylor cone, which is continuously elongated and whipped. As the jet travels towards the metallic collector, the solvent evaporates and the nanofibers are continuously deposited in the collector (Costa et al. 2022; Xue et al. 2019).
Representation of a typical electrospinning setup. Image retrieved with permission from (Costa et al. 2022)
There are multiple strategies for incorporating bioactive compounds into electrospun fibers, including blend, emulsion and co-axial electrospinning, as well as surface immobilization (Fahimirad and Ajalloueian 2019). This versatile technique allows the production of different types of fibers (superficial porous, hollow, with a core–shell structure, etc.) and fibrous structures (non-woven, aligned fibers, multilayer, etc.). Its applications include drug delivery systems, wound dressings, tissue engineering scaffolding, biosensors, filtration membranes, etc. (Li and Wang 2021).
The formation of fibers from the polymer jet depends on the solution (type of polymer and its blend with other polymer(s), polymer’s molecular weight and concentration; type, volatility and concentration of the solvent(s); solution’s surface tension, viscosity and conductivity), process (applied voltage, flow rate, distance between the spinneret and collector, needle diameter, and type of collector) and the environmental (temperature and humidity) parameters (Gruppuso et al. 2021).
Regarding the solution parameters, the polymer molecular weight and concentration, as well as the type of solvent, are very important to the characteristics of the final membranes, since the intrinsic viscosity of the solution is related to the molecular weight and concentration of the polymer and the conductivity depends on the specific solvent mixture. Solutions with higher polymer concentration, and consequently, higher viscosity, promote the formation of larger fibers with reduced defects. Solutions with high conductivity leads to the formation of homogeneous nanofibers with smaller diameters, due to the increase of charge density, resulting in stronger elongation forces in ejected jet (Costa et al. 2022; Bombin et al. 2020). Considering the process conditions, the collector and the spinneret are connected to electrodes, acquiring opposite charges. The voltage applied at the spinneret might be positive or negative. As the voltage increases, the spherical morphology of the droplet transitions into a more elongated, conical shape. As the voltage is further increased, the electric forces dominate over the surface tension and the electrically charged polymer solution is extruded towards the grounded collector. Regarding the flow rate, when it is too low, polymer ejection is difficult to be achieved, because the replacement of the solution ejected from the tip of the capillary is not sufficient. On the other hand, when the flow rate is too high, beaded nanofiber formation can occur as a result of Taylor cone deformation. It is also necessary to provide a minimum distance between the spinneret and the collector, so that the solvent can evaporate before reaching the collector. However, too long distances can promote the formation of other defects, such as non-uniform or sharp-beaded nanofibers (Bombin et al. 2020; Xue et al. 2019). Finally, the environmental parameters, such as temperature and humidity, should also be considered. An increase in temperature promotes a decrease in the viscosity of the polymer solution, and consequently, a decrease in the diameters of the electrospun nanofibers. The relative humidity will affect the evaporation rate of the solvent, and consequently, the solidification rate of the jet. Generally, lower relative humidity values cause rapid solvent evaporation, resulting in the production of fibers with higher diameters. Therefore, all of these parameters need to be properly adjusted and optimized in order to achieve the highest quality of the final nanofibers for the desirable application (Costa et al. 2022; Xue et al. 2019).
Cellulose and its derivatives for the production of electrospun nanofibers to be applied in biomedical applications
Electrospun nanofibers have been extensively researched and used for multifaceted biomedical applications, such as delivery of therapeutic agents, tissue engineering, wound dressings, enzyme immobilization and so on. As previously mentioned, the exceptional properties of cellulose, like biodegradability, biocompatibility, abundant availability of renewable resources and cost-effectiveness, make this polymer very attractive for medical field. However, biomedical applications of cellulose have some obstacles, such as the limited solubility of organic solvent in general and its inability to melt due to inter and intra-molecular hydrogen bonding, which difficult its use for example in electrospinning. Thus, cellulose derivatives, have been extensively investigated to overcome these obstacles and generate electrospun nanofibers, that can be transformed into cellulose fibers by various processes (Khoshnevisan et al. 2018, 2019).
To date, and to the best of authors’ knowledge, only very few studies have reported the use of algae-based cellulose for the manufacture of nanofibrous scaffolds for medical field. Nevertheless, cellulose and its derivatives extracted from other sources than algae have already demonstrated great potential and advantages to be applied in electrospinning, aiming the production of membranes for biomedical applications. Therefore, the next section aims to give an overview of the potential of electrospun cellulose-based membranes for several medical applications, using cellulose and its derivatives extracted from algae and other sources.
CA is one of the most widely used cellulose derivatives in biomedical applications (Wsoo et al. 2020). The extraction of cellulose from green seaweeds, its subsequently chemical modification into CA, and the use of this polymer in electrospinning was already reported by Madub et al. The obtained CA was used in electrospinning alone and blended with poly-L-lactide (PLLA), poly-DL-lactide (PDLLA) or polydioxanone (PDX) to develop nanofibrous mats. Deacetylation of the mats was also performed to obtain regenerative cellulose nanofibers. These cellulose-based scaffolds were evaluated in vitro, showing good cell-material interaction with fibroblast cells with formation of actin filament, filopodial and lamelipodial protrusions from the cells. In vivo biocompatibility studies using a Wistar rat model indicated no foreign body response and enhanced angiogenesis. Thus, these results demonstrated the potential of the use of nanofibrous mats with cellulose from Ulva sp. in improving diabetic wound healing (Madub et al. 2021). In another study, CNCs were extracted from green seaweeds by mercerization, bleaching and acid hydrolysis, which were then mixed with polyethylene oxide (PEO)/Eudragit E-100. The addition of different CNC contents allowed the successfully production of nanofibers, being the formation of nano-net dependent on CNC content. The addition of CNCs is expected to reinforce the mechanical properties of the developed mats to be applied in tissue engineering and biotechnological fields (Ko et al. 2019).
Electrospinning of CA extracted from seaweeds is still being explored, so there are not many studies published yet. However, there are already several studies that prove the successful production of CA nanofibers from other sources than algae for biomedical applications. For example, commercial CA was used to prepare porous fibrous membranes containing different amounts of thymol (THY) by electrospinning technique. The results indicated that nanopores were generated on the surface of the fibers in situ, in which air was trapped and resulted in an increase in the hydrophobicity of the porous fibrous membranes. The in vitro drug release tests demonstrated that the porous fibrous membranes loaded with THY had slower initial drug release and longer drug release time, when compared with nonporous THY-loaded fibrous membrane, showing that surface structure had significant impact on drug release profile. Moreover, porous THY-CA nanofibers presented higher antibacterial activity against gram-positive and gram-negative bacteria as well as better cytocompatibility comparing to nonporous ones. Therefore, these results show the potential of porous CA-based nanofibers as new wound healing materials (Chen et al. 2020). The blend of commercial CA with other polymer for the development of electrospun mats for wound dressing applications was also reported. CA was blended with poly (ɛ-caprolactone) (PCL) and dextran. The incorporation of tetracycline hydrochloride (TCH), an antibacterial drug, improved the cell proliferation, enhanced blood clotting ability and cell attachment as well as antimicrobial activity of the membranes. PCL/CA/dextran/TCH mats showed good bioactivity, high cell attachment and proliferation as well as effective antibacterial activity, indicating the potential of these mats to be used as bioactive dressings (Liao et al. 2015).
In other study, Farahani et al. developed multifunctional nanofibrous CA/gelatin/Zataria multiflora-nanoemulsion (CA/Gel/ZM-nano) for wound dressing, where ZM-nano represents a nanoemulsion of a natural antibacterial active plant. The developed nanofibers incorporated with ZM-nano, with lower CA/Gel ratios, presented a more controlled drug release profile than higher ratios and adequate antibacterial activity against E. coli and Staphylococcus aureus (S. aureus). Additionally, nanofibers with lower CA/Gel ratio promoted higher adhesion and proliferation of L929 fibroblast cells. In vivo wound healing evaluation revealed that the nanofibrous mats incorporated with ZM-nano, considerably accelerated the wound healing process, being the CA/Gel ratio of 50:50 the one with best healing performance, as demonstrated in Fig. 9 (Farahani et al. 2020).
a Representative images of burn wound healing process in rats treated with (a) commercial gauze, (b) CA/Gel (100:0) nanofibers, (c) CA/Gel (50:50) nanofibers, (d) drug-loaded CA/Gel (100:0) nanofibers, (e) drug-loaded CA/Gel (50:50) nanofibers. b H&E staining histological images 22 days post-operation. c Relative wound areas measured at 5, 15 and 22 days post-operation. Image retrieved with permission from (Farahani et al. 2020)
There are also some examples of published studies where other cellulose derivatives from various sources are used to develop electrospun nanofibers for biomedical applications. For instance, the addition of CNCs into electrospun nanofibers can contribute to the improvement of the mechanical, thermal and hydrophilic properties of the mats as well as to achieve long-term sustained drug release (Ali et al. 2022; Bellani et al. 2016; Cheng et al. 2017). For example, Cheng et al. prepared electrospun nanofibers of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and CNCs, loaded with tetracycline hydrochloride (TH). The CNCs were used to improve the mechanical, thermal, and hydrophilic properties of the PHBV membranes. It was found that 6 wt% CNC content increased the tensile strength by 125%, Young's modulus by 110% and maximum decomposition temperature (Tmax) by 24.3 °C. In addition, the hydrophilicity of the membranes gradually improved with CNC introduction, which is advantageous for biomedical applications. The maximum drug loading was 25% and the drug loading efficiency was 98.8%, respectively, and more than 86% drug was delivered within 540 h from PHBV/CNC membranes (Cheng et al. 2017).
In another study, Ribeiro et al. developed nanofibers based on chitosan (CS), poly (ethylene oxide) (PEO), CNCs and acacia plant-based extract by electrospinning, which showed great potential to act as localized drug delivery systems for wound care applications. The authors showed that the incorporation of CNCs improved the physical integrity of the nanofibers, their morphology, diameters, water vapor transmission rate, and thermal properties. In addition, they showed that the introduction of acacia into the CS/PEO/CNC system, revealed antibacterial effect while maintaining antifungal activity, demonstrating its great effect against a wide range of microorganisms, which is crucial for preventing or treating infections. All developed systems demonstrated no cytotoxicity in non-tumor cells, suggesting their biocompatibility (Ribeiro et al. 2021a, b).
Regarding the use of MCC in electrospinning, there are some studies that mentioned the advantages of using MCC to improve the mechanical properties of the electrospun membranes. However, there’s only few which report the possible applications of those mats in biomedical applications. Guzman-Puyol et al. demonstrated that MCC/fibroin nanofibers presented higher stiffness, showing elastic modulus of 293 MPa, when compared to only fibroin nanofibers (125 MPa). Nevertheless, the addition of MCC promoted a decrease in elongation, suggesting that MCC can act as a reinforcing agent rather than a plasticizer. Moreover, MCC/fibroin nanofibers exhibited excellent cell compatibility (higher than the control) and no cytotoxic effects, demonstrating the potential applicability in biomedical sector (Guzman-Puyol et al. 2016). CA and cellulose composite scaffolds containing MCC and cellulose whisker (CW) were electrospun from CA solutions, followed by deacetylation of the mats. Electrospun scaffolds containing MCC exhibited higher hydrophilicity than CW. After deacetylation, cellulose scaffolds presented better biocompatibility than the CA scaffolds. Nevertheless, the addition of both MCC and CW promoted an improvement in biocompatibility when compared to pure cellulose nanofibers, as well as to those nanofibers with only one of the fillers. The scaffolds with micro- and nano-scale organization were able to better mimic the ECM, which can promote an enhancement of cell viability, adhesion and proliferation, demonstrating the advantages of cellulose-based structures in biomedical field (Jia et al. 2013).
CMC also shows great potential for the development of electrospun mats for biomedical applications, since it is reported that CMC can improve the properties of the membranes, including the mechanical ones. For example, the work performed by Kazeminava et al., showed a green, simple and cost-effective protocol to prepare highly efficient antimicrobial nanofibers based on PVA mixed with CMC, and crosslinked with citric acid-based quantum dots (CA-QDs). Colistin (CL), which is an antibacterial agent, was also incorporated into the polymeric formulation. The addition of CMC promoted the formation of nanofibers with larger diameters and with improved mechanical properties. The developed CMC/PVA/CL/CA-QDs nanofibers demonstrated the desired hydrophilicity, cell proliferation, and cytotoxicity, maintaining the cell viability of the samples above 80% even after 5 days, against human skin fibroblast cell lines (HFF-1), and remarkable antibacterial activity for different bacterial strains (S. aureus, K. pneumoniae, P. aeruginosa, and E. coli bacteria). Nanofibers’ cytocompatibility can be related to the good cytotoxic nature of CMC and CA-QDs. Thus, the developed nanofibrous membranes can act as wound dressings to control microbial infection in wounds (Kazeminava et al. 2022). In another study, Allafchian et al., developed CMC/PVA nanofibers loaded with flufenamic acid (FFA) for drug delivery applications. It was found that the CMC/PVA ratio controlled the diameter of the fibers. Furthermore, the drug release was more prolonged in nanofibers containing the cross-linker EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) (Allafchian et al. 2020).
In conclusion, it can be said that the combination of electrospinning technology and cellulose (and its derivatives) provides a feasible approach to produce nanostructured fibrous structures with promising functionalities, flexibility, renewability and biodegradability for biomedical applications. At the same time, it enables value-added applications of cellulose and its derivatives that are obtained from nature or even biomass waste.
Conclusions
Marine macroalgae are becoming a promising renewable source of cellulose, due to its high abundance and availability. Moreover, their atypical proliferation and accumulation in some cases leads to negative environmental impacts as well as health concerns and may pose a serious danger to the aquatic ecosystem. Therefore, the use and valorization of this resource, including when presented in the form of waste, is a suitable strategy to overcome these issues and, at the same time, to obtain different valuable compounds, in line with the circular economy concept. In fact, macroalgae are a very interesting biomass due to its high content of polysaccharides and bioactive compounds, demonstrating high potential as natural sources of high added-value compounds.
Cellulose presents great properties such as biocompatibility, biodegradability, renewability and non-toxicity, which make it a very attractive material for various biomedical applications. Cellulose may contain unique and distinct properties in terms of dimensions, morphology, aspect ratio, crystallinity and physicochemical properties. Moreover, cellulose presents several derivatives, such as MCC, CNCs, CNFs, CA and CMC, that can be extracted from algae or modified from the extracted cellulose. The main extraction methodologies of cellulose, and some derivatives, from algae are based on a combination of alkaline and bleaching treatments, followed by acid hydrolysis. However, it presents environmental disadvantages, since it uses harmful solvents, generates harmful residues, requires the use of high temperatures, time and energy. Hence, the search for greener and more sustainable methodologies for extraction of cellulose and its derivatives from marine seaweeds is of extreme importance.
Nanofibers produced by electrospinning have demonstrated great potential to be applied in different biomedical applications due to their unique properties. The combination of cellulose with electrospinning allows the production of ultrafine nanofibrous membranes with highly porous structure, biocompatibility, biodegradability and flexibility. However, the poor solubility of cellulose in commonly used solvents limits its use in electrospinning. Thus, cellulose derivatives have been widely used to provide a viable approach to produce nanostructured fibrous materials with promising functionalities for biomedical applications.
Regarding future perspectives, it can be concluded that there are several studies reporting the development of nanofibers by electrospinning of cellulose extracted from other sources than algae, many of which are already being used in biomedical applications. However, when it comes to cellulose and its derivatives extracted from algae, it is still a field that needs more research and development, especially when it comes to the use of greener extraction methods. However, given the potentiality of the results so far, and as we move towards a more sustainable world, electrospun nanofibers with cellulose extracted from algae can be a promising approach to develop new solutions for biomedical field.
Data availability
Not applicable.
References
Ali MS, Ali MS, Orasugh JT, Chattopadhyay D (2022) Synthesis of nanohybrid reinforced electrospun methylcellulose/polyvinyl alcohol/polyethylene glycol nanofiber: study of material properties for possible biomedical applications. In: IOP conference series: materials science and engineering 1263(1):12033. https://doi.org/10.1088/1757-899X/1263/1/012033
Allafchian A, Hosseini H, Ghoreishi SM (2020) Electrospinning of PVA-carboxymethyl cellulose nanofibers for flufenamic acid drug delivery. Int J Biol Macromol 163:1780–1786. https://doi.org/10.1016/j.ijbiomac.2020.09.129
Andryukov BG, Besednova NN, Kuznetsova TA et al (2020) Sulfated polysaccharides from marine algae as a basis of modern biotechnologies for creating wound dressings: current achievements and future prospects. Biomedicines 8(9):301. https://doi.org/10.3390/biomedicines8090301
Araújo D, Castro MCR, Figueiredo A, Vilarinho M, Machado A (2020) Green synthesis of cellulose acetate from corncob: physicochemical properties and assessment of environmental impacts. J Clean Prod 260:120865. https://doi.org/10.1016/j.jclepro.2020.120865
Baghel RS, Reddy CRK, Singh RP (2021) Seaweed-based cellulose: applications, and future perspectives. Carbohydr Polym 267:118241. https://doi.org/10.1016/j.carbpol.2021.118241
Bar-Shai N, Sharabani-Yosef O, Zollmann M, Lesman A, Golberg A (2021) Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci Rep 11(1):11843. https://doi.org/10.1038/s41598-021-90903-2
Baruah J, Deka RC, Kalita E (2020) Greener production of microcrystalline cellulose (MCC) from Saccharum spontaneum (Kans grass): statistical optimization. Int J Biol Macromol 154:672–682. https://doi.org/10.1016/j.ijbiomac.2020.03.158
Bellani CF, Pollet E, Hebraud A, Pereira FV, Schlatter G, Avérous L et al (2016) Morphological, thermal, and mechanical properties of poly(ε-caprolactone)/poly(ε-caprolactone)-grafted-cellulose nanocrystals mats produced by electrospinning. J Appl Polym Sci. https://doi.org/10.1002/app.43445
Birgersson PS, Oftebro M, Strand WI, Aarstad OA, Sætrom GI, Sletta H et al (2023) Sequential extraction and fractionation of four polysaccharides from cultivated brown algae Saccharina latissima and Alaria esculenta. Algal Res 69:102928. https://doi.org/10.1016/j.algal.2022.102928
Bombin ADJ, Dunne NJ, McCarthy HO (2020) Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C 114:110994. https://doi.org/10.1016/j.msec.2020.110994
Chen Y, Qiu Y, Chen W, Wei Q (2020) Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater Sci Eng C 109:110536. https://doi.org/10.1016/j.msec.2019.110536
Cheng M, Qin Z, Hu S, Dong S, Ren Z, Yu H (2017) Achieving long-term sustained drug delivery for electrospun biopolyester nanofibrous membranes by introducing cellulose nanocrystals. ACS Biomater Sci Eng 3(8):1666–1676. https://doi.org/10.1021/acsbiomaterials.7b00169
Costa SM, Fangueiro R, Ferreira DP (2022) Drug delivery systems for photodynamic therapy: the potentiality and versatility of electrospun nanofibers. Macromol Biosci 22(5):2100512. https://doi.org/10.1002/mabi.202100512
Doh H, Whiteside WS (2020) Isolation of cellulose nanocrystals from brown seaweed, Sargassum fluitans, for development of alginate nanocomposite film. Polym Cryst 3(4):e10133. https://doi.org/10.1002/pcr2.10133
Doh H, Hyeock M, Scott W (2020) Physicochemical characteristics of cellulose nanocrystals isolated from seaweed biomass. Food Hydrocoll 102:105542. https://doi.org/10.1016/j.foodhyd.2019.105542
El M, Kassab Z, Aboulkas A, Gaillard C, Barakat A (2018) Reuse of red algae waste for the production of cellulose nanocrystals and its application in polymer nanocomposites. Int J Biol Macromol 106:681–691. https://doi.org/10.1016/j.ijbiomac.2017.08.067
El-Newehy MH, El-Naggar ME, Alotaiby S, El-Hamshary H, Moydeen M, Al-Deyab S (2016) Preparation of biocompatible system based on electrospun CMC/PVA nanofibers as controlled release carrier of diclofenac sodium. J Macromol Sci Part A 53(9):566–573. https://doi.org/10.1080/10601325.2016.1201752
El-Sheekh MM, Yousuf WE, Kenawy E-R, Mohamed TM (2023) Biosynthesis of cellulose from Ulva lactuca, manufacture of nanocellulose and its application as antimicrobial polymer. Sci Rep 13(1):10188. https://doi.org/10.1038/s41598-023-37287-7
Fahimirad S, Ajalloueian F (2019) Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int J Pharm 566:307–328. https://doi.org/10.1016/j.ijpharm.2019.05.053
Farahani H, Barati A, Arjomandzadegan M, Vatankhah E (2020) Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int J Biol Macromol 162:762–773. https://doi.org/10.1016/j.ijbiomac.2020.06.175
Flórez-Fernández N, Torres MD, González-Muñoz MJ, Domínguez H (2019) Recovery of bioactive and gelling extracts from edible brown seaweed Laminaria ochroleuca by non-isothermal autohydrolysis. Food Chem 277:353–361. https://doi.org/10.1016/j.foodchem.2018.10.096
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24(11):4605–4609. https://doi.org/10.1007/s10570-017-1450-3
Gomri C, Cretin M, Semsarilar M (2022) Recent progress on chemical modification of cellulose nanocrystal (CNC) and its application in nanocomposite films and membranes-A comprehensive review. Carbohydr Polym 294:119790. https://doi.org/10.1016/j.carbpol.2022.119790
Gruppuso M, Turco G, Marsich E, Porrelli D (2021) Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl Mater Today 24:101148. https://doi.org/10.1016/j.apmt.2021.101148
Guzman-Puyol S, Heredia-Guerrero JA, Ceseracciu L, Hajiali H, Canale C, Scarpellini A et al (2016) Low-cost and effective fabrication of biocompatible nanofibers from silk and cellulose-rich materials. ACS Biomater Sci Eng 2(4):526–534. https://doi.org/10.1021/acsbiomaterials.5b00500
Hamouda RA, Abdel-Hamid MS (2022) A comparative study of cellulose nanocomposite derived from algae and bacteria and its applications. In: Shalan AE, Hamdy Makhlouf AS, Lanceros-Méndez S (eds) Advances in nanocomposite materials for environmental and energy harvesting applications. Engineering Materials. Springer, Cham, pp 151–187
He Q, Wang Q, Zhou H, Ren D, He Y, Cong H et al (2018) Highly crystalline cellulose from brown seaweed Saccharina japonica: isolation, characterization and microcrystallization. Cellulose 25(10):5523–5533. https://doi.org/10.1007/s10570-018-1966-1
Hosny KM, Alkhalidi HM, Alharbi WS, Md S, Sindi AM, Ali SA et al (2022) Recent trends in assessment of cellulose derivatives in designing novel and nanoparticulate-based drug delivery systems for improvement of oral health. Polymers 14(1):92. https://doi.org/10.3390/polym14010092
Iliou K, Kikionis S, Ioannou E, Roussis V (2022) Marine biopolymers as bioactive functional ingredients of electrospun nanofibrous scaffolds for biomedical applications. Mar Drugs 20(5):314. https://doi.org/10.3390/md20050314
Ilyas R, Atikah MSN (2021) Production of nanocellulose from sustainable algae marine biomass. In: Seminar on advanced bio- and mineral based natural fibre composites (SBMC2021). Institute of Tropical Forestry and Forest Products (INTROP), Universiti Putra Malaysia
Janmohammadi M, Nazemi Z, Salehi AOM, Seyfoori A, John JV, Nourbakhsh MS et al (2023) Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact Mater 20:137–163. https://doi.org/10.1016/j.bioactmat.2022.05.018
Jia B, Li Y, Yang B, Xiao D, Zhang S, Rajulu AV et al (2013) Effect of microcrystal cellulose and cellulose whisker on biocompatibility of cellulose-based electrospun scaffolds. Cellulose 20(4):1911–1923. https://doi.org/10.1007/s10570-013-9952-0
Jia F, Liu H, Zhang G (2016) Preparation of carboxymethyl cellulose from corncob. Environ Sci 31:98–102. https://doi.org/10.1016/j.proenv.2016.02.013
Jonasson S, Bünder A, Niittylä T, Oksman K (2020) Isolation and characterization of cellulose nanofibers from aspen wood using derivatizing and non-derivatizing pretreatments. Cellulose 27(1):185–203. https://doi.org/10.1007/s10570-019-02754-w
Jönsson M, Allahgholi L, Sardari RRR, Hreggviðsson GO, Nordberg Karlsson E (2020) Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 25(4):930. https://doi.org/10.3390/molecules25040930
Kanikireddy V, Varaprasad K, Jayaramudu T, Karthikeyan C, Sadiku R (2020) Carboxymethyl cellulose-based materials for infection control and wound healing: a review. Int J Biol Macromol 164:963–975. https://doi.org/10.1016/j.ijbiomac.2020.07.160
Kazeminava F, Javanbakht S, Nouri M, Adibkia K, Ganbarov K, Yousefi M et al (2022) Electrospun nanofibers based on carboxymethyl cellulose/polyvinyl alcohol as a potential antimicrobial wound dressing. Int J Biol Macromol 214:111–119. https://doi.org/10.1016/j.ijbiomac.2022.05.175
Khoshnevisan K, Maleki H, Samadian H, Shahsavari S, Sarrafzadeh MH, Larijani B et al (2018) Cellulose acetate electrospun nanofibers for drug delivery systems: applications and recent advances. Carbohydr Polym 198:131–141. https://doi.org/10.1016/j.carbpol.2018.06.072
Khoshnevisan K, Maleki H, Samadian H, Doostan M, Khorramizadeh MR (2019) Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int J Biol Macromol 140:1260–1268. https://doi.org/10.1016/j.ijbiomac.2019.08.207
Ko SW, Lee JY, Aguilar LE, Oh YM, Park CH, Kim CS (2019) Fabrication of a micro/nano-net membrane using cellulose nanocrystals derived from seaweed. J Nanosci Nanotechnol 19(4):2232–2235. https://doi.org/10.1166/jnn.2019.15989
Lakshmi DS, Trivedi N, Reddy CRK (2017) Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr Polym 157:1604–1610. https://doi.org/10.1016/j.carbpol.2016.11.042
Langan P, Nishiyama Y, Chanzy H (2001) X-ray Structure of mercerized cellulose II at 1 Å resolution. Biomacromol 2(2):410–416. https://doi.org/10.1021/bm005612q
Leandro A, Pereira L, Gonçalves AMM (2019) Diverse applications of marine macroalgae. Mar Drugs 18(1):17. https://doi.org/10.3390/md18010017
Li H, Wang M (2021) 18—Electrospinning and nanofibrous structures for biomedical applications. In: Osaka A, Narayan RBTB (eds) Bioceramics. Elsevier, pp 401–436
Liao N, Unnithan AR, Joshi MK, Tiwari AP, Hong ST, Park C-H et al (2015) Electrospun bioactive poly (ɛ-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf A Physicochem Eng Asp 469:194–201. https://doi.org/10.1016/j.colsurfa.2015.01.022
Liu Z, Li X, Xie W, Deng H (2017) Extraction, isolation and characterization of nanocrystalline cellulose from industrial kelp (Laminaria japonica) waste. Carbohydr Polym 173:353–359. https://doi.org/10.1016/j.carbpol.2017.05.079
Madub K, Goonoo N, Gimié F, Ait Arsa I, Schönherr H, Bhaw-Luximon A (2021) Green seaweeds ulvan-cellulose scaffolds enhance in vitro cell growth and in vivo angiogenesis for skin tissue engineering. Carbohydr Polym 251:117025. https://doi.org/10.1016/j.carbpol.2020.117025
Mali P, Sherje AP (2022) Cellulose nanocrystals: fundamentals and biomedical applications. Carbohydr Polym 275:118668. https://doi.org/10.1016/j.carbpol.2021.118668
Mitbumrung W, Rungraung N, Muangpracha N, Akanitkul P, Winuprasith T (2022) Approaches for extracting nanofibrillated cellulose from oat bran and its emulsion capacity and stability. Polymers 14(2):327. https://doi.org/10.3390/polym14020327
Mohan AA, Antony AR, Greeshma K, Yun J-H, Ramanan R, Kim H-S (2022) Algal biopolymers as sustainable resources for a net-zero carbon bioeconomy. Bioresour Technol 344:126397. https://doi.org/10.1016/j.biortech.2021.126397
Morais ES, Lopes AMDC, Freire MG, Freire CSR, Coutinho JAP, Silvestre AJD (2020) Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 25(16):3652. https://doi.org/10.3390/molecules25163652
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124(31):9074–9082. https://doi.org/10.1021/ja0257319
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125(47):14300–14306. https://doi.org/10.1021/ja037055w
Nunes RCR (2017) 13—Rubber nanocomposites with nanocellulose. In Thomas S, Maria HJ (eds) Progress in rubber nanocomposites. Woodhead publishing series in composites science and engineering, pp 463–494
Onyianta AJ, O’Rourke D, Sun D, Popescu C-M, Dorris M (2020) High aspect ratio cellulose nanofibrils from macroalgae Laminaria hyperborea cellulose extract via a zero-waste low energy process. Cellulose 27(14):7997–8010. https://doi.org/10.1007/s10570-020-03223-5
Oprea M, Voicu SI (2020) Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr Polym 247:116683. https://doi.org/10.1016/j.carbpol.2020.116683
Otero P, Carpena M, Garcia-Oliveira P, Echave J, Soria-Lopez A, Garcia-Perez P et al (2023) Seaweed polysaccharides: emerging extraction technologies, chemical modifications and bioactive properties. Crit Rev Food Sci Nutr 63(13):1901–1929. https://doi.org/10.1080/10408398.2021.1969534
Pardilhó S, Costa E, Melo D, Machado S, Espírito Santo L, Oliveira MB et al (2021) Comprehensive characterisation of marine macroalgae waste and impact of oil extraction, focusing on the biomass recovery potential. Algal Res 58:102416. https://doi.org/10.1016/j.algal.2021.102416
Pardilhó S, Cotas J, Pereira L, Oliveira MB, Dias JM (2022) Marine macroalgae in a circular economy context: a comprehensive analysis focused on residual biomass. Biotechnol Adv 60:107987. https://doi.org/10.1016/j.biotechadv.2022.107987
Pereira AG, Fraga-Corral M, Garcia-Oliveira P, Lourenço-Lopes C, Carpena M, Prieto MA et al (2021) The use of invasive algae species as a source of secondary metabolites and biological activities: Spain as case-study. Mar Drugs 19(4):178. https://doi.org/10.3390/md19040178
Pinteus S, Lemos MFL, Alves C, Neugebauer A, Silva J, Thomas OP et al (2018) Marine invasive macroalgae: turning a real threat into a major opportunity—the biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res 34:217–234. https://doi.org/10.1016/j.algal.2018.06.018
Pradeep HK, Patel DH, Onkarappa HS, Pratiksha CC, Prasanna GD (2022) Role of nanocellulose in industrial and pharmaceutical sectors—a review. Int J Biol Macromol 207:1038–1047. https://doi.org/10.1016/j.ijbiomac.2022.03.171
Rahman MS, Hasan MS, Nitai AS, Nam S, Karmakar AK, Ahsan MS et al (2021) Recent developments of carboxymethyl cellulose. Polymers 13(8):1345. https://doi.org/10.3390/polym13081345
Ribeiro AS, Costa SM, Ferreira DP, Abidi H, Fangueiro R (2021a) Development of chitosan–gelatin nanofibers with cellulose Nanocrystals for Skin Protection applications. Key Eng Mater 893:45–55. https://doi.org/10.4028/www.scientific.net/KEM.893.45
Ribeiro AS, Costa SM, Ferreira DP, Calhelha RC, Barros L, Stojković D et al (2021b) Chitosan/nanocellulose electrospun fibers with enhanced antibacterial and antifungal activity for wound dressing applications. React Funct Polym 159:104808. https://doi.org/10.1016/j.reactfunctpolym.2020.104808
Robledo D, Vázquez-Delfín E, Freile-Pelegrín Y, Vásquez-Elizondo RM, Qui-Minet ZN, Salazar-Garibay A (2021) Challenges and opportunities in relation to sargassum events along the Caribbean Sea. Front Mar Sci 8:699664. https://doi.org/10.3389/fmars.2021.699664
Roca M, Dunbar MB, Román A, Caballero I, Zoffoli ML, Gernez P et al (2022) Monitoring the marine invasive alien species Rugulopteryx okamurae using unmanned aerial vehicles and satellites. Front Mar Sci 9:1004012. https://doi.org/10.3389/fmars.2022.1004012
Ross IL, Shah S, Hankamer B, Amiralian N (2021) Microalgal nanocellulose—opportunities for a circular bioeconomy. Trends Plant Sci 26(9):924–939. https://doi.org/10.1016/j.tplants.2021.05.004
Salminen R, Reza M, Pääkkönen T, Peyre J, Kontturi E (2017) TEMPO-mediated oxidation of microcrystalline cellulose: limiting factors for cellulose nanocrystal yield. Cellulose 24(4):1657–1667. https://doi.org/10.1007/s10570-017-1228-7
Samadian H, Zamiri S, Ehterami A, Farzamfar S, Vaez A, Khastar H et al (2020) Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: in vitro and in vivo studies. Sci Rep 10(1):8312. https://doi.org/10.1038/s41598-020-65268-7
Samiee S, Ahmadzadeh H, Hosseini M, Lyon S (2019) Chapter 17—algae as a source of microcrystalline cellulose. In: Hosseini M (ed) Advanced bioprocessing for alternative fuels, biobased chemicals, and bioproducts technologies and approaches for scale-up and commercialization. Woodhead Publishing, pp 331–350
Sankhla S, Sardar HH, Neogi S (2021) Greener extraction of highly crystalline and thermally stable cellulose micro-fibers from sugarcane bagasse for cellulose nano-fibrils preparation. Carbohydr Polym 251:117030. https://doi.org/10.1016/j.carbpol.2020.117030
Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, Bacabac RG et al (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28(4):1893–1931. https://doi.org/10.1007/s10570-020-03674-w
Shannon E, Abu-Ghannam N (2016) Antibacterial derivatives of marine algae: an overview of pharmacological mechanisms and applications. Mar Drugs 14(4):81. https://doi.org/10.3390/md14040081
Shokri J, Adibkia K (2013) Application of cellulose and cellulose derivatives in pharmaceutical industries. In: van de Ven T, Godbout L (eds) Cellulose—medical, pharmaceutical and electronic applications. Rijeka, IntechOpen
Singh S, Gaikwad KK, Park S-I, Lee YS (2017) Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int J Biol Macromol 99:506–510. https://doi.org/10.1016/j.ijbiomac.2017.03.004
Singh S, Gaikwad KK, Lee YS (2018) Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. Int J Biol Macromol 107:1879–1887. https://doi.org/10.1016/j.ijbiomac.2017.10.057
Singh S, Verma DK, Thakur M, Tripathy S, Patel AR, Shah N et al (2021) Supercritical fluid extraction (SCFE) as green extraction technology for high-value metabolites of algae, its potential trends in food and human health. Food Res Int 150(Pt A):110746. https://doi.org/10.1016/j.foodres.2021.110746
Surendran G, Sherje AP (2022) Cellulose nanofibers and composites: an insight into basics and biomedical applications. J Drug Deliv Sci Technol 75:103601. https://doi.org/10.1016/j.jddst.2022.103601
Tarchoun AF, Trache D, Klapötke TM (2019) Microcrystalline cellulose from Posidonia oceanica brown algae: extraction and characterization. Int J Biol Macromol 138:837–845. https://doi.org/10.1016/j.ijbiomac.2019.07.176
Tayeb AH, Amini E, Ghasemi S, Tajvidi M (2018) Cellulose nanomaterials-binding properties and applications: a review. Molecules 23(10):2684. https://doi.org/10.3390/molecules23102684
Teixeira MA, Paiva MC, Amorim MTP, Felgueiras AHP (2020) Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomater (basel, Switzerland) 10(3):557. https://doi.org/10.3390/nano10030557
Teramoto Y (2015) Functional thermoplastic materials from derivatives of cellulose and related structural polysaccharides. Molecules 20(4):5487–5527. https://doi.org/10.3390/molecules20045487
Thielemans K, De Bondt Y, Van den Bosch S, Bautil A, Roye C, Deneyer A et al (2022) Decreasing the degree of polymerization of microcrystalline cellulose by mechanical impact and acid hydrolysis. Carbohydr Polym 294:119764. https://doi.org/10.1016/j.carbpol.2022.119764
Torres FG, De-la-Torre GE (2022) Green algae as a sustainable source for energy generation and storage technologies. Sustain Energy Technol Assess 53:102658. https://doi.org/10.1016/j.seta.2022.102658
Trache D, Tarchoun AF, Derradji M, Hamidon TS, Masruchin N, Brosse N et al (2020) Nanocellulose: from fundamentals to advanced applications. Front Chem 8:392. https://doi.org/10.3389/fchem.2020.00392
Wada M, Chanzy H, Nishiyama Y, Langan P (2004) Cellulose IIII crystal structure and hydrogen bonding by synchrotron X-ray and neutron fiber diffraction. Macromolecules 37(23):8548–8555. https://doi.org/10.1021/ma0485585
Wada M, Heux L, Nishiyama Y, Langan P (2009) X-ray crystallographic, scanning microprobe X-ray diffraction, and cross-polarized/magic angle spinning 13C NMR studies of the structure of cellulose IIIII. Biomacromol 10(2):302–309. https://doi.org/10.1021/bm8010227
Wahlström N, Edlund U, Pavia H, Toth G, Jaworski A, Pell AJ et al (2020) Cellulose from the green macroalgae Ulva lactuca: isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 27(7):3707–3725. https://doi.org/10.1007/s10570-020-03029-5
Wan M, Wang Z, Mai G, Ma Z, Xia X, Tan Y et al (2022) Photosynthetic characteristics of Macroalgae Ulva fasciata and Sargassum thunbergii in the Daya Bay of the South China Sea, with special reference to the effects of light quality. Sustainability 14(13):8063. https://doi.org/10.3390/su14138063
Wang H-MD, Li X-C, Lee D-J, Chang J-S (2017) Potential biomedical applications of marine algae. Bioresour Technol 244(Pt 2):1407–1415. https://doi.org/10.1016/j.biortech.2017.05.198
Wang H, Xie H, Du H, Wang X, Liu W, Duan Y et al (2020) Highly efficient preparation of functional and thermostable cellulose nanocrystals via H2SO4 intensified acetic acid hydrolysis. Carbohydr Polym 239:116233. https://doi.org/10.1016/j.carbpol.2020.116233
Wsoo MA, Shahir S, Mohd Bohari SP, Nayan NHM, Razak SIA (2020) A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: a new perspective. Carbohydr Res 491:107978. https://doi.org/10.1016/j.carres.2020.107978
Xiang Z, Gao W, Chen L, Lan W, Zhu JY, Runge T (2016) A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 23(1):493–503. https://doi.org/10.1007/s10570-015-0840-7
Xue J, Wu T, Dai Y, Xia Y (2019) Electrospinning and electrospun nanofibers : methods, materials, and applications. Chem Rev 119:5298–5415. https://doi.org/10.1021/acs.chemrev.8b00593
Yan F, Li L, Yu D, Cui C, Zang S, Xu Z et al (2021) Physiological responses of Sargassum muticum, a potential golden tide species, to different levels of light and nitrogen. Front Ecol Evol 9:759732. https://doi.org/10.3389/fevo.2021.759732
Young CS, Lee C-S, Sylvers LH, Venkatesan AK, Gobler CJ (2022) The invasive red seaweed, Dasysiphonia japonica, forms harmful algal blooms: mortality in early life stage fish and bivalves and identification of putative toxins. Harmful Algae 118:102294. https://doi.org/10.1016/j.hal.2022.102294
Youssouf L, Lallemand L, Giraud P, Soulé F, Bhaw-Luximon A, Meilhac O et al (2017) Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr Polym 166:55–63. https://doi.org/10.1016/j.carbpol.2017.01.041
Yu S, Sun J, Shi Y, Wang Q, Wu J, Liu J (2021) Nanocellulose from various biomass wastes: its preparation and potential usages towards the high value-added products. Environ Sci Ecotechnology 5:100077. https://doi.org/10.1016/j.ese.2020.100077
Zamel D, Khan AU (2021) Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: mini-review. Inorg Chem Commun 131:108766. https://doi.org/10.1016/j.inoche.2021.108766
Zanchetta E, Damergi E, Patel B, Borgmeyer T, Pick H, Pulgarin A et al (2021) Algal cellulose, production and potential use in plastics: challenges and opportunities. Algal Res 56:102288. https://doi.org/10.1016/j.algal.2021.102288
Zhang Y, Zhang C, Wang Y (2021) Recent progress in cellulose-based electrospun nanofibers as multifunctional materials. Nanoscale Adv 3(21):6040–6047. https://doi.org/10.1039/D1NA00508A
Funding
Open access funding provided by FCT|FCCN (b-on). The authors are thankful to project UID/CTM/00264/2023 of 2C2T—Centro de Ciência e Tecnologia Têxtil, funded by National Founds through FCT/MCTES- Fundação para a Ciência e a Tecnologia. Sofia M. Costa is thankful to FCT PhD Scholarship (SFRH/BD/147517/2019) and Diana P. Ferreira is thankful to CEECIND/02803/2017, founded by National Founds through FCT/MCTES.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by BM and SMC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors consent to the publication of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Machado, B., Costa, S.M., Costa, I. et al. The potential of algae as a source of cellulose and its derivatives for biomedical applications. Cellulose 31, 3353–3376 (2024). https://doi.org/10.1007/s10570-024-05816-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-05816-w