Abstract
Objectives
To deregulate the purine operon of the purine biosynthetic pathway and optimize energy generation of the respiratory chain to improve the yield of guanosine in Bacillus amyloliquefaciens XH7.
Results
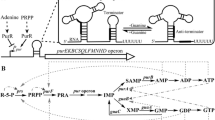
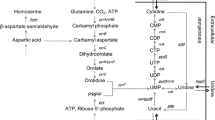
The 5′-untranslated region of the purine operon, which contains the guanine-sensing riboswitch, was disrupted. The native promoter Pw in B. amyloliquefaciens XH7 was replaced by different strong promoters. Among the promoter replacement mutants, XH7purE::P41 gave the highest guanosine yield (16.3 g/l), with an increase of 23% compared with B. amyloliquefaciens XH7. The relative expression levels of the purine operon genes (purE, purF, and purD) in the XH7purE::P41 mutant were upregulated. The concentration of inosine monophosphate (IMP), the primary intermediate in the purine pathway, was also significantly increased in the XH7purE::P41 mutant. Combined modification of the low-coupling branched respiratory chains (cytochrome bd oxidase) improved guanosine production synergistically. The final guanosine yield in the XH7purE::P41△cyd mutant increased by 41% to 19 g/l compared with B. amyloliquefaciens XH7.
Conclusion
The combined modification strategy used in this study is a novel approach to improve the production of guanosine in industrial bacterial strains.




Similar content being viewed by others
References
Asahara T, Mori Y, Zakataeva NP, Livshits VA, Yoshida K, Matsuno K (2010) Accumulation of gene-targeted Bacillus subtilis mutations that enhance fermentative inosine production. Appl Microbiol Biotechnol 87:2195–2207
Asakura Y, Kimura E, Usuda Y, Kawahara Y, Matsui K, Osumi T, Nakamatsu T (2007) Altered metabolic flux due to deletion of odhA causes L-glutamate overproduction in Corynebacterium glutamicum. Appl Environ Microbiol 73:1308–1319
Eiteman MA, Altman E (2006) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24:530–536
Kröger C, Dillona SC, Camerona ADS, Papenfort K, Sivasankarana SK, Liu L, Liu Y, Shin H, Chen RR, Wang NS, Li J, Du G, Chen J (2013) Developing Bacillus spp. as a cell factory for production of microbial enzymes and industrially important biochemicals in the context of systems and synthetic biology. Appl Microbiol Biotechnol 97:6113–6127
Li XJ, Chen T, Chen X, Zhao XM (2006) Redirection electron flow to high coupling efficiency of terminal oxidase to enhance riboflavin biosynthesis. Appl Microbiol Biotechnol 73:374–383
Liao Y, Huang L, Wang B, Zhou F, Pan L (2015) The global transcriptional landscape of Bacillus amyloliquefaciens XH7 and high-throughput screening of strong promoters based on RNA-seq data. Gene 571:252–262
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods 25:402–408
Lobanov KV, Korol’kova NV, Eremina SY, Lopes LE, Mironov AS (2011) Mutation analysis of the purine operon leader region in Bacillus subtilis. Russ J Genet 47:890–899
Mironov AS, Karelov DV, Solovieva IM, Eremina SYu, Errais-Lopes L, Krenevab PA, Perumovb DA (2008) Relationship between the secondary structure and the regulatory activity of the leader region of the riboflavin biosynthesis operon in Bacillus subtilis. Russ J Genet 44:399–404
Peifer S, Schneider K, Nürenberg G, Volmer DA, Heinzle E (2012) Quantitation of intracellular purine intermediates in different Corynebacteria using electrospray LC-MS/MS. Anal Bioanal Chem 404:2295–2305
Shi T, Wang Y, Wang Z, Wang G, Liu D, Fu J, Chen T, Zhao X (2014) Deregulation of purine pathway in Bacillus subtilis and its use in riboflavin biosynthesis. Microb Cell Fact 13:101
Wang J, He K, Xu Q, Chen N (2014) Mutagenetic study of a novel inosine monophosphate dehydrogenase from Bacillus amyloliquefaciens and its possible application in guanosine production. Biotechnol Biotechnol Equip 28:102–106
Yang H, Liao Y, Wang B, Lin Y, Pan L (2011) Complete genome sequence of Bacillus amyloliquefaciens XH7, which exhibits production of purine nucleosides. J Bacteriol 193:5593–5594
Zakataeva NP, Romanenkov DV, Skripnikova VS, Vitushkina MV, Livshits VA, Kivero AD, Novikova AE (2012) Wild-type and feedback-resistant phosphoribosylpyrophosphate synthetases from Bacillus amyloliquefaciens: purification, characterization, and application to increase purine nucleoside production. Appl Microbiol Biotechnol 93:2023–2033
Zamboni N, Sauer U (2003) Knockout of the high-coupling cytochrome aa3 oxidase reduces TCA cycle fluxes in Bacillus subtilis. FEMS Microbiol Lett 226:121–126
Acknowledgements
This work was supported by the Science and Technology Planning Project of Guangdong Province (Grant Numbers 2016A050503016, 2016A010105004 and 2013B010404007), the Science and Technology Planning Project of Guangzhou City (Grant Number 201510010191), the National High-tech R&D Program (863 Program) (Grant Number 2014AA021304) and the China Scholarship Council Fund (201606155032).
Supporting information
Supplementary Table 1—Bacterial strains and plasmids used.
Supplementary Table 2—Primers used.
Supplementary Fig. 1—Construction of the expression plasmid pBE-Pw-bgaB.
Supplementary Fig. 2—Construction of the integrative plasmids pKS2-T1 and pKS2-T2.
Supplementary Fig. 3—Construction of the integrative plasmids pKS2-P41, pKS2-P r2 , and pKS2-P43.
Supplementary Fig. 4—Construction of the knockout plasmid pKS2-cyd.
Supplementary Fig. 5—Genetic modification of the purine operon in Bacillus amyloliquefaciens XH7.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, Y., Ye, Y., Wang, B. et al. Optimization of the purine operon and energy generation in Bacillus amyloliquefaciens for guanosine production. Biotechnol Lett 39, 1675–1682 (2017). https://doi.org/10.1007/s10529-017-2412-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2412-4