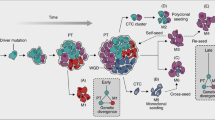
Abstract
The metastatic cascade is a complex process with multiple factors contributing to the seeding and growth of cancer cells at metastatic sites. Within this complex process, several genes have been identified as metastasis suppressors, playing a role in the inhibition of metastasis. Interestingly, some of these genes have been shown to also play a role in regulating the tumor microenvironment. In this review, we comment on the recent developments in the biology of metastasis suppressor genes and their crosstalk with the microenvironment.


Similar content being viewed by others
Data availability
N/A.
References
Ganesh, K., & Massagué, J. (2021). Targeting metastatic cancer. Nature Medicine, 27(1), 34–44. https://doi.org/10.1038/S41591-020-01195-4
Lyden, D., Ghajar, C. M., Correia, A. L., et al. (2022). Metastasis. Cancer Cell, 40(8), 787–791. https://doi.org/10.1016/j.ccell.2022.07.010
Massagué, J., & Obenauf, A. C. (2016). Metastatic colonization by circulating tumour cells. Nature, 529(7586), 298–306. https://doi.org/10.1038/nature17038
Sosa, M. S., Bravo-Cordero, J. J. (2020). Understanding the mechanistic features of cancer metastasis. Cancer Reports, 3(1). https://doi.org/10.1002/CNR2.1238
Di Martino, J. S., Akhter, T., & Bravo-Cordero, J. J. (2021). Remodeling the ECM: Implications for metastasis and tumor dormancy. Cancers, 13(19), 4916. https://doi.org/10.3390/CANCERS13194916
Horak, C. E., Lee, J. H., Marshall, J. C., Shreeve, S. M., & Steeg, P. S. (2008). The role of metastasis suppressor genes in metastatic dormancy. APMIS, 116(7–8), 586–601. https://doi.org/10.1111/j.1600-0463.2008.01027.x
Cairns, J. (1975). Mutation selection and the natural history of cancer. Nature, 255(5505), 197–200. https://doi.org/10.1038/255197A0
Greaves, M., & Maley, C. C. (2012). Clonal evolution in cancer. Nature, 481(7381), 306. https://doi.org/10.1038/NATURE10762
Rhim, A. D., Mirek, E. T., Aiello, N. M., et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148(1–2), 349–361. https://doi.org/10.1016/j.cell.2011.11.025
Hosseini, H., Obradovic, M. M. S., Hoffmann, M., et al. (2016). Early dissemination seeds metastasis in breast cancer. Nature, 540(7634), 552–558. https://doi.org/10.1038/nature20785
Gupta, P. B., Mani, S., Yang, J., Hartwell, K., & Weinberg, R. A. (2005). The evolving portrait of cancer metastasis. Cold Spring Harb Symp Quant Biol, 70, 291–7. https://doi.org/10.1101/sqb.2005.70.033
Pantel, K., Brakenhoff, R. H., & Brandt, B. (2008). Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature Reviews Cancer, 8(5), 329–340. https://doi.org/10.1038/nrc2375
Klein, C. A. (2009). Parallel progression of primary tumours and metastases. Nature Reviews Cancer, 9(4), 302–312. https://doi.org/10.1038/nrc2627
Hu, Z., Ding, J., Ma, Z., et al. (2019). Quantitative evidence for early metastatic seeding in colorectal cancer. Nature Genetics, 51(7), 1113–1122. https://doi.org/10.1038/s41588-019-0423-x
Ray, A., Callaway, M. K., Rodríguez-Merced, N. J., et al., (2022). Stromal architecture directs early dissemination in pancreatic ductal adenocarcinoma. JCI Insight, 7(3). https://doi.org/10.1172/JCI.INSIGHT.150330
Di Martino, J. S., Akhter, T., & Bravo-Cordero, J. J. (2021). Remodeling the ECM: Implications for metastasis and tumor dormancy. Cancers (Basel)., 13(19), 4916. https://doi.org/10.3390/CANCERS13194916
Quail, D. F., & Joyce, J. A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nature Medicine, 19(11), 1423–1437. https://doi.org/10.1038/NM.3394
Friedl, P., & Gilmour, D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews Molecular Cell Biology, 10(7), 445–457. https://doi.org/10.1038/NRM2720
Mukherjee, A., & Bravo-Cordero, J. J. (2023). Regulation of dormancy during tumor dissemination: The role of the ECM. Cancer Metastasis Reviews., 42, 99–112. https://doi.org/10.1007/s10555-023-10094-2. Published online March 1, 2023.
Bravo-Cordero, J. J., Hodgson, L., & Condeelis, J. (2012). Directed cell invasion and migration during metastasis. Current Opinion in Cell Biology, 24(2), 277–283. https://doi.org/10.1016/j.ceb.2011.12.004
Thiery, J. P., Acloque, H., Huang, R. Y. J., & Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139(5), 871–890. https://doi.org/10.1016/J.CELL.2009.11.007
Nieto, M. A., Huang, R. Y. Y. J., Jackson, R. A. A., & Thiery, J. P. P. (2016). EMT: 2016. Cell, 166(1), 21–45. https://doi.org/10.1016/J.CELL.2016.06.028
Mondal, C., Gacha-Garay, M. J., Larkin, K. A., et al. (2022). A proliferative to invasive switch is mediated by srGAP1 downregulation through the activation of TGF-β2 signaling. Cell Reports 40 (12). https://doi.org/10.1016/J.CELREP.2022.111358/ATTACHMENT/AD571673-CC1F-4AC5-9309-522AAC0B5913/MMC2
Mondal, C., Di Martino, J. S., & Bravo-Cordero, J. J. (2021). Actin dynamics during tumor cell dissemination. International Review of Cell and Molecular Biology, 360, 65–98. https://doi.org/10.1016/BS.IRCMB.2020.09.004
Gligorijevic, B., Wyckoff, J., Yamaguchi, H., Wang, Y., Roussos, E. T., & Condeelis, J. (2012). N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. Journal of Cell Science, 125(Pt 3), 724–734. https://doi.org/10.1242/JCS.092726
Sharma, V. P., Eddy, R., Entenberg, D., Kai, M., Gertler, F. B., & Condeelis, J. (2013). Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Current Biology, 23(21), 2079–2089. https://doi.org/10.1016/J.CUB.2013.08.044
Oser, M., & Condeelis, J. (2009). The cofilin activity cycle in lamellipodia and invadopodia. Journal of Cellular Biochemistry, 108(6), 1252. https://doi.org/10.1002/JCB.22372
Oser, M., Yamaguchi, H., Mader, C. C., et al. (2009). Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. Journal of Cell Biology, 186(4), 571–587. https://doi.org/10.1083/JCB.200812176
Risson, E., Nobre, A. R., Maguer-Satta, V., & Aguirre-Ghiso, J. A. (2020). The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nature Cancer., 1(7), 672–680. https://doi.org/10.1038/s43018-020-0088-5
Sosa, M. S., Bragado, P., & Aguirre-Ghiso, J. A. (2014). Mechanisms of disseminated cancer cell dormancy: An awakening field. Nature Reviews Cancer, 14(9), 611–622. https://doi.org/10.1038/NRC3793
Sosa, M. S., Bragado, P., & Aguirre-Ghiso, J. A. (2014). Mechanisms of disseminated cancer cell dormancy: An awakening field. Nature Reviews Cancer, 14(9), 611–622. https://doi.org/10.1038/nrc3793
Giancotti, F. G. (2013). Mechanisms governing metastatic dormancy and reactivation. Cell, 155(4), 750. https://doi.org/10.1016/j.cell.2013.10.029
Butturini, E., de Prati, A. C., Boriero, D., & Mariotto, S. (2019). Tumor dormancy and interplay with hypoxic tumor microenvironment. International Journal of Molecular Sciences., 20(17), 4305. https://doi.org/10.3390/IJMS20174305
Ferrer, A., Roser, C. T., El-Far, M. H., et al. (2020). Hypoxia-mediated changes in bone marrow microenvironment in breast cancer dormancy. Cancer Letters, 488, 9–17. https://doi.org/10.1016/J.CANLET.2020.05.026
Ghajar, C. M., Peinado, H., Mori, H., et al. (2013). The perivascular niche regulates breast tumour dormancy. Nature Cell Biology, 15(7), 807–817. https://doi.org/10.1038/NCB2767
Indraccolo, S., Minuzzo, S., Masiero, M., et al. (2009). Cross-talk between tumor and endothelial cells involving the Notch3-Dll4 interaction marks escape from tumor dormancy. Cancer Research, 69(4), 1314–1323. https://doi.org/10.1158/0008-5472.CAN-08-2791
Bragado, P., Estrada, Y., Parikh, F., et al. (2013). TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nature Cell Biology, 15(11), 1351–1361. https://doi.org/10.1038/NCB2861
Fane, M. E., Chhabra, Y., Alicea, G. M., et al. (2022). Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature, 606(7913), 396–405. https://doi.org/10.1038/S41586-022-04774-2
Di Martino, J. S., Akhter, T., & Bravo-Cordero, J. J. (2021). Remodeling the ecm: Implications for metastasis and tumor dormancy. Cancers (Basel)., 13(19), 4916. https://doi.org/10.3390/cancers13194916
Di Martino, J. S., Nobre, A. R., Mondal, C., et al. (2022). A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nature Cancer., 3(1), 90–107. https://doi.org/10.1038/S43018-021-00291-9
Dasgupta, A., Lim, A. R., & Ghajar, C. M. (2017). Circulating and disseminated tumor cells: Harbingers or initiators of metastasis? Molecular Oncology, 11(1), 40. https://doi.org/10.1002/1878-0261.12022
Kang, Y., & Pantel, K. (2013). Tumor cell dissemination: Emerging biological insights from animal models and cancer patients. Cancer Cell, 23(5), 573. https://doi.org/10.1016/J.CCR.2013.04.017
Sosa, M. S., Parikh, F., Maia, A. G., et al. (2015). NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nature Communications, 6. https://doi.org/10.1038/NCOMMS7170
Faubert, B., Solmonson, A., DeBerardinis, R. J. (2020). Metabolic reprogramming and cancer progression. Science (80- ), 368(6487). https://doi.org/10.1126/SCIENCE.AAW5473/ASSET/043E2E47-0328-47B9-927A-622370C71FB7/ASSETS/GRAPHIC/368_AAW5473_F4.JPEG
Lehúede, C., Dupuy, F., Rabinovitch, R., Jones, R. G., & Siegel, P. M. (2016). Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Research, 76(18), 5201–5208. https://doi.org/10.1158/0008-5472.CAN-16-0266/660578/P/METABOLIC-PLASTICITY-AS-A-DETERMINANT-OF-TUMOR
Berger, J. C., Vander Griend, D. J., Robinson, V. L., Hickson, J. A., & Rinker-Schaeffer, C. W. (2005). Metastasis suppressor genes: From gene identification to protein function and regulation. Cancer Biology & Therapy, 4(8), 805–812. https://doi.org/10.4161/cbt.4.8.1865
Stafford, L. J., Vaidya, K. S., & Welch, D. R. (2008). Metastasis suppressors genes in cancer. International Journal of Biochemistry & Cell Biology, 40(5), 874–891. https://doi.org/10.1016/j.biocel.2007.12.016
Hurst, D. R., & Welch, D. R. (2011). Metastasis suppressor genes At the interface between the environment and tumor cell growth. Vol. 286. Elsevier Inc. https://doi.org/10.1016/B978-0-12-385859-7.00003-3
Cook, L. M., Hurst, D. R., & Welch, D. R. (2011). Metastasis suppressors and the tumor microenvironment. Seminars in Cancer Biology, 21(2), 113–122. https://doi.org/10.1016/j.semcancer.2010.12.005
Steeg, P. S., Bevilacqua, G., Kopper, L., et al. (1988). Evidence for a novel gene associated with low tumor metastatic potential. Journal of the National Cancer Institute, 80(3), 200–204. https://doi.org/10.1093/JNCI/80.3.200
Hur, J., Il, Choi J., Lee, H., et al. (2016). CD82/KAI1 maintains the dormancy of long-term hematopoietic stem cells through interaction with DARC-expressing macrophages. Cell Stem Cell., 18(4), 508–521. https://doi.org/10.1016/J.STEM.2016.01.013
Lee, J. H., Miele, M. E., Hicks, D. J., et al. (1996). KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. Journal of the National Cancer Institute, 88(23), 1731–1737. https://doi.org/10.1093/jnci/88.23.1731
Ly, T., Harihar, S., & Welch, D. R. (2020). KISS1 in metastatic cancer research and treatment: Potential and paradoxes. Cancer and Metastasis Reviews, 39(3), 739–754. https://doi.org/10.1007/s10555-020-09868-9
Harihar, S., Ray, S., Narayanan, S., Santhoshkumar, A., Ly, T., & Welch, D. R. (2020). Role of the tumor microenvironment in regulating the anti-metastatic effect of KISS1. Clinical & Experimental Metastasis, 37(2), 209–223. https://doi.org/10.1007/s10585-020-10030-6
Jiang, Y., Berk, M., Singh, L. S., et al. (2005). KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clinical & Experimental Metastasis, 22(5), 369–376. https://doi.org/10.1007/S10585-005-8186-4
McNally, L. R., Welch, D. R., Beck, B. H., et al. (2010). KISS1 over-expression suppresses metastasis of pancreatic adenocarcinoma in a xenograft mouse model. Clinical & Experimental Metastasis, 27(8), 591–600. https://doi.org/10.1007/S10585-010-9349-5
Nash, K. T., Phadke, P. A., Navenot, J. M., et al. (2007). Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. Journal of the National Cancer Institute, 99(4), 309–321. https://doi.org/10.1093/JNCI/DJK053
Buxton, I. L. O., & Yokdang, N. (2011). Extracellular NM23 signaling in breast cancer: Incommodus verum. Cancers (Basel)., 3(3), 2844–2857. https://doi.org/10.3390/cancers3032844
Knopeke, M. T., Ritschdorff, E. T., Clark, R., et al. (2011). Building on the foundation of daring hypotheses: Using the MKK4 metastasis suppressor to develop models of dormancy and metastatic colonization. FEBS Letters, 585(20), 3159–3165. https://doi.org/10.1016/J.FEBSLET.2011.09.007
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D., & Ossowski, L. (2003). ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Research, 63(7), 1684–1695. https://doi.org/10.1016/J.UROLONC.2003.12.012
Ranganathan, A. C., Adam, A. P., Zhang, L., & Aguirre-Ghiso, J. A. (2006). Tumor cell dormancy induced by p38 SAPK and ER-stress signaling: An adaptive advantage for metastatic cells? Growth cessation and adaptation to stress as a selective advantage for multi-cellular organisms. Cancer Biology & Therapy, 5(7), 729–735.
Fu, Z., Kitagawa, Y., Shen, R., et al. (2006). Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate, 66(3), 248–256. https://doi.org/10.1002/PROS.20319
Schoentgen, F., & Jonic, S. (2020). PEBP1/RKIP behavior: A mirror of actin-membrane organization. Cellular and Molecular Life Sciences, 77(5), 859–874. https://doi.org/10.1007/S00018-020-03455-5
Sakurai-Yageta, M., Recchi, C., Le Dez, G., et al. (2008). The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. Journal of Cell Biology, 181(6), 985–998. https://doi.org/10.1083/JCB.200709076
Bravo-Cordero, J. J., Cordani, M., Soriano, S. F., et al. (2016). A novel high-content analysis tool reveals Rab8-driven cytoskeletal reorganization through Rho GTPases, calpain and MT1-MMP. Journal of Cell Science, 129(8), 1734–1749. https://doi.org/10.1242/JCS.174920
Yun, J., Frankenberger, C. A., Kuo, W. L., et al. (2011). Signalling pathway for RKIP and Let-7 regulates and predicts metastatic breast cancer. EMBO Journal, 30(21), 4500. https://doi.org/10.1038/EMBOJ.2011.312
Datar, I., Feng, J., Qiu, X., et al. (2015). RKIP inhibits local breast cancer invasion by antagonizing the transcriptional activation of MMP13. PLoS ONE, 10(8), e0134494. https://doi.org/10.1371/JOURNAL.PONE.0134494
Sica, A., Larghi, P., Mancino, A., et al. (2008). Macrophage polarization in tumour progression. Seminars in Cancer Biology, 18(5), 349–355. https://doi.org/10.1016/j.semcancer.2008.03.004
Lin, E. Y., & Pollard, J. W. (2004). Macrophages: Modulators of breast cancer progression. Novartis Foundation Symposium, 256, 158–172. https://doi.org/10.1002/0470856734.CH12
Kalpana, G., Figy, C., Feng, J., et al. (2021). The RhoA dependent anti-metastatic function of RKIP in breast cancer. Scientific Reports 11(1):17455. https://doi.org/10.1038/S41598-021-96709-6
Bravo-Cordero, J. J., Hodgson, L., & Condeelis, J. (2012). Directed cell invasion and migration during metastasis. Current Opinion in Cell Biology, 24(2), 277–283. https://doi.org/10.1016/J.CEB.2011.12.004
Vennin, C., Herrmann, D., Lucas, M. C., & Timpson, P. (2016). Intravital imaging reveals new ancillary mechanisms co-opted by cancer cells to drive tumor progression. F1000Research., 5, 892. https://doi.org/10.12688/F1000RESEARCH.8090.1
Linde, N., Casanova-Acebes, M., Sosa, M. S., et al. (2018). Macrophages orchestrate breast cancer early dissemination and metastasis. Nature Communications, 9(1). https://doi.org/10.1038/S41467-017-02481-5
Borriello, L., Coste, A., Traub, B., et al. (2022). Primary tumor associated macrophages activate programs of invasion and dormancy in disseminating tumor cells. Nature Communications. 13(1). https://doi.org/10.1038/S41467-022-28076-3
Lawson, C. D., & Ridley, A. J. (2018). Rho GTPase signaling complexes in cell migration and invasion. Journal of Cell Biology, 217(2), 447–457. https://doi.org/10.1083/JCB.201612069
Ridley, A. J. (2006). Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends in Cell Biology, 16(10), 522–529. https://doi.org/10.1016/J.TCB.2006.08.006
Wu, Y., Moissoglu, K., Wang, H., et al. (2009). Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proceedings of the National Academy of Science of the United States of America, 106(14), 5807. https://doi.org/10.1073/PNAS.0810094106
Harding, M. A., & Theodorescu, D. (2007). RhoGDI2: A new metastasis suppressor gene: Discovery and clinical translation. Urologic Oncology, 25(5), 401–406. https://doi.org/10.1016/J.UROLONC.2007.05.006
Moissoglu, K., McRoberts, K. S., Meier, J. A., Theodorescu, D., & Schwartz, M. A. (2009). Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Research, 69(7), 2838–2844. https://doi.org/10.1158/0008-5472.CAN-08-1397/654444/P/RHO-GDP-DISSOCIATION-INHIBITOR-2-SUPPRESSES
Griner, E. M., Dancik, G. M., Costello, J. C., et al. (2015). RhoC is an unexpected target of RhoGDI2 in prevention of lung colonization of bladder cancer. Molecular Cancer Research, 13(3), 483. https://doi.org/10.1158/1541-7786.MCR-14-0420
Naba, A., Clauser, K. R., Ding, H., Whittaker, C. A., Carr, S. A., & Hynes, R. O. (2016). The extracellular matrix: Tools and insights for the “omics” era. Matrix Biology, 49, 10–24. https://doi.org/10.1016/J.MATBIO.2015.06.003
Hynes, R. O. (2009). The extracellular matrix: Not just pretty fibrils. Science, 326(5957), 1216–1219. https://doi.org/10.1126/SCIENCE.1176009
Said, N., Sanchez-Carbayo, M., Smith, S. C., & Theodorescu, D. (2012). RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. The Journal of Clinical Investigation, 122(4), 1503–1518. https://doi.org/10.1172/JCI61392
Ahmed, M., Sottnik, J. L., Dancik, G. M., et al. (2016). An osteopontin/CD44 axis in RhoGDI2-mediated metastasis suppression. Cancer Cell, 30(3), 432–443. https://doi.org/10.1016/J.CCELL.2016.08.002
Boyerinas, B., Zafrir, M., Yesilkanal, A. E., Price, T. T., Hyjek, E. M., & Sipkins, D. A. (2013). Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood, 121(24), 4821–4831. https://doi.org/10.1182/BLOOD-2012-12-475483
Zimmermann, R. C., & Welch, D. R. (2020). BRMS1: A multi-functional signaling molecule metastasis. Cancer and Metastasis Reviews, 39(3), 755. https://doi.org/10.1007/S10555-020-09871-0
Acknowledgements
We would like to thank Swagata Basu for critical reading and editing of the manuscript.
Funding
This work was supported by an NCI R01 (CA244780), NCI R03 (CA270679), NCI R61 (CA278402) the Irma T. Hirschl Trust, the Emerging Leader Award from the Mark Foundation (to J.J.B.C), and the Tisch Cancer Institute NIH Cancer Center grant (P30 CA196521). C.M. received support from the Ramon Areces Foundation.
Author information
Authors and Affiliations
Contributions
CM and JJBC wrote and edit the manuscript. CM made the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
N/A.
Ethical approval
N/A.
Informed consent
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Megino-Luque, C., Bravo-Cordero, J.J. Metastasis suppressor genes and their role in the tumor microenvironment. Cancer Metastasis Rev 42, 1147–1154 (2023). https://doi.org/10.1007/s10555-023-10155-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10155-6