Abstract
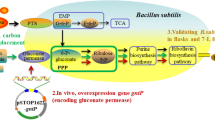
The metabolic impact of redirection electron flow to high coupling efficiency of terminal oxidases on riboflavin biosynthetic ability was quantitatively assessed during batch culture in this paper. While disruption of the low coupling bd oxidase of the riboflavin overproducing B. subtilis PK, the apparent phenotype with more rapid specific growth rate and higher biomass yield was achieved. Compared to by-products formation, a discernible shift to less acetate and more acetoin in cyd mutant was observed. As the overflow metabolism was decreased in B. subtilis PK cyd, more carbon source was directed to biomass and riboflavin biosynthetic pathway, which resulted in higher biomass and about 30% improvement of riboflavin biosynthetic ability. The higher product-corrected biomass yield in mutant showed that the efficient energy generation is an important factor for exponential growth of riboflavin overproducing B. subtilis strain in batch culture.






Similar content being viewed by others
References
Aristidou AA, Bennett GN, San KY (1994) Modification of the central metabolic pathways Escherichia coli to reduce the acetate accumulation by the heterologous expression of the bacillus subtilis acetolactate synthase gene. Biotechnol Bioeng 44(8):944–951
Aristidou AA, San KY, Bennett GN (1999) Metabolic flux analysis of Escherichia coli expressing the Bacillus subtilis acetolactate synthase in batch and continuous culture. Biotechnol Bioeng 63(6):737–749
Bai DM, Zhao XM, Li XG, Xu SM (2004a) Strain improvement and metabolic flux analysis in the wild-type and a mutant Lactobacillus lactis atrain for L(+)-lactic acid production. Biotechnol Bioeng 88(6):681–689
Bai DM, Zhao XM, Li XG, Xu SM (2004b) Strain improvement of Rhizopus oryzae for over-production of L(+)-lactic acid and metabolic flux analysis of mutants. Biochem Eng J 18:41–48
Bernofsky C, Swan M (1973) An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem 53:452–458
Bulthuis BA, Koningstein GM, Stouthamer AH, Verseveld (1989) A comparison between aerobic growth of Bacillus licheniformis in continuous culture and partial-recycling fermentor, with contributions to the discussion on maintenance energy demand. Arch Microbiol 152:499–507
Chen T, Wang JY, Zhou SQ, Chen X, Zhao XM (2004) Trait improvement of riboflavin-producing Bacillus subtilis by genome shuffling and metabolic flux analysis. J Chem Ind Eng China 55(11):1842–1848
Chen T, Chen X, Wang JG, Ban R, Zhao XM (2005) Effect of riboflavin operon dosage on riboflavin productivity in Bacillus subtilis. Trans Tianjin Univ 11(1):1–5
Cherrington CA, Hinton M, Chopra I (1990) Effects of short-chain organic acids on macromolecular synthesis in Escherichia coli. J Appl Bacteriol 68:69–74
Cherrington CA, Hinton M, Pearson GR, Chopra I (1991) Short-chain organic acids at pH 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J Appl Bacteriol 70:161–165
Dauner M, Sauer U (2001) Stoichiometric growth model for riboflavin-producing Bacillus subtilis. Biotechnol Bioeng 76(2):132–143
Dauner M, Sonderegger M, Hochuli M, Szyperski T, Wüthrich K, Hohmann HP, Sauer U, Bailey JE (2002) Intracellular carbon fluxes in riboflavin-producing Bacillus subtilis during growth on two-carbon substrate mixtures. Appl Environ Microbiol 68:1760–1771
Demain AL (1972) Riboflavin oversynthesis. Annu Rev Microbiol 26:369–388
Gelfand RM, Mironov AA, Jomantas J, Kozlov Y, Perumov DA (1999) A conserved RNA structure element involved in the regulation of bacterial synthesis genes. Trends Genet 15:439–442
Goel A, Ferrance J, Jeong J, Ataai MM (1993) Analysis of metabolic fluxes in batch and continuous cultures of Bacillus subtilis. Biotechnol Bioeng 42:686–696
Grundy FJ, Waters DA, Takova TY, Henkin TM (1993) Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol 10:259–271
Holms WH (1986) The central metabolic pathway of Escherichia coli: relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr Top Cell Regul 28:69–105
Hümbelin M, Griesser V, Keller T, Schurter W, Haiker M, Hohmann HP, Ritz H, Richter G, Bacher A (1999) GTP cyclohydrolase II and 3,4-dihydroxy-2-butanone 4-phosphate synthase are rate-limiting enzymes in riboflavin synthesis of an industrial Bacillus subtilis strain used for riboflavin production. J Ind Microbiol Biotech 22:1–7
Jense KF, Pederson S (1990) Metabolic growth rate control in Escherichia coli may be a consequence of subsaturation of macromolecular apparatus with substrates and catalytic components. Microbiol Rev 54:89–100
Jünemann S (1997) Cytochrome bd terminal oxidase. Biochim Biophys Acta 1321:107–127
Lee J, Goel A, AtaaI MM, Domach MM (1997) Supply-side analysis of growth of Bacillus subtilis on glucose-citrate medium: feasible network alternatives and yield optimality. Appl Environ Microbiol 63(2):710–718
Li XJ, Zhou SQ, Chen T, Chen X, Zhao XM (2005a) Effect of transcriptional modified riboflavin operon on riboflavin biosynthetic ability in Bacillus subtilis. 2nd Chinese National Chemical and Biochemical Engineering Annual Meeting. Beijing, China
Li XJ, Chen T, Chen X, Zhao XM (2005b) Effect of ribA amplification on riboflavin production in B. subtilis. 2nd Chinese National Chemical and Biochemical Engineering Annual Meeting. Beijing, China
Majewski RA, Domach MM (1990) Simple constrained optimization view of acetate overflow in Escherichia coli. Biotechnol Bioeng 35:732–738
Maria CR, Najimudin N, Leslie RW, Zahler SA (1993) Regulation of the Bacillus sibtilis alsD and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol 175(12):3863–3874
Oh DK, Kim SY, Kim JH (1998) Increase of xylitol production rate by controlling redox potential in Candida parapsilosis. Biotechnol Bioeng 58:440–444
Perkins JB, Pero JG, Sloma A (1991) Riboflavin overproducing strains of bacteria. European patent application: 0405370
Perkins JB, Sloma A, Hermann T, Theriault K, Zachgo E, Erdenberger T, Hannett N, Chatterjee1 NP, Williams V, Rufo GA Jr, Hatch R, Pero1 J (1999) Genetic engineering of Bacillus subtilis for the commercial production of riboflavin. J Ind Microbiol Biotech 22:8–18
Russell JB, Cook GM (1995) Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev 59:48–62
Santana M, Kunst F, Hullo MF, Rapoport G, Danchin A, Glaser P (1992) olecular cloning, sequencing, and physiological characterization of the qox operon from Bacillus subtilis encoding the aa3-600 oxidase. J Biochem 267:10225–10231
Sauer U, Baily JE (1999) Estimation of P-to-O ratio in Bacillus subtilis and its influence on maximum riboflavin yield. Biotechnol Bioeng 64:750–754
Sauer U, Cameron DC, Baily JE (1998) Metabolic capacity of Bacillus subtilis for the production of purine nucleosides, riboflavin, and folic acid. Biotechnol Bioeng 59(2):227–238
Stahmann KP, Revuelta JL, Seulberger H (2000) Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl Microbiol Biotechnol 53:509–516
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic engineering. Academic, San Diego
Stouthamer AH, Verseveld HW (1987) Microbial energetics should be considered in manipulating metabolism for biotechnological purposes. Trends Biotechnol 5:149–155
Tappe W, Laverman A, Bohland M, Braster M, Rittershaus S, Groeneweg J, Verseveld HW (1999) Maintenance energy demand and starvation recovery dynamics of Nitrosomonas eurpaea and Nitrobacter winogradskyi cultivation in a retentostat with complete biomass retention. Appl Environ Microbiol 65(6):2471–2477
Trumpower BL, Gennis RB (1994) Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu Rev Biochem 63:675–716
Verseveld HW, Chesbro WR, Braster M, Stouthamer AH (1984) Eubacteria have 3 growth modes keyed to nutrient flow-consequences for the concept of maintenance and maximal growth yield. Arch Microbiol 137:176–184
Vitreschank AG, Rodionov DA, Mironov AA, Gelfand MS (2002) Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res 30:3141–3151
Winstedt L, Wachenfeldt C (2000) Terminal osidases of Bacillus subtilis strain 168: one quinol oxidases, cytochrome aa 3 or bd, is required for aerobic growth. J Bacteriol 182:6557–6564
Winstedt L, Yoshida K, Fujita Y, Wachenfeldt C (1998) Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J Bacteriol 180:6571–6580
Yasbin RE, Wilson GA, Young FE (1975) Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol 121(1):296–304
Zamboni N (2003) Metabolic engineering of respiration for improved riboflavin production and elucidation of NADPH metabolism in Bacillus subtilis. Ph.D. thesis. Switzerland
Zamboni N, Mouncey N, Hohmann HP, Sauer U (2003) Reducing maintenance metabolism by metabolic engineering of respiration improves riboflavin by Bacillus subtilis. Metab Eng 5:49–55
Acknowledgements
Financial support from the National Natural Science Foundation of China (NSFC-20536040), the National Project of Key Fundamental Research (2003CB716003), and the Development Project of Science and Technology of Tianjin (05YFGZGX04500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XJ., Chen, T., Chen, X. et al. Redirection electron flow to high coupling efficiency of terminal oxidase to enhance riboflavin biosynthesis. Appl Microbiol Biotechnol 73, 374–383 (2006). https://doi.org/10.1007/s00253-006-0482-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0482-7