Abstract
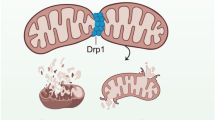
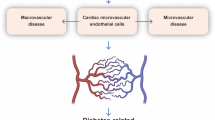
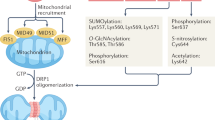
Coronary microvascular endothelial dysfunction is both a culprit and a victim of diabetes, and can accelerate diabetes-related microvascular and macrovascular complications by promoting vasoconstrictive, pro-inflammatory and pro-thrombotic responses. Perturbed mitochondrial function induces oxidative stress, disrupts metabolism and activates apoptosis in endothelial cells, thus exacerbating the progression of coronary microvascular complications in diabetes. The mitochondrial quality surveillance (MQS) system responds to stress by altering mitochondrial metabolism, dynamics (fission and fusion), mitophagy and biogenesis. Dysfunctional mitochondria are prone to fission, which generates two distinct types of mitochondria: one with a normal and the other with a depolarized mitochondrial membrane potential. Mitochondrial fusion and mitophagy can restore the membrane potential and homeostasis of defective mitochondrial fragments. Mitophagy-induced decreases in the mitochondrial population can be reversed by mitochondrial biogenesis. MQS abnormalities induce pathological mitochondrial fission, delayed mitophagy, impaired metabolism and defective biogenesis, thus promoting the accumulation of unhealthy mitochondria and the activation of mitochondria-dependent apoptosis. In this review, we examine the effects of MQS on mitochondrial fitness and explore the association of MQS disorders with coronary microvascular endothelial dysfunction in diabetes. We also discuss the potential to treat diabetes-related coronary microvascular endothelial dysfunction using novel MQS-altering drugs.






Similar content being viewed by others
References
Joslin EP (2021) The prevention of diabetes mellitus. JAMA 325(2):190. https://doi.org/10.1001/jama.2020.17738
American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32(Suppl 1):S62–S67. https://doi.org/10.2337/dc09-S062
Forbes JM, Cooper ME (2013) Mechanisms of diabetic complications. Physiol Rev 93(1):137–188. doi:https://doi.org/10.1152/physrev.00045.2011
Escaned J, Lerman LO (2020) Coronary microcirculation and hypertensive heart failure. Eur Heart J 41(25):2376–2378. doi:https://doi.org/10.1093/eurheartj/ehaa437
Lüscher TF (2018) Assessing myocardial ischaemia in epicardial coronaries and the microcirculation. Eur Heart J 39(46):4047–4050. doi:https://doi.org/10.1093/eurheartj/ehy816
Taqueti VR, Di Carli MF (2018) Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol 72(21):2625–2641. https://doi.org/10.1016/j.jacc.2018.09.042
Vancheri F, Longo G, Vancheri S, Henein M (2020) Coronary microvascular dysfunction. J Clin Med. https://doi.org/10.3390/jcm9092880
Cuijpers I, Simmonds SJ, van Bilsen M, Czarnowska E, González Miqueo A, Heymans S, Kuhn AR, Mulder P, Ratajska A, Jones EAV, Brakenhielm E (2020) Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic Res Cardiol 115(4):39. doi:https://doi.org/10.1007/s00395-020-0798-y
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. doi:https://doi.org/10.1016/j.jacc.2013.02.092
Jia G, Hill MA, Sowers JR (2018) Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circul Res 122(4):624–638. doi:https://doi.org/10.1161/circresaha.117.311586
Shivu GN, Phan TT, Abozguia K, Ahmed I, Wagenmakers A, Henning A, Narendran P, Stevens M, Frenneaux M (2010) Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation 121(10):1209–1215. doi:https://doi.org/10.1161/circulationaha.109.873273
Kibel A, Selthofer-Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, Gros M (2017) Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res 45(6):1901–1929. doi:https://doi.org/10.1177/0300060516675504
Akbari M, Kirkwood TBL, Bohr VA (2019) Mitochondria in the signaling pathways that control longevity and health span. Ageing Res Rev 54:100940. doi:https://doi.org/10.1016/j.arr.2019.100940
Kalyanaraman B, Cheng G, Hardy M, Ouari O, Lopez M, Joseph J, Zielonka J, Dwinell MB (2018) A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biol 14:316–327. doi:https://doi.org/10.1016/j.redox.2017.09.020
Wang J, Toan S, Zhou H (2020) New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis 23(3):299–314. doi:https://doi.org/10.1007/s10456-020-09720-2
Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM (2020) Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol 34:101517. doi:https://doi.org/10.1016/j.redox.2020.101517
Chowdhury AR, Zielonka J, Kalyanaraman B, Hartley RC, Murphy MP, Avadhani NG (2020) Mitochondria-targeted paraquat and metformin mediate ROS production to induce multiple pathways of retrograde signaling: a dose-dependent phenomenon. Redox Biol 36:101606. https://doi.org/10.1016/j.redox.2020.101606
Zhu H, Toan S, Mui D, Zhou H (2021) Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta Physiol 231(3):e13590. https://doi.org/10.1111/apha.13590
Zhou H, Ren J, Toan S, Mui D (2021) Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res Rev 66:101250. doi:https://doi.org/10.1016/j.arr.2020.101250
Cho HM, Ryu JR, Jo Y, Seo TW, Choi YN, Kim JH, Chung JM, Cho B, Kang HC, Yu SW, Yoo SJ, Kim H, Sun W (2019) Drp1-Zip1 Interaction Regulates Mitochondrial Quality Surveillance System. Mol Cell 73(2):364–376e368. doi:https://doi.org/10.1016/j.molcel.2018.11.009
Guarini G, Huqi A, Morrone D, Capozza P, Todiere G, Marzilli M (2014) Pharmacological approaches to coronary microvascular dysfunction. Pharmacol Ther 144(3):283–302. doi:https://doi.org/10.1016/j.pharmthera.2014.06.008
Tabit CE, Chung WB, Hamburg NM, Vita JA (2010) Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 11(1):61–74. doi:https://doi.org/10.1007/s11154-010-9134-4
Chang X, Lochner A, Wang HH, Wang S, Zhu H, Ren J, Zhou H (2021) Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics 11(14):6766–6785. doi:https://doi.org/10.7150/thno.60143
Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM (2016) Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 166(5):1324–1337e1311. doi:https://doi.org/10.1016/j.cell.2016.07.040
Qiao K, Liu Y, Xu Z, Zhang H, Zhang H, Zhang C, Chang Z, Lu X, Li Z, Luo C, Liu Y, Yang C, Sun T (2020) RNA m6A methylation promotes the formation of vasculogenic mimicry in hepatocellular carcinoma via Hippo pathway. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09744-8
Ollauri-Ibáñez C, Núñez-Gómez E, Egido-Turrión C, Silva-Sousa L, Díaz-Rodríguez E, Rodríguez-Barbero A, López-Novoa JM, Pericacho M (2020) Continuous endoglin (CD105) overexpression disrupts angiogenesis and facilitates tumor cell metastasis. Angiogenesis 23(2):231–247. doi:https://doi.org/10.1007/s10456-019-09703-y
Nguyen QL, Okuno N, Hamashima T, Dang ST, Fujikawa M, Ishii Y, Enomoto A, Maki T, Nguyen HN, Nguyen VT, Fujimori T, Mori H, Andrae J, Betsholtz C, Takao K, Yamamoto S, Sasahara M (2020) Vascular PDGFR-alpha protects against BBB dysfunction after stroke in mice. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09742-w
Nawaz MI, Rezzola S, Tobia C, Coltrini D, Belleri M, Mitola S, Corsini M, Sandomenico A, Caporale A, Ruvo M, Presta M (2020) D-Peptide analogues of Boc-Phe-Leu-Phe-Leu-Phe-COOH induce neovascularization via endothelial N-formyl peptide receptor 3. Angiogenesis 23(3):357–369. doi:https://doi.org/10.1007/s10456-020-09714-0
Nakamoto S, Ito Y, Nishizawa N, Goto T, Kojo K, Kumamoto Y, Watanabe M, Majima M (2020) Lymphangiogenesis and accumulation of reparative macrophages contribute to liver repair after hepatic ischemia-reperfusion injury. Angiogenesis 23(3):395–410. doi:https://doi.org/10.1007/s10456-020-09718-w
Moon EH, Kim YH, Vu PN, Yoo H, Hong K, Lee YJ, Oh SP (2020) TMEM100 is a key factor for specification of lymphatic endothelial progenitors. Angiogenesis 23(3):339–355. doi:https://doi.org/10.1007/s10456-020-09713-1
Pennathur S, Heinecke JW (2007) Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 7(4):257–264. doi:https://doi.org/10.1007/s11892-007-0041-3
De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154(3):651–663. doi:https://doi.org/10.1016/j.cell.2013.06.037
Margadant C (2020) Positive and negative feedback mechanisms controlling tip/stalk cell identity during sprouting angiogenesis. Angiogenesis 23(2):75–77. doi:https://doi.org/10.1007/s10456-020-09706-0
Ma Q, Reiter RJ, Chen Y (2020) Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 23(2):91–104. doi:https://doi.org/10.1007/s10456-019-09689-7
Lustgarten Guahmich N, Farber G, Shafiei S, McNally D, Redmond D, Kallinos E, Stuhlmann H, Dufort D, James D, Blobel CP (2020) Endothelial deletion of ADAM10, a key regulator of Notch signaling, causes impaired decidualization and reduced fertility in female mice. Angiogenesis 23(3):443–458. doi:https://doi.org/10.1007/s10456-020-09723-z
Hwang MH, Kim S (2014) Type 2 diabetes: endothelial dysfunction and exercise. J Exerc Nutr Biochem 18(3):239–247. https://doi.org/10.5717/jenb.2014.18.3.239
Latacz E, Caspani E, Barnhill R, Lugassy C, Verhoef C, Grünhagen D, Van Laere S, Fernández Moro C, Gerling M, Dirix M, Dirix LY, Vermeulen PB (2020) Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis 23(1):43–54. doi:https://doi.org/10.1007/s10456-019-09690-0
Sabbatinelli J, Prattichizzo F, Olivieri F, Procopio AD, Rippo MR, Giuliani A (2019) Where metabolism meets senescence: focus on endothelial cells. Front Physiol 10:1523. https://doi.org/10.3389/fphys.2019.01523
Dagher Z, Ruderman N, Tornheim K, Ido Y (2001) Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circul Res 88(12):1276–1282. doi:https://doi.org/10.1161/hh1201.092998
Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo J (2001) Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. Faseb j 15(10):1771–1773. doi:https://doi.org/10.1096/fj.00-0893fje
Merchan JR, Kovács K, Railsback JW, Kurtoglu M, Jing Y, Piña Y, Gao N, Murray TG, Lehrman MA, Lampidis TJ (2010) Antiangiogenic activity of 2-deoxy-D-glucose. PLoS ONE 5(10):e13699. doi:https://doi.org/10.1371/journal.pone.0013699
Kuczynski EA, Reynolds AR (2020) Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis 23(1):55–74. doi:https://doi.org/10.1007/s10456-019-09698-6
McElwain CJ, Tuboly E, McCarthy FP, McCarthy CM (2020) Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front Endocrinol 11:655. https://doi.org/10.3389/fendo.2020.00655
Knapp M, Tu X, Wu R (2019) Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin 40(1):1–8. doi:https://doi.org/10.1038/s41401-018-0042-6
Tang X, Luo YX, Chen HZ, Liu DP (2014) Mitochondria, endothelial cell function, and vascular diseases. Front Physiol 5:175. doi:https://doi.org/10.3389/fphys.2014.00175
Drummond GR, Sobey CG (2014) Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends in endocrinology and metabolism. TEM 25(9):452–463. doi:https://doi.org/10.1016/j.tem.2014.06.012
Koh JH, Johnson ML, Dasari S, LeBrasseur NK, Vuckovic I, Henderson GC, Cooper SA, Manjunatha S, Ruegsegger GN, Shulman GI, Lanza IR, Nair KS (2019) TFAM Enhances Fat Oxidation and Attenuates High-Fat Diet-Induced Insulin Resistance in Skeletal Muscle. Diabetes 68(8):1552–1564. doi:https://doi.org/10.2337/db19-0088
D’Souza K, Nzirorera C, Kienesberger PC (2016) Lipid metabolism and signaling in cardiac lipotoxicity. Biochim Biophys Acta 1861(10):1513–1524. doi:https://doi.org/10.1016/j.bbalip.2016.02.016
Ko VH, Yu LJ, Dao DT, Li X, Secor JD, Anez-Bustillos L, Cho BS, Pan A, Mitchell PD, Kishikawa H, Puder M (2020) Roxadustat (FG-4592) accelerates pulmonary growth, development, and function in a compensatory lung growth model. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09735-9
Pangare M, Makino A (2012) Mitochondrial function in vascular endothelial cell in diabetes. J Smooth Muscle Res 48(1):1–26. https://doi.org/10.1540/jsmr.48.1
Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA (2011) Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124(4):444–453. doi:https://doi.org/10.1161/circulationaha.110.014506
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97(22):12222–12226. doi:https://doi.org/10.1073/pnas.97.22.12222
Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig 112(7):1049–1057. doi:https://doi.org/10.1172/jci18127
Devalaraja-Narashimha K, Padanilam BJ (2009) PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol 20(1):95–103. https://doi.org/10.1681/asn.2008030325
Jiang L, Li N (2020) B-cell non-Hodgkin lymphoma: importance of angiogenesis and antiangiogenic therapy. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09729-7
Ceriello A, Ihnat MA, Thorpe JE (2009) Clinical review 2: The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab 94(2):410–415. doi:https://doi.org/10.1210/jc.2008-1824
Wautier JL, Schmidt AM (2004) Protein glycation: a firm link to endothelial cell dysfunction. Circul Res 95(3):233–238. doi:https://doi.org/10.1161/01.Res.0000137876.28454.64
Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R (2002) Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106(4):466–472. doi:https://doi.org/10.1161/01.cir.0000023043.02648.51
Das Evcimen N, King GL (2007) The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res 55(6):498–510. doi:https://doi.org/10.1016/j.phrs.2007.04.016
Chang T, Wang R, Wu L (2005) Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radic Biol Med 38(2):286–293. doi:https://doi.org/10.1016/j.freeradbiomed.2004.10.034
Sena CM, Matafome P, Crisóstomo J, Rodrigues L, Fernandes R, Pereira P, Seiça RM (2012) Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res 65(5):497–506. doi:https://doi.org/10.1016/j.phrs.2012.03.004
Islam MT (2020) Angiostatic effects of ascorbic acid: current status and future perspectives. Angiogenesis 23(3):275–277. doi:https://doi.org/10.1007/s10456-020-09719-9
Matafome P, Sena C, Seiça R (2013) Methylglyoxal, obesity, and diabetes. Endocrine 43(3):472–484. doi:https://doi.org/10.1007/s12020-012-9795-8
Pan M, Han Y, Basu A, Dai A, Si R, Willson C, Balistrieri A, Scott BT, Makino A (2018) Overexpression of hexokinase 2 reduces mitochondrial calcium overload in coronary endothelial cells of type 2 diabetic mice. Am J Physiol Cell Physiol 314(6):C732–c740. doi:https://doi.org/10.1152/ajpcell.00350.2017
Alvarado-Vásquez N, Zapata E, Alcázar-Leyva S, Massó F, Montaño LF (2007) Reduced NO synthesis and eNOS mRNA expression in endothelial cells from newborns with a strong family history of type 2 diabetes. Diabetes/Metab Res Rev 23(7):559–566. https://doi.org/10.1002/dmrr.743
Moruzzi N, Del Sole M, Fato R, Gerdes JM, Berggren PO, Bergamini C, Brismar K (2014) Short and prolonged exposure to hyperglycaemia in human fibroblasts and endothelial cells: metabolic and osmotic effects. Int J Biochem Cell Biol 53:66–76. doi:https://doi.org/10.1016/j.biocel.2014.04.026
Kim EH, Koh EH, Park JY, Lee KU (2010) Adenine nucleotide translocator as a regulator of mitochondrial function: implication in the pathogenesis of metabolic syndrome. Korean Diabetes J 34(3):146–153. https://doi.org/10.4093/kdj.2010.34.3.146
Gerő D, Torregrossa R, Perry A, Waters A, Le-Trionnaire S, Whatmore JL, Wood M, Whiteman M (2016) The novel mitochondria-targeted hydrogen sulfide (H(2)S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol Res 113(Pt A):186–198. doi:https://doi.org/10.1016/j.phrs.2016.08.019
Broniarek I, Koziel A, Jarmuszkiewicz W (2016) The effect of chronic exposure to high palmitic acid concentrations on the aerobic metabolism of human endothelial EA.hy926 cells. Pflug Arch 468(9):1541–1554. https://doi.org/10.1007/s00424-016-1856-z
Shah GN, Morofuji Y, Banks WA, Price TO (2013) High glucose-induced mitochondrial respiration and reactive oxygen species in mouse cerebral pericytes is reversed by pharmacological inhibition of mitochondrial carbonic anhydrases: Implications for cerebral microvascular disease in diabetes. Biochem Biophys Res Commun 440(2):354–358. doi:https://doi.org/10.1016/j.bbrc.2013.09.086
Shah GN, Price TO, Banks WA, Morofuji Y, Kovac A, Ercal N, Sorenson CM, Shin ES, Sheibani N (2013) Pharmacological inhibition of mitochondrial carbonic anhydrases protects mouse cerebral pericytes from high glucose-induced oxidative stress and apoptosis. J Pharmacol Exp Ther 344(3):637–645. doi:https://doi.org/10.1124/jpet.112.201400
Chan DC (2020) Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol 15:235–259. doi:https://doi.org/10.1146/annurev-pathmechdis-012419-032711
Giacomello M, Pyakurel A, Glytsou C, Scorrano L (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21(4):204–224. doi:https://doi.org/10.1038/s41580-020-0210-7
Xian H, Liou YC (2021) Functions of outer mitochondrial membrane proteins: mediating the crosstalk between mitochondrial dynamics and mitophagy. Cell Death Differ 28(3):827–842. doi:https://doi.org/10.1038/s41418-020-00657-z
Whitley BN, Engelhart EA, Hoppins S (2019) Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion 49:269–283. doi:https://doi.org/10.1016/j.mito.2019.06.002
Vásquez-Trincado C, García-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, Lavandero S (2016) Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 594(3):509–525. doi:https://doi.org/10.1113/jp271301
Mishra P, Chan DC (2016) Metabolic regulation of mitochondrial dynamics. J Cell Biol 212(4):379–387. doi:https://doi.org/10.1083/jcb.201511036
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.005328
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. doi:https://doi.org/10.1016/j.redox.2017.11.004
Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J (2015) Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116(2):264–278. doi:https://doi.org/10.1161/circresaha.116.303356
Song M, Franco A, Fleischer JA, Zhang L, Dorn GW II (2017) Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab 26(6):872-883e875. https://doi.org/10.1016/j.cmet.2017.09.023
Papanicolaou KN, Ngoh GA, Dabkowski ER, O’Connell KA, Ribeiro RF Jr, Stanley WC, Walsh K (2012) Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol 302(1):H167–179. doi:https://doi.org/10.1152/ajpheart.00833.2011
Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K (2011) Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31(6):1309–1328. doi:https://doi.org/10.1128/mcb.00911-10
Hall AR, Burke N, Dongworth RK, Kalkhoran SB, Dyson A, Vicencio JM, Dorn GW, Yellon II, Hausenloy DM DJ (2016) Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis 7(5):e2238. doi:https://doi.org/10.1038/cddis.2016.139
Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101(45):15927–15932. doi:https://doi.org/10.1073/pnas.0407043101
Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L (2015) The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab 21(6):834–844. doi:https://doi.org/10.1016/j.cmet.2015.05.007
Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M (2015) Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab 21(6):845–854. doi:https://doi.org/10.1016/j.cmet.2015.04.016
Bravo-San Pedro JM, Kroemer G, Galluzzi L (2017) Autophagy and Mitophagy in Cardiovascular Disease. Circ Res 120(11):1812–1824. doi:https://doi.org/10.1161/circresaha.117.311082
Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T, Matoba S (2013) Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun 4:2308. doi:https://doi.org/10.1038/ncomms3308
Rana A, Rera M, Walker DW (2013) Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A 110(21):8638–8643. doi:https://doi.org/10.1073/pnas.1216197110
Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW (2011) PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A 108(23):9572–9577. doi:https://doi.org/10.1073/pnas.1106291108
Siddall HK, Yellon DM, Ong SB, Mukherjee UA, Burke N, Hall AR, Angelova PR, Ludtmann MH, Deas E, Davidson SM, Mocanu MM, Hausenloy DJ (2013) Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS ONE 8(4):e62400. doi:https://doi.org/10.1371/journal.pone.0062400
Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW (2007) Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest 117(10):2825–2833. doi:https://doi.org/10.1172/jci32490
Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW IInd (2002) Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med 8(7):725–730. doi:https://doi.org/10.1038/nm719
Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X, Huang K, Xie Z, Zou MH (2017) Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation 136(23):2248–2266. https://doi.org/10.1161/circulationaha.117.030235
García-Quintans N, Sánchez-Ramos C, Prieto I, Tierrez A, Arza E, Alfranca A, Redondo JM, Monsalve M (2016) Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1α-deficient mice. Angiogenesis 19(2):217–228. doi:https://doi.org/10.1007/s10456-016-9502-0
Riehle C, Abel ED (2012) PGC-1 proteins and heart failure. Trends Cardiovasc Med 22(4):98–105. doi:https://doi.org/10.1016/j.tcm.2012.07.003
Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305(5685):858–862. https://doi.org/10.1126/science.1099793
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160(2):189–200. https://doi.org/10.1083/jcb.200211046
Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. Embo j 25(13):2966–2977. doi:https://doi.org/10.1038/sj.emboj.7601184
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204(6):919–929. doi:https://doi.org/10.1083/jcb.201308006
Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS (2006) Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 281(49):37972–37979. doi:https://doi.org/10.1074/jbc.M606059200
Bakuy V, Unal O, Gursoy M, Kunt A, Ozisik K, Sargon M, Emir M, Sener E (2014) Electron microscopic evaluation of internal thoracic artery endothelial morphology in diabetic coronary bypass patients. Ann Thorac Surg 97(3):851–857. doi:https://doi.org/10.1016/j.athoracsur.2013.09.102
Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR (2012) Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metabol 15(2):186–200. doi:https://doi.org/10.1016/j.cmet.2012.01.009
Di Tomo P, Alessio N, Falone S, Pietrangelo L, Lanuti P, Cordone V, Santini SJ, Di Pietrantonio N, Marchisio M, Protasi F, Di Pietro N, Formoso G, Amicarelli F, Galderisi U, Pandolfi A (2021) Endothelial cells from umbilical cord of women affected by gestational diabetes: A suitable in vitro model to study mechanisms of early vascular senescence in diabetes. Faseb j 35(6):e21662. doi:https://doi.org/10.1096/fj.202002072RR
Xing H, Zhang Z, Shi G, He Y, Song Y, Liu Y, Harrington EO, Sellke FW, Feng J (2021) Chronic Inhibition of mROS Protects Against Coronary Endothelial Dysfunction in Mice With Diabetes. Front Cell Dev Biol 9:643810. doi:https://doi.org/10.3389/fcell.2021.643810
Makino A, Scott BT, Dillmann WH (2010) Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53(8):1783–1794. doi:https://doi.org/10.1007/s00125-010-1770-4
Durand MJ, Ait-Aissa K, Levchenko V, Staruschenko A, Gutterman DD, Beyer AM (2019) Visualization and quantification of mitochondrial structure in the endothelium of intact arteries. Cardiovascular Res 115(10):1546–1556. doi:https://doi.org/10.1093/cvr/cvy294
García-Martínez E, Redondo A, Piulats JM, Rodríguez A, Casado A (2020) Are antiangiogenics a good ‘partner’ for immunotherapy in ovarian cancer? Angiogenesis. https://doi.org/10.1007/s10456-020-09734-w
García-Gómez P, Valiente M (2020) Vascular co-option in brain metastasis. Angiogenesis 23(1):3–8. doi:https://doi.org/10.1007/s10456-019-09693-x
Fukada K, Kajiya K (2020) Age-related structural alterations of skeletal muscles and associated capillaries. Angiogenesis 23(2):79–82. doi:https://doi.org/10.1007/s10456-020-09705-1
Iannantuoni F, Abad-Jiménez AMdM, Canet Z, Díaz-Pozo F, López-Domènech P, Morillas S, Rocha C, Víctor MVM (2020) Mitochondrial alterations and enhanced human leukocyte/endothelial cell interactions in type 1 diabetes. J Clin Med. https://doi.org/10.3390/jcm9072155
Chen W, Xiang H, Chen R, Yang J, Yang X, Zhou J, Liu H, Zhao S, Xiao J, Chen P, Chen AF, Chen S, Lu H (2019) S1PR2 antagonist ameliorate high glucose-induced fission and dysfunction of mitochondria in HRGECs via regulating ROCK1. BMC Nephrol 20(1):135. doi:https://doi.org/10.1186/s12882-019-1323-0
Lee HZ, Yeh FT, Wu CH (2004) The effect of elevated extracellular glucose on adherens junction proteins in cultured rat heart endothelial cells. Life Sci 74(17):2085–2096. doi:https://doi.org/10.1016/j.lfs.2003.06.046
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J (2018) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15:335–346. doi:https://doi.org/10.1016/j.redox.2017.12.019
Kim D, Sesaki H, Roy S (2021) Reduced levels of Drp1 protect against development of retinal vascular lesions in diabetic retinopathy. Cells. https://doi.org/10.3390/cells10061379
Kim D, Roy S (2020) Effects of diabetes on mitochondrial morphology and its implications in diabetic retinopathy. Investig Ophthalmol Vis Sci 61(10):10. https://doi.org/10.1167/iovs.61.10.10
Kowluru RA, Mohammad G (2020) Epigenetics and Mitochondrial Stability in the Metabolic Memory Phenomenon Associated with Continued Progression of Diabetic Retinopathy. Sci Rep 10(1):6655. doi:https://doi.org/10.1038/s41598-020-63527-1
Duraisamy AJ, Mohammad G, Kowluru RA (2019) Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim Biophys Acta 1865(6):1617–1626. https://doi.org/10.1016/j.bbadis.2019.03.013
Shi Y, Fan S, Wang D, Huyan T, Chen J, Chen J, Su J, Li X, Wang Z, Xie S, Yun C, Li X, Tie L (2018) FOXO1 inhibition potentiates endothelial angiogenic functions in diabetes via suppression of ROCK1/Drp1-mediated mitochondrial fission. Biochim Biophys Acta 1864(7):2481–2494. https://doi.org/10.1016/j.bbadis.2018.04.005
Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, Kitajewski J, Rehman J, Yoon Y, Cho J, Fukai T, Ushio-Fukai M (2018) Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Rep 23(12):3565–3578. doi:https://doi.org/10.1016/j.celrep.2018.05.054
Lemasters JJ (2014) Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (Type 3). Redox Biol 2:749–754. doi:https://doi.org/10.1016/j.redox.2014.06.004
Czaja MJ, Ding WX, Donohue TM Jr, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM (2013) Functions of autophagy in normal and diseased liver. Autophagy 9(8):1131–1158. doi:https://doi.org/10.4161/auto.25063
Lemasters JJ, Zhong Z (2018) Mitophagy in hepatocytes: Types, initiators and role in adaptive ethanol metabolism☆. Liver Res 2(3):125–132. doi:https://doi.org/10.1016/j.livres.2018.09.005
Kim I, Lemasters JJ (2011) Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol 300(2):C308–317. doi:https://doi.org/10.1152/ajpcell.00056.2010
Rodriguez-Enriquez S, Kim I, Currin RT, Lemasters JJ (2006) Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy 2(1):39–46. doi:https://doi.org/10.4161/auto.2229
McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA (2014) Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. Embo j 33(4):282–295. doi:https://doi.org/10.1002/embj.201385902
Cai Q, Jeong YY (2020) Mitophagy in Alzheimer’s disease and other age-related neurodegenerative diseases. Cells. https://doi.org/10.3390/cells9010150
Fivenson EM, Lautrup S, Sun N, Scheibye-Knudsen M, Stevnsner T, Nilsen H, Bohr VA, Fang EF (2017) Mitophagy in neurodegeneration and aging. Neurochem Int 109:202–209. doi:https://doi.org/10.1016/j.neuint.2017.02.007
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14. doi:https://doi.org/10.1038/nrm3028
Fritsch LE, Moore ME, Sarraf SA, Pickrell AM (2020) Ubiquitin and Receptor-Dependent Mitophagy Pathways and Their Implication in Neurodegeneration. J Mol Biol 432(8):2510–2524. doi:https://doi.org/10.1016/j.jmb.2019.10.015
Yamano K, Matsuda N, Tanaka K (2016) The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation. EMBO Rep 17(3):300–316. doi:https://doi.org/10.15252/embr.201541486
Morales PE, Arias-Durán C, Ávalos-Guajardo Y, Aedo G, Verdejo HE, Parra V, Lavandero S (2020) Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med 71:100822. doi:https://doi.org/10.1016/j.mam.2019.09.006
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524(7565):309–314. doi:https://doi.org/10.1038/nature14893
Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, Chen Q (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14(2):177–185. doi:https://doi.org/10.1038/ncb2422
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ 25(6):1080–1093. doi:https://doi.org/10.1038/s41418-018-0086-7
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y (2017) Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13:498–507. doi:https://doi.org/10.1016/j.redox.2017.07.007
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 113(4):23. doi:https://doi.org/10.1007/s00395-018-0682-1
Wang J, Zhu P, Li R, Ren J, Zhou H (2020) Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol 30:101415. doi:https://doi.org/10.1016/j.redox.2019.101415
Gao A, Jiang J, Xie F, Chen L (2020) Bnip3 in mitophagy: Novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction. Clin Chim Acta 506:72–83. doi:https://doi.org/10.1016/j.cca.2020.02.024
Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA (2007) NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA 104(49):19500–19505. doi:https://doi.org/10.1073/pnas.0708818104
Liu L, Sakakibara K, Chen Q, Okamoto K (2014) Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res 24(7):787–795. doi:https://doi.org/10.1038/cr.2014.75
Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A (1999) Nix and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem 274(1):7–10. doi:https://doi.org/10.1074/jbc.274.1.7
Xi J, Rong Y, Zhao Z, Huang Y, Wang P, Luan H, Xing Y, Li S, Liao J, Dai Y, Liang J, Wu F (2021) Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy. J Ethnopharmacol 271:113855. doi:https://doi.org/10.1016/j.jep.2021.113855
Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, Cui Z, Cui T, Wang XL, Shen YH (2015) PINK1-Parkin-Mediated Mitophagy Protects Mitochondrial Integrity and Prevents Metabolic Stress-Induced Endothelial Injury. PLoS ONE 10(7):e0132499. doi:https://doi.org/10.1371/journal.pone.0132499
Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, Crncec I, Kramer N, Unger C, Hengstschläger M, Eferl R, Moriggl R, Sommergruber W, Gerner C, Dolznig H (2020) Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 23(2):159–177. doi:https://doi.org/10.1007/s10456-019-09688-8
Umapathy A, Chamley LW, James JL (2020) Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 23(2):105–117. doi:https://doi.org/10.1007/s10456-019-09694-w
Tomita Y, Cakir B, Liu CH, Fu Z, Huang S, Cho SS, Britton WR, Sun Y, Puder M, Hellström A, Talukdar S, Smith LEH (2020) Free fatty acid receptor 4 activation protects against choroidal neovascularization in mice. Angiogenesis 23(3):385–394. doi:https://doi.org/10.1007/s10456-020-09717-x
Tacconi C, He Y, Ducoli L, Detmar M (2020) Epigenetic regulation of the lineage specificity of primary human dermal lymphatic and blood vascular endothelial cells. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09743-9
Szulcek R, Sanchez-Duffhues G, Rol N, Pan X, Tsonaka R, Dickhoff C, Yung LM, Manz XD, Kurakula K, Kiełbasa SM, Mei H, Timens W, Yu PB, Bogaard HJ, Goumans MJ (2020) Exacerbated inflammatory signaling underlies aberrant response to BMP9 in pulmonary arterial hypertension lung endothelial cells. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09741-x
Szaraz P, Mander P, Gasner N, Librach M, Iqbal F, Librach C (2020) Glucose withdrawal induces Endothelin 1 release with significant angiogenic effect from first trimester (FTM), but not term human umbilical cord perivascular cells (HUCPVC). Angiogenesis 23(2):131–144. doi:https://doi.org/10.1007/s10456-019-09682-0
Smadja DM, Guerin CL, Chocron R, Yatim N, Boussier J, Gendron N, Khider L, Hadjadj J, Goudot G, Debuc B, Juvin P, Hauw-Berlemont C, Augy JL, Peron N, Messas E, Planquette B, Sanchez O, Charbit B, Gaussem P, Duffy D, Terrier B, Mirault T, Diehl JL (2020) Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. https://doi.org/10.1007/s10456-020-09730-0
Wang X, Zhang JQ, Xiu CK, Yang J, Fang JY, Lei Y (2020) Ginseng-Sanqi-Chuanxiong (GSC) extracts ameliorate diabetes-induced endothelial cell senescence through regulating mitophagy via the AMPK pathway. Oxid Med Cell Longev 2020:7151946. https://doi.org/10.1155/2020/7151946
Jin H, Zhu Y, Li Y, Ding X, Ma W, Han X, Wang B (2019) BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis 24(5–6):511–528. doi:https://doi.org/10.1007/s10495-019-01535-x
Yoshibayashi M, Kume S, Yasuda-Yamahara M, Yamahara K, Takeda N, Osawa N, Chin-Kanasaki M, Nakae Y, Yokoi H, Mukoyama M, Asanuma K, Maegawa H, Araki SI (2020) Protective role of podocyte autophagy against glomerular endothelial dysfunction in diabetes. Biochem Biophys Res Commun 525(2):319–325. doi:https://doi.org/10.1016/j.bbrc.2020.02.088
Vaeyens MM, Jorge-Peñas A, Barrasa-Fano J, Steuwe C, Heck T, Carmeliet P, Roeffaers M, Van Oosterwyck H (2020) Matrix deformations around angiogenic sprouts correlate to sprout dynamics and suggest pulling activity. Angiogenesis 23(3):315–324. doi:https://doi.org/10.1007/s10456-020-09708-y
Zhu W, Yuan Y, Liao G, Li L, Liu J, Chen Y, Zhang J, Cheng J, Lu Y (2018) Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis 9(8):837. doi:https://doi.org/10.1038/s41419-018-0861-x
Hill JH, Chen Z, Xu H (2014) Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant. Nat Genet 46(4):389–392. doi:https://doi.org/10.1038/ng.2920
Fournier P, Viallard C, Dejda A, Sapieha P, Larrivée B, Royal I (2020) The protein tyrosine phosphatase PTPRJ/DEP-1 contributes to the regulation of the Notch-signaling pathway and sprouting angiogenesis. Angiogenesis 23(2):145–157. doi:https://doi.org/10.1007/s10456-019-09683-z
Domingues A, Boisson-Vidal C, Marquet de Rouge P, Dizier B, Sadoine J, Mignon V, Vessières E, Henrion D, Escriou V, Bigey P, Chaussain C, Smadja DM, Nivet-Antoine V (2020) Targeting endothelial thioredoxin-interacting protein (TXNIP) protects from metabolic disorder-related impairment of vascular function and post-ischemic revascularisation. Angiogenesis 23(2):249–264. doi:https://doi.org/10.1007/s10456-019-09704-x
Dieterich LC, Tacconi C, Menzi F, Proulx ST, Kapaklikaya K, Hamada M, Takahashi S, Detmar M (2020) Lymphatic MAFB regulates vascular patterning during developmental and pathological lymphangiogenesis. Angiogenesis 23(3):411–423. doi:https://doi.org/10.1007/s10456-020-09721-1
di Somma M, Vliora M, Grillo E, Castro B, Dakou E, Schaafsma W, Vanparijs J, Corsini M, Ravelli C, Sakellariou E, Mitola S (2020) Role of VEGFs in metabolic disorders. Angiogenesis 23(2):119–130. doi:https://doi.org/10.1007/s10456-019-09700-1
Depoix CL, Colson A, Hubinont C, Debieve F (2020) Impaired vascular endothelial growth factor expression and secretion during in vitro differentiation of human primary term cytotrophoblasts. Angiogenesis 23(2):221–230. doi:https://doi.org/10.1007/s10456-019-09702-z
Chen J, Lin FL, Leung JYK, Tu L, Wang JH, Chuang YF, Li F, Shen HH, Dusting GJ, Wong VHY, Lisowski L, Hewitt AW, Bui BV, Zhong J, Liu GS (2020) A drug-tunable Flt23k gene therapy for controlled intervention in retinal neovascularization. Angiogenesis. doi:https://doi.org/10.1007/s10456-020-09745-7
Capasso TL, Li B, Volek HJ, Khalid W, Rochon ER, Anbalagan A, Herdman C, Yost HJ, Villanueva FS, Kim K, Roman BL (2020) BMP10-mediated ALK1 signaling is continuously required for vascular development and maintenance. Angiogenesis 23(2):203–220. doi:https://doi.org/10.1007/s10456-019-09701-0
Wallace DC (2008) Mitochondria as chi. Genetics 179(2):727–735. doi:https://doi.org/10.1534/genetics.104.91769
Fox TD (2012) Mitochondrial protein synthesis, import, and assembly. Genetics 192(4):1203–1234. doi:https://doi.org/10.1534/genetics.112.141267
Handschin C, Spiegelman BM (2006) Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27(7):728–735. doi:https://doi.org/10.1210/er.2006-0037
Fan W, Evans R (2015) PPARs and ERRs: molecular mediators of mitochondrial metabolism. Curr Opin Cell Biol 33:49–54. doi:https://doi.org/10.1016/j.ceb.2014.11.002
Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813(7):1269–1278. doi:https://doi.org/10.1016/j.bbamcr.2010.09.019
Huo L, Scarpulla RC (2001) Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol Cell Biol 21(2):644–654. doi:https://doi.org/10.1128/MCB.21.2.644-654.2001
Ristevski S, O’Leary DA, Thornell AP, Owen MJ, Kola I, Hertzog PJ (2004) The ETS transcription factor GABPalpha is essential for early embryogenesis. Mol Cell Biol 24(13):5844–5849. doi:https://doi.org/10.1128/MCB.24.13.5844-5849.2004
Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ (1998) Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem 273(10):5678–5684. doi:https://doi.org/10.1074/jbc.273.10.5678
Leone TC, Weinheimer CJ, Kelly DP (1999) A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A 96(13):7473–7478. doi:https://doi.org/10.1073/pnas.96.13.7473
Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP (2002) The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109(1):121–130. doi:https://doi.org/10.1172/JCI14080
Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, Subbiah R, Chatham J, Zhelyabovska O, Yang Q (2010) Peroxisome proliferator-activated receptor {delta} is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res 106(5):911–919. doi:https://doi.org/10.1161/CIRCRESAHA.109.206185
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP (2007) Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117(12):3930–3939. doi:https://doi.org/10.1172/JCI32578
Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM (2005) Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res 97(4):372–379. doi:https://doi.org/10.1161/01.RES.0000179226.34112.6d
Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ (2007) Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest 117(10):2791–2801. doi:https://doi.org/10.1172/JCI30335
Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP (2007) The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6(1):25–37. doi:https://doi.org/10.1016/j.cmet.2007.06.005
Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM (2007) ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6(1):13–24. doi:https://doi.org/10.1016/j.cmet.2007.06.007
Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM (2011) Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metab 13(3):283–293. doi:https://doi.org/10.1016/j.cmet.2011.01.019
Blattler SM, Verdeguer F, Liesa M, Cunningham JT, Vogel RO, Chim H, Liu H, Romanino K, Shirihai OS, Vazquez F, Ruegg MA, Shi Y, Puigserver P (2012) Defective mitochondrial morphology and bioenergetic function in mice lacking the transcription factor Yin Yang 1 in skeletal muscle. Mol Cell Biol 32(16):3333–3346. doi:https://doi.org/10.1128/MCB.00337-12
Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, Wang Y, Jin ES, Jeffrey FM, Portman M, Maclellan WR (2010) Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. J Clin Invest 120(5):1494–1505. doi:https://doi.org/10.1172/JCI38331
Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP (2014) A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res 114(4):626–636. doi:https://doi.org/10.1161/CIRCRESAHA.114.302562
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106(7):847–856. doi:https://doi.org/10.1172/JCI10268
Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM (2006) Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A 103(26):10086–10091. doi:https://doi.org/10.1073/pnas.0603615103
Chen W, Zhang X, Birsoy K, Roeder RG (2010) A muscle-specific knockout implicates nuclear receptor coactivator MED1 in the regulation of glucose and energy metabolism. Proc Natl Acad Sci U S A 107(22):10196–10201. doi:https://doi.org/10.1073/pnas.1005626107
Neupert W, Herrmann JM (2007) Translocation of proteins into mitochondria. Annu Rev Biochem 76:723–749. doi:https://doi.org/10.1146/annurev.biochem.76.052705.163409
Schmidt O, Pfanner N, Meisinger C (2010) Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol 11(9):655–667. doi:https://doi.org/10.1038/nrm2959
Saitoh T, Igura M, Obita T, Ose T, Kojima R, Maenaka K, Endo T, Kohda D (2007) Tom20 recognizes mitochondrial presequences through dynamic equilibrium among multiple bound states. Embo J 26(22):4777–4787. doi:https://doi.org/10.1038/sj.emboj.7601888
Zhang F, Zhang L, Qi Y, Xu H (2016) Mitochondrial cAMP signaling. Cellular and molecular life sciences. CMLS 73(24):4577–4590. doi:https://doi.org/10.1007/s00018-016-2282-2
Zhang F, Qi Y, Zhou K, Zhang G, Linask K, Xu H (2015) The cAMP phosphodiesterase Prune localizes to the mitochondrial matrix and promotes mtDNA replication by stabilizing TFAM. EMBO Rep 16(4):520–527. doi:https://doi.org/10.15252/embr.201439636
Vasseur A, Cabel L, Tredan O, Chevrier M, Dubot C, Lorgis V, Jacot W, Goncalves A, Debled M, Levy C, Ferrero JM, Jouannaud C, Luporsi E, Mouret-Reynier MA, Dalenc F, Lemonnier J, Savignoni A, Tanguy ML, Bidard FC, Pierga JY (2020) Prognostic value of CEC count in HER2-negative metastatic breast cancer patients treated with bevacizumab and chemotherapy: a prospective validation study (UCBG COMET). Angiogenesis 23(2):193–202. doi:https://doi.org/10.1007/s10456-019-09697-7
Santos JM, Tewari S, Kowluru RA (2012) A compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free Radic Biol Med 53(9):1729–1737. doi:https://doi.org/10.1016/j.freeradbiomed.2012.08.588
Santos JM, Kowluru RA (2011) Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Investig Ophthalmol Vis Sci 52(12):8791–8798. doi:https://doi.org/10.1167/iovs.11-8203
Santos JM, Tewari S, Goldberg AF, Kowluru RA (2011) Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radic Biol Med 51(10):1849–1860. doi:https://doi.org/10.1016/j.freeradbiomed.2011.08.017
Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E (2006) Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55(1):120–127
Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z (2009) Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297(1):H13–20. doi:https://doi.org/10.1152/ajpheart.00368.2009
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z (2019) Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol 24:101192. doi:https://doi.org/10.1016/j.redox.2019.101192
Wang H, Ramshekar A, Kunz E, Sacks DB, Hartnett ME (2020) IQGAP1 causes choroidal neovascularization by sustaining VEGFR2-mediated Rac1 activation. Angiogenesis. https://doi.org/10.1007/s10456-020-09740-y
Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A (2009) Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297(5):H1876–1881. doi:https://doi.org/10.1152/ajpheart.00375.2009
Santos JM, Mishra M, Kowluru RA (2014) Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: implications in diabetic retinopathy and metabolic memory phenomenon. Exp Eye Res 121:168–177. doi:https://doi.org/10.1016/j.exer.2014.02.010
Mishra M, Kowluru RA (2014) Retinal mitochondrial DNA mismatch repair in the development of diabetic retinopathy, and its continued progression after termination of hyperglycemia. Investig Ophthalmol Vis Sci 55(10):6960–6967. doi:https://doi.org/10.1167/iovs.14-15020
Yue P, Jing S, Liu L, Ma F, Zhang Y, Wang C, Duan H, Zhou K, Hua Y, Wu G, Li Y (2018) Association between mitochondrial DNA copy number and cardiovascular disease: Current evidence based on a systematic review and meta-analysis. PLoS ONE 13(11):e0206003. doi:https://doi.org/10.1371/journal.pone.0206003
Ait-Aissa K, Kadlec AO, Hockenberry J, Gutterman DD, Beyer AM (2018) Telomerase reverse transcriptase protects against angiotensin II-induced microvascular endothelial dysfunction. Am J Physiol Heart Circ Physiol 314(5):H1053–h1060. doi:https://doi.org/10.1152/ajpheart.00472.2017
Kajihara N, Kukidome D, Sada K, Motoshima H, Furukawa N, Matsumura T, Nishikawa T, Araki E (2017) Low glucose induces mitochondrial reactive oxygen species via fatty acid oxidation in bovine aortic endothelial cells. J Diabetes Invest 8(6):750–761. doi:https://doi.org/10.1111/jdi.12678
Xie X, Chowdhury SR, Sangle G, Shen GX (2010) Impact of diabetes-associated lipoproteins on oxygen consumption and mitochondrial enzymes in porcine aortic endothelial cells. Acta Biochim Pol 57(4):393–398
Sangle GV, Chowdhury SK, Xie X, Stelmack GL, Halayko AJ, Shen GX (2010) Impairment of mitochondrial respiratory chain activity in aortic endothelial cells induced by glycated low-density lipoprotein. Free Radic Biol Med 48(6):781–790. doi:https://doi.org/10.1016/j.freeradbiomed.2009.12.017
Author information
Authors and Affiliations
Contributions
HZ, HZ and XC collected and analyzed all literatures. XC and HZ wrote and revised the original draft. AL, HHW, SW and JR contributed to discussion and revisions of the manuscript. All authors approved the submission of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, D., Wang, J., Toan, S. et al. Molecular mechanisms of coronary microvascular endothelial dysfunction in diabetes mellitus: focus on mitochondrial quality surveillance. Angiogenesis 25, 307–329 (2022). https://doi.org/10.1007/s10456-022-09835-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-022-09835-8