Abstract
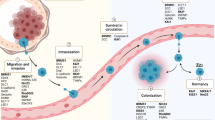
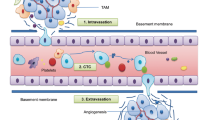
Metastasis is the leading cause of cancer patient mortality. Metastasis suppressors are genes that, upon reexpression in metastatic tumor cells to levels observed in their nonmetastatic counterparts, significantly reduce metastasis without affecting the growth of the primary tumor. Analysis of > 30 metastasis suppressors revealed complex mechanisms of action that include multiple signaling pathways, transcriptional patterns, posttranscriptional regulatory mechanisms, and potential contributions of genomic stability. Clinical testing of strategies to re-establish a validated metastasis suppressor pathway in tumors is best directed to the adjuvant setting, with the goal of inhibiting the outgrowth of occult micrometastases.
Similar content being viewed by others
References
Leone, A., Flatow, U., King, C. R., Sandeen, M. A., Margulies, I. M. K., Liotta, L. A., et al. (1991). Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell, 65, 25–35. https://doi.org/10.1016/0092-8674(91)90404-M
Steeg, P. S., Bevilacqua, G., Kopper, L., Thorgeirsson, U. P., Talmadge, J. E., Liotta, L. A., et al. (1988). Evidence for a novel gene associated with low tumor metastatic potential. J. Nat′l. Cancer Inst., 80, 200–204. https://doi.org/10.1093/jnci/80.3.200
Hurst, D. R., Edmonds, M. D., Scott, G. K., Benz, C. C., Vaidya, K. S., & Welch, D. R. (2009). Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Research, 69(4), 1279–1283. https://doi.org/10.1158/0008-5472.CAN-08-3559
Geleta, B. R., Tout, F., Lim, S. C., Sahni, S., Jansson, P. J., Apte, M. V., et al. (2022). Targeting Wnt/tenascin C-mediated cross talk between pancreatic cancer cells and stellate cells via activation of the metastasis suppressor NDRG1. Journal of Biological Chemistry, 298(3). https://doi.org/10.1016/j.jbc.2022.101608
Dammai, V., Adryan, B., Lavenburg, K. R., & Hsu, T. (2003). Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes & Development, 17(22), 2812–2824. https://doi.org/10.1101/gad.1096903
Boissan, M., Montagnac, G., Shen, Q., Griparic, L., Guitton, J., Romao, M., et al. (2014). Membrane trafficking. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science, 344(6191), 1510–1515. https://doi.org/10.1126/science.1253768
Khan, I., Gril, B., & Steeg, P. S. (2019). Metastasis suppressors NME1 and NME2 promote dynamin 2 oligomerization and regulate tumor cell endocytosis, motility, and metastasis. Cancer Research, 79(18), 4689–4702. https://doi.org/10.1158/0008-5472.CAN-19-0492
Lodillinsky, C., Fuhrmann, L., Irondelle, M., Pylypenko, O., Li, X. Y., Bonsang-Kitzis, H., et al. (2021). Metastasis-suppressor NME1 controls the invasive switch of breast cancer by regulating MT1-MMP surface clearance. Oncogene, 40(23), 4019–4032. https://doi.org/10.1038/s41388-021-01826-1
Menezes, S. V., Kovacevic, Z., & Richardson, D. R. (2019). The metastasis suppressor NDRG1 down-regulates the epidermal growth factor receptor via a lysosomal mechanism by up-regulating mitogen-inducible gene 6. Journal of Biological Chemistry, 294(11), 4045–4064. https://doi.org/10.1074/jbc.RA118.006279
Ma, L., Reinhardt, F., Pan, E., Soutschek, J., Bhat, B., Marcusson, E. G., et al. (2010). Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nature Biotechnology, 28(4), 341–U367. https://doi.org/10.1038/nbt.1618
Palmieri, D., Halverson, D. O., Ouatas, T., Horak, C. E., Salerno, M., Johnson, J., et al. (2005). Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst, 97(9), 632–642. https://doi.org/10.1093/jnci/dji111
Miller, K. D., Althouse, S. K., Nabell, L., Rugo, H., Carey, L., Kimmick, G., et al. (2014). A phase II study of medroxyprogesterone acetate in patients with hormone receptor negative metastatic breast cancer: Translational breast cancer research consortium trial 007. Breast Cancer Research and Treatment, 148(1), 99–106. https://doi.org/10.1007/s10549-014-3131-3
Park, K. C., Paluncic, J., Kovacevic, Z., & Richardson, D. R. (2020). Pharmacological targeting and the diverse functions of the metastasis suppressor, NDRG1, in cancer. Free Radical Biology and Medicine, 157, 154–175. https://doi.org/10.1016/j.freeradbiomed.2019.05.020
Marshall, J. C. A., Collins, J. W., Nakayama, J., Horak, C. E., Liewehr, D. J., Steinberg, S. M., et al. (2012). Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. Journal of the National Cancer Institute, 104(17), 1306–1319. https://doi.org/10.1093/jnci/djs319
Brooks, D., Zimmer, A., Wakefield, L., Lyle, T., Difilippantonio, S., Tucci, F., et al. (2018). Limited fibrosis accompanies triple-negative breast cancer metastasis in multiple model systems and is not a preventive target. Oncotarget, 9(34). https://doi.org/10.18632/oncotarget.25231
Khan, I., Gril, B., Hoshino, A., Yang, H. H., Lee, M. P., Difilippantonio, S., et al. (2022). Metastasis suppressor NME1 in exosomes or liposomes conveys motility and migration inhibition in breast cancer model systems. Clinical & Experimental Metastasis, 39(5), 815–831. https://doi.org/10.1007/s10585-022-10182-7
Ahmed, M., Lai, T. H., Kim, W., & Kim, D. R. (2021). A functional network model of the metastasis suppressor PEBP1/RKIP and its regulators in breast cancer cells. Cancers, 13(23). https://doi.org/10.3390/cancers13236098
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, I., Steeg, P.S. A perspective on the metastasis suppressor field. Cancer Metastasis Rev 42, 1061–1063 (2023). https://doi.org/10.1007/s10555-023-10131-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10131-0