Abstract
Vessel co-option is a non-angiogenic mechanism of tumour vascularisation in which cancer cells utilise pre-existing blood vessels instead of inducing new blood vessel formation. Vessel co-option has been observed across a range of different tumour types, in both primary cancers and metastatic disease. Importantly, vessel co-option is now implicated as a major mechanism that mediates resistance to conventional anti-angiogenic drugs and this may help to explain the limited efficacy of this therapeutic approach in certain clinical settings. This includes the use of anti-angiogenic drugs to treat advanced-stage/metastatic disease, treatment in the adjuvant setting and the treatment of primary disease. In this article, we review the available evidence linking vessel co-option with resistance to anti-angiogenic therapy in numerous tumour types, including breast, colorectal, lung and pancreatic cancer, glioblastoma, melanoma, hepatocellular carcinoma, and renal cell carcinoma. The finding that vessel co-option is a significant mechanism of resistance to anti-angiogenic therapy may have important implications for the future of anti-cancer therapy, including (a) predicting response to anti-angiogenic drugs, (b) the need to develop therapies that target both angiogenesis and vessel co-option in tumours, and (c) predicting the response to other therapeutic modalities, including immunotherapy.

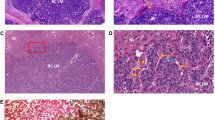
These images were first published in Bridgeman et al. [114] and are reproduced here with the permission of the copyright holder. Scale bar = 50 μm

This figure is adapted from Kuczynski et al. [10] with the permission of the copyright holder

This figure is adapted from Kuczynski et al. [10] with the permission of the copyright holder

This figure is adapted from Kuczynski et al. [10] with the permission of the copyright holder
Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. https://doi.org/10.1056/NEJM197111182852108
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400. https://doi.org/10.1038/nrd1381
Gerber HP, Ferrara N (2005) Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 65(3):671–680
Kerbel R, Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2(10):727–739. https://doi.org/10.1038/nrc905
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307. https://doi.org/10.1038/nature10144
Vasudev NS, Reynolds AR (2014) Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis 17(3):471–494. https://doi.org/10.1007/s10456-014-9420-y
Ebos JM, Kerbel RS (2011) Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8(4):210–221. https://doi.org/10.1038/nrclinonc.2011.21
Jayson GC, Kerbel R, Ellis LM, Harris AL (2016) Antiangiogenic therapy in oncology: current status and future directions. Lancet 388(10043):518–529. https://doi.org/10.1016/S0140-6736(15)01088-0
Roviello G, Bachelot T, Hudis CA, Curigliano G, Reynolds AR, Petrioli R, Generali D (2017) The role of bevacizumab in solid tumours: a literature based meta-analysis of randomised trials. Eur J Cancer 75:245–258. https://doi.org/10.1016/j.ejca.2017.01.026
Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR (2019) Vessel co-option in cancer. Nat Rev Clin Oncol 16(8):469–493. https://doi.org/10.1038/s41571-019-0181-9
Porta C, Cosmai L, Leibovich BC, Powles T, Gallieni M, Bex A (2019) The adjuvant treatment of kidney cancer: a multidisciplinary outlook. Nat Rev Nephrol 15(7):423–433. https://doi.org/10.1038/s41581-019-0131-x
Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8(8):592–603. https://doi.org/10.1038/nrc2442
Sennino B, McDonald DM (2012) Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 12(10):699–709. https://doi.org/10.1038/nrc3366
van Beijnum JR, Nowak-Sliwinska P, Huijbers EJ, Thijssen VL, Griffioen AW (2015) The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev 67(2):441–461. https://doi.org/10.1124/pr.114.010215
Leenders WP, Küsters B, de Waal RM (2002) Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium 9(2):83–87
Dome B, Hendrix MJC, Paku S, Tovari J, Timar J (2007) Alternative vascularization mechanisms in cancer—pathology and therapeutic implications. Am J Pathol 170(1):1–15
Donnem T, Reynolds AR, Kuczynski EA, Gatter K, Vermeulen PB, Kerbel RS, Harris AL, Pezzella F (2018) Non-angiogenic tumours and their influence on cancer biology. Nat Rev Cancer 18(5):323–336. https://doi.org/10.1038/nrc.2018.14
Donnem T, Hu J, Ferguson M, Adighibe O, Snell C, Harris AL, Gatter KC, Pezzella F (2013) Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med 2(4):427–436. https://doi.org/10.1002/cam4.105
Winkler F (2017) Hostile takeover: how tumours hijack pre-existing vascular environments to thrive. J Pathol 242(3):267–272. https://doi.org/10.1002/path.4904
Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, Leenders WP, van Furth WR (2009) Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer 9:444. https://doi.org/10.1186/1471-2407-9-444
Gilbert MR (2016) Antiangiogenic therapy for glioblastoma: complex biology and complicated results. J Clin Oncol 34(14):1567–1569. https://doi.org/10.1200/jco.2016.66.5364
Gerstner ER, Duda DG, di Tomaso E, Ryg PA, Loeffler JS, Sorensen AG, Ivy P, Jain RK, Batchelor TT (2009) VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol 6(4):229–236. https://doi.org/10.1038/nrclinonc.2009.14
Khasraw M, Ameratunga M, Grommes C (2014) Bevacizumab for the treatment of high-grade glioma: an update after phase III trials. Expert Opinion Biol Ther 14(5):729–740. https://doi.org/10.1517/14712598.2014.898060
Zhuang H, Shi S, Yuan Z, Chang JY (2019) Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer 18(1):21. https://doi.org/10.1186/s12943-019-0950-1
Berghoff AS, Preusser M (2018) Anti-angiogenic therapies in brain metastases. Memo 11(1):14–17. https://doi.org/10.1007/s12254-018-0384-2
Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284(5422):1994–1998. https://doi.org/10.1126/science.284.5422.1994
Baker GJ, Yadav VN, Motsch S, Koschmann C, Calinescu AA, Mineharu Y, Camelo-Piragua SI, Orringer D, Bannykh S, Nichols WS, deCarvalho AC, Mikkelsen T, Castro MG, Lowenstein PR (2014) Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia 16(7):543–561. https://doi.org/10.1016/j.neo.2014.06.003
Sakariassen PO, Prestegarden L, Wang J, Skaftnesmo KO, Mahesparan R, Molthoff C, Sminia P, Sundlisaeter E, Misra A, Tysnes BB, Chekenya M, Peters H, Lende G, Kalland KH, Oyan AM, Petersen K, Jonassen I, van der Kogel A, Feuerstein BG, Terzis AJ, Bjerkvig R, Enger PO (2006) Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci USA 103(44):16466–16471. https://doi.org/10.1073/pnas.0607668103
Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H (2014) Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 5:4196. https://doi.org/10.1038/ncomms5196
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16(1):116–122. https://doi.org/10.1038/nm.2072
Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, Peault B, Barnhill RL, Lugassy C (2016) Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci Rep 6:23834. https://doi.org/10.1038/srep23834
Kusters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ, Peters JP, van Der Kogel AJ, de Waal RM (2002) Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res 62(2):341–345
Dome B, Timar J, Paku S (2003) A novel concept of glomeruloid body formation in experimental cerebral metastases. J Neuropathol Exp Neurol 62(6):655–661. https://doi.org/10.1093/jnen/62.6.655
Lugassy C, Haroun RI, Brem H, Tyler BM, Jones RV, Fernandez PM, Patierno SR, Kleinman HK, Barnhill RL (2002) Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol 24(6):473–478. https://doi.org/10.1007/s12307-014-0156-4
Bugyik E, Dezso K, Reiniger L, Laszlo V, Tovari J, Timar J, Nagy P, Klepetko W, Dome B, Paku S (2011) Lack of angiogenesis in experimental brain metastases. J Neuropathol Exp Neurol 70(11):979–991. https://doi.org/10.1097/NEN.0b013e318233afd7
Simonsen TG, Gaustad JV, Rofstad EK (2015) Intertumor heterogeneity in vascularity and invasiveness of artificial melanoma brain metastases. J Exp Clin Cancer Res 34(1):150. https://doi.org/10.1186/s13046-015-0264-0
Wesseling P, van der Laak JA, de Leeuw H, Ruiter DJ, Burger PC (1994) Quantitative immunohistological analysis of the microvasculature in untreated human glioblastoma multiforme. Computer-assisted image analysis of whole-tumor sections. J Neurosurg 81(6):902–909. https://doi.org/10.3171/jns.1994.81.6.0902
Bernsen H, Van der Laak J, Kusters B, Van der Ven A, Wesseling P (2005) Gliomatosis cerebri: quantitative proof of vessel recruitment by cooptation instead of angiogenesis. J Neurosurg 103(4):702–706. https://doi.org/10.3171/jns.2005.103.4.0702
Claes A, Idema AJ, Wesseling P (2007) Diffuse glioma growth: a guerilla war. Acta Neuropathol 114(5):443–458. https://doi.org/10.1007/s00401-007-0293-7
Scherer HJ (1938) Structural development in gliomas. Am J Cancer 34(3):333–351
Siam L, Bleckmann A, Chaung HN, Mohr A, Klemm F, Barrantes-Freer A, Blazquez R, Wolff HA, Luke F, Rohde V, Stadelmann C, Pukrop T (2015) The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget 6(30):29254–29267. https://doi.org/10.18632/oncotarget.4201
Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA, Goodman SL, Weller M, Schittenhelm J, Preusser M (2013) Invasion patterns in brain metastases of solid cancers. Neuro-oncology 15(12):1664–1672. https://doi.org/10.1093/neuonc/not112
Carbonell WS, Ansorge O, Sibson N, Muschel R (2009) The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4(6):e5857. https://doi.org/10.1371/journal.pone.0005857
Rodewald AK, Rushing EJ, Kirschenbaum D, Mangana J, Mittmann C, Moch H, Lugassy C, Barnhill RL, Mihic-Probst D (2019) Eight autopsy cases of melanoma brain metastases showing angiotropism and pericytic mimicry. Implications for extravascular migratory metastasis. J Cutan Pathol 46(8):570–578. https://doi.org/10.1111/cup.13465
Montana V, Sontheimer H (2011) Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci 31(13):4858–4867. https://doi.org/10.1523/JNEUROSCI.3825-10.2011
Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, Krishnan S, Lindberg OR, Yuen TJ, Tien AC, Sabo JK, Wang N, Chen I, Kloepper J, Larrouquere L, Ghosh M, Tirosh I, Huillard E, Alvarez-Buylla A, Oldham MC, Persson AI, Weiss WA, Batchelor TT, Stemmer-Rachamimov A, Suva ML, Phillips JJ, Aghi MK, Mehta S, Jain RK, Rowitch DH (2018) A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 33(5):874-889.e877. https://doi.org/10.1016/j.ccell.2018.03.020
Yadav VN, Zamler D, Baker GJ, Kadiyala P, Erdreich-Epstein A, DeCarvalho AC, Mikkelsen T, Castro MG, Lowenstein PR (2016) CXCR4 increases in vivo glioma perivascular invasion, and reduces radiation induced apoptosis: a genetic knockdown study. Oncotarget 7(50):83701–83719. https://doi.org/10.18632/oncotarget.13295
Voutouri C, Kirkpatrick ND, Chung E, Mpekris F, Baish JW, Munn LL, Fukumura D, Stylianopoulos T, Jain RK (2019) Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc Natl Acad Sci USA 116(7):2662–2671. https://doi.org/10.1073/pnas.1818322116
Jabouille A, Delugin M, Pineau R, Dubrac A, Soulet F, Lhomond S, Pallares-Lupon N, Prats H, Bikfalvi A, Chevet E, Touriol C, Moenner M (2015) Glioblastoma invasion and cooption depend on IRE1alpha endoribonuclease activity. Oncotarget 6(28):24922–24934. https://doi.org/10.18632/oncotarget.4679
Otani Y, Ichikawa T, Kurozumi K, Inoue S, Ishida J, Oka T, Shimizu T, Tomita Y, Hattori Y, Uneda A, Matsumoto Y, Michiue H, Date I (2018) Fibroblast growth factor 13 regulates glioma cell invasion and is important for bevacizumab-induced glioma invasion. Oncogene 37(6):777–786. https://doi.org/10.1038/onc.2017.373
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156(5):1002–1016. https://doi.org/10.1016/j.cell.2014.01.040
Caspani EM, Crossley PH, Redondo-Garcia C, Martinez S (2014) Glioblastoma: a pathogenic crosstalk between tumor cells and pericytes. PLoS ONE 9(7):e101402. https://doi.org/10.1371/journal.pone.0101402
Teglasi V, Csury DT, Dezso K, Bugyik E, Szabo V, Szallasi Z, Paku S, Reiniger L (2019) Origin and distribution of connective tissue and pericytes impacting vascularization in brain metastases with different growth patterns. J Neuropathol Exp Neurol 78(4):326–339. https://doi.org/10.1093/jnen/nlz007
Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fillbrandt R, Stavrou D, Westphal M, Lamszus K (2001) Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res 61(18):6624–6628
Paez-Ribes M, Allen E, Hudock J, Takeda J, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15(3):220–231. https://doi.org/10.1016/j.ccr.2009.01.027
Lucio-Eterovic AK, Piao Y, de Groot JF (2009) Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 15(14):4589–4599. https://doi.org/10.1158/1078-0432.CCR-09-0575
de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA (2010) Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-oncology 12(3):233–242. https://doi.org/10.1093/neuonc/nop027
Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, Miletic H, Wang J, Stieber D, Stuhr L, Moen I, Rygh CB, Bjerkvig R, Niclou SP (2011) Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA 108(9):3749–3754. https://doi.org/10.1073/pnas.1014480108
Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, Park M, Bergers G (2012) VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22(1):21–35. https://doi.org/10.1016/j.ccr.2012.05.037
Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, Shuman MA (2000) Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2(4):306–314. https://doi.org/10.1038/sj.neo.7900102
Navis AC, Bourgonje A, Wesseling P, Wright A, Hendriks W, Verrijp K, van der Laak JA, Heerschap A, Leenders WP (2013) Effects of dual targeting of tumor cells and stroma in human glioblastoma xenografts with a tyrosine kinase inhibitor against c-MET and VEGFR2. PLoS ONE 8(3):e58262. https://doi.org/10.1371/journal.pone.0058262
Leenders WP, Kusters B, Verrijp K, Maass C, Wesseling P, Heerschap A, Ruiter D, Ryan A, de Waal R (2004) Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res 10(18 Pt 1):6222–6230. https://doi.org/10.1158/1078-0432.CCR-04-0823
Falchetti ML, D’Alessandris QG, Pacioni S, Buccarelli M, Morgante L, Giannetti S, Lulli V, Martini M, Larocca LM, Vakana E, Stancato L, Ricci-Vitiani L, Pallini R (2019) Glioblastoma endothelium drives bevacizumab-induced infiltrative growth via modulation of PLXDC1. Int J Cancer 144(6):1331–1344. https://doi.org/10.1002/ijc.31983
Gomez-Manzano C, Holash J, Fueyo J, Xu J, Conrad CA, Aldape KD, de Groot JF, Bekele BN, Yung WK (2008) VEGF Trap induces antiglioma effect at different stages of disease. Neuro-oncology 10(6):940–945. https://doi.org/10.1215/15228517-2008-061
Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C, Galanis E, Sarkaria JN, Anastasiadis PZ (2013) Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS ONE 8(2):e56505. https://doi.org/10.1371/journal.pone.0056505
di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, Hedley-Whyte ET, Sorensen AG, Batchelor TT, Jain RK (2011) Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res 71(1):19–28. https://doi.org/10.1158/0008-5472.CAN-10-2602
Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70(10):779–787. https://doi.org/10.1212/01.wnl.0000304121.57857.38
Kleinschmidt-DeMasters BK, Damek DM (2010) The imaging and neuropathological effects of Bevacizumab (Avastin) in patients with leptomeningeal carcinomatosis. J Neurooncol 96(3):375–384. https://doi.org/10.1007/s11060-009-9969-2
Mandelcorn ED, Palestine AG, Dubovy S, Davis JL (2010) Vascular co-option in lung cancer metastatic to the eye after treatment with bevacizumab. J Ophthalmic Inflamm Infect 1(1):35–38. https://doi.org/10.1007/s12348-010-0013-7
Hardian RF, Goto T, Kuwabara H, Hanaoka Y, Kobayashi S, Kanno H, Shimojo H, Horiuchi T, Hongo K (2019) An autopsy case of widespread brain dissemination of glioblastoma unnoticed by magnetic resonance imaging after treatment with bevacizumab. Surg Neurol Int 10:137. https://doi.org/10.25259/sni-183-2019
Wick W, Wick A, Weiler M, Weller M (2011) Patterns of progression in malignant glioma following anti-VEGF therapy: perceptions and evidence. Curr Neurol Neurosci Rep 11(3):305–312. https://doi.org/10.1007/s11910-011-0184-0
Nowosielski M, Ellingson BM, Chinot OL, Garcia J, Revil C, Radbruch A, Nishikawa R, Mason WP, Henriksson R, Saran F, Kickingereder P, Platten M, Sandmann T, Abrey LE, Cloughesy TF, Bendszus M, Wick W (2018) Radiologic progression of glioblastoma under therapy-an exploratory analysis of AVAglio. Neuro-oncology 20(4):557–566. https://doi.org/10.1093/neuonc/nox162
Chamberlain MC (2011) Radiographic patterns of relapse in glioblastoma. J Neurooncol 101(2):319–323. https://doi.org/10.1007/s11060-010-0251-4
Lovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. https://doi.org/10.1056/NEJMoa0708857
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66. https://doi.org/10.1016/s0140-6736(16)32453-9
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M, Investigators R-s (2019) Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(2):282–296. https://doi.org/10.1016/S1470-2045(18)30937-9
Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klumpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379(1):54–63. https://doi.org/10.1056/NEJMoa1717002
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth H, Helm W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342. https://doi.org/10.1056/NEJMoa032691
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312. https://doi.org/10.1016/s0140-6736(12)61900-x
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30(28):3499–3506. https://doi.org/10.1200/JCO.2012.42.8201
Khan K, Cunningham D, Chau I (2017) Targeting angiogenic pathways in colorectal cancer: complexities, challenges and future directions. Curr Drug Targ 18(1):56–71. https://doi.org/10.2174/1389450116666150325231555
Llovet JM, Montal R, Sia D, Finn RS (2018) Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 15(10):599–616. https://doi.org/10.1038/s41571-018-0073-4
Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N et al (1987) Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 60(4):810–819. https://doi.org/10.1002/1097-0142(19870815)60%3A4%3C810%3A%3AAID-CNCR2820600417%3E3.0.CO%3B2-1
Sugihara S, Kojiro M, Nakashima T (1985) Ultrastructural study of hepatocellular carcinoma with replacing growth pattern. Acta Pathol Jpn 35(3):549–559. https://doi.org/10.1111/j.1440-1827.1985.tb00597.x
Nakashima T, Kojiro M, Kawano Y, Shirai F, Takemoto N, Tomimatsu Y, Kawasaki H, Okuda K (1982) Histologic growth pattern of hepatocellular carcinoma: relationship to orcein (hepatitis B surface antigen)-positive cells in cancer tissue. Hum Pathol 13(6):563–568. https://doi.org/10.1016/S0046-8177(82)80272-4
Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY, Van Marck E (2001) Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol 195(3):336–342. https://doi.org/10.1002/path.966
Stessels F, Van den Eynden G, Van der Auwera I, Salgado R, Van den Heuvel E, Harris AL, Jackson DG, Colpaert CG, van Marck EA, Dirix LY, Vermeulen PB (2004) Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 90(7):1429–1436. https://doi.org/10.1038/sj.bjc.6601727
van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, Majeed AW, Eefsen RL, Coebergh van den Braak RRJ, Lazaris A, Fernandez MC, Galjart B, Laerum OD, Rayes R, Grunhagen DJ, Van de Paer M, Sucaet Y, Mudhar HS, Schvimer M, Nystrom H, Kockx M, Bird NC, Vidal-Vanaclocha F, Metrakos P, Simoneau E, Verhoef C, Dirix LY, Van Laere S, Gao ZH, Brodt P, Reynolds AR, Vermeulen PB (2017) International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer 117(10):1427–1441. https://doi.org/10.1038/bjc.2017.334
Terayama N, Terada T, Nakanuma Y (1996) Histologic growth patterns of metastatic carcinomas of the liver. Jpn J Clin Oncol 26(1):24–29. https://doi.org/10.1093/oxfordjournals.jjco.a023174
Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, Daley F, Vermeulen PB, Yousef GM, Foster FS, Reynolds AR, Kerbel RS (2016) Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. J Natl Cancer Inst 108(8):djw030. https://doi.org/10.1093/jnci/djw030
Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan MR, Wotherspoon A, Gao ZH, Shi Y, Van den Eynden G, Daley F, Peckitt C, Tan X, Salman A, Lazaris A, Gazinska P, Berg TJ, Eltahir Z, Ritsma L, van Rheenen J, Khashper A, Brown G, Nystrom H, Sund M, Van Laere S, Loyer E, Dirix L, Cunningham D, Metrakos P, Reynolds AR (2016) Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 22(11):1294–1302. https://doi.org/10.1038/nm.4197
Lazaris A, Amri A, Petrillo SK, Zoroquiain P, Ibrahim N, Salman A, Gao ZH, Vermeulen PB, Metrakos P (2018) Vascularization of colorectal carcinoma liver metastasis: insight into stratification of patients for anti-angiogenic therapies. J Pathol Clin Res 4(3):184–192. https://doi.org/10.1002/cjp2.100
Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N (2018) Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359(6378):920–926. https://doi.org/10.1126/science.aao2774
Held T, Verbeke CS, Strobel O, Rutkowski W, Villard C, Moro CF, Del Chiaro M, Buchler M, Heuchel R, Lohr M (2019) Immunohistochemical profiling of liver metastases and matched-pair analysis in patients with metastatic pancreatic ductal adenocarcinoma. Pancreatology. https://doi.org/10.1016/j.pan.2019.09.005
Barnhill R, Vermeulen P, Daelemans S, van Dam PJ, Roman-Roman S, Servois V, Hurbain I, Gardrat S, Raposa G, Nicolas A, Dendale R, Pierron G, Desjardins L, Cassoux N, Piperno-Neumann S, Mariani P, Lugassy C (2018) Replacement and desmoplastic histopathological growth patterns: a pilot study of prediction of outcome in patients with uveal melanoma liver metastases. J Pathol Clin Res 4(4):227–240. https://doi.org/10.1002/cjp2.105
Kozaka K, Sasaki M, Fujii T, Harada K, Zen Y, Sato Y, Sawada S, Minato H, Matsui O, Nakanuma Y (2007) A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: ‘bile ductular carcinoma’. Histopathology 51(3):390–400. https://doi.org/10.1111/j.1365-2559.2007.02735.x
Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 15(2):143–155. https://doi.org/10.1016/s1470-2045(13)70586-2
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. https://doi.org/10.1056/NEJMoa061884
Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Perol M (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384(9944):665–673. https://doi.org/10.1016/S0140-6736(14)60845-X
Alshangiti A, Chandhoke G, Ellis PM (2018) Antiangiogenic therapies in non-small-cell lung cancer. Curr Oncol 25(Suppl 1):S45–S58. https://doi.org/10.3747/co.25.3747
Janning M, Loges S (2018) Anti-angiogenics: their value in lung cancer therapy. Oncol Res Treat 41(4):172–180. https://doi.org/10.1159/000488119
Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M, Rilke F (1997) Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol 151(5):1417–1423
Passalidou E, Trivella M, Singh N, Ferguson M, Hu J, Cesario A, Granone P, Nicholson AG, Goldstraw P, Ratcliffe C, Tetlow M, Leigh I, Harris AL, Gatter KC, Pezzella F (2002) Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. Br J Cancer 86(2):244–249. https://doi.org/10.1038/sj.bjc.6600015
Yousem SA (2009) Peripheral squamous cell carcinoma of lung: patterns of growth with particular focus on airspace filling. Hum Pathol 40(6):861–867. https://doi.org/10.1016/j.humpath.2008.11.008
Adighibe O, Micklem K, Campo L, Ferguson M, Harris A, Pozos R, Gatter K, Pezzella F (2006) Is nonangiogenesis a novel pathway for cancer progression? A study using 3-dimensional tumour reconstructions. Br J Cancer 94(8):1176–1179. https://doi.org/10.1038/sj.bjc.6603039
Sardari Nia P, Colpaert C, Vermeulen P, Weyler J, Pezzella F, Van Schil P, Van Marck E (2008) Different growth patterns of non-small cell lung cancer represent distinct biologic subtypes. Ann Thorac Surg 85(2):395–405. https://doi.org/10.1016/j.athoracsur.2007.08.054
Offersen BV, Pfeiffer P, Hamilton-Dutoit S, Overgaard J (2001) Patterns of angiogenesis in nonsmall-cell lung carcinoma. Cancer 91(8):1500–1509
Adighibe O, Leek RD, Fernandez-Mercado M, Hu J, Snell C, Gatter KC, Harris AL, Pezzella F (2016) Why some tumours trigger neovascularisation and others don’t: the story thus far. Chin J Cancer 35:18. https://doi.org/10.1186/s40880-016-0082-6
Rosenblatt MB, Lisa JR, Collier F (1967) Primary and metastatic bronciolo-alveolar carcinoma. Dis Chest 52(2):147–152. https://doi.org/10.1378/chest.52.2.147
Pezzella F, Di Bacco A, Andreola S, Nicholson AG, Pastorino U, Harris AL (1996) Angiogenesis in primary lung cancer and lung secondaries. Eur J Cancer (Oxford, England: 1990) 32(14):2494–2500. https://doi.org/10.1016/S0959-8049(96)00377-2
Sardari Nia P, Hendriks J, Friedel G, Van Schil P, Van Marck E (2007) Distinct angiogenic and non-angiogenic growth patterns of lung metastases from renal cell carcinoma. Histopathology 51(3):354–361. https://doi.org/10.1111/j.1365-2559.2007.02800.x
Breast-Cancer-Progression-Working-Party, (2000) Evidence for novel non-angiogenic pathway in breast-cancer metastasis. Lancet 355(9217):1787–1788
Bridgeman VL, Vermeulen PB, Foo S, Bilecz A, Daley F, Kostaras E, Nathan MR, Wan E, Frentzas S, Schweiger T, Hegedus B, Hoetzenecker K, Renyi-Vamos F, Kuczynski EA, Vasudev NS, Larkin J, Gore M, Dvorak HF, Paku S, Kerbel RS, Dome B, Reynolds AR (2017) Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol 241(3):362–374. https://doi.org/10.1002/path.4845
Mizuuchi H, Suda K, Kitahara H, Shimamatsu S, Kohno M, Okamoto T, Maehara Y (2015) Solitary pulmonary metastasis from malignant melanoma of the bulbar conjunctiva presenting as a pulmonary ground glass nodule: report of a case. Thorac Cancer 6(1):97–100. https://doi.org/10.1111/1759-7714.12124
Szabo V, Bugyik E, Dezso K, Ecker N, Nagy P, Timar J, Tovari J, Laszlo V, Bridgeman VL, Wan E, Frentzas S, Vermeulen PB, Reynolds AR, Dome B, Paku S (2015) Mechanism of tumour vascularization in experimental lung metastases. J Pathol 235(3):384–396. https://doi.org/10.1002/path.4464
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23(4):792–799. https://doi.org/10.1200/jco.2005.05.098
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676. https://doi.org/10.1056/NEJMoa072113
Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol 28(20):3239–3247. https://doi.org/10.1200/jco.2008.21.6457
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29(10):1252–1260. https://doi.org/10.1200/jco.2010.28.0982
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29(32):4286–4293. https://doi.org/10.1200/jco.2010.34.1255
Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V (2013) AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol 31(14):1719–1725. https://doi.org/10.1200/jco.2012.44.7912
Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, Pivot X, Iwata H, Aogi K, Lugo-Quintana R, Harbeck N, Brickman MJ, Zhang K, Kern KA, Martin M (2010) Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 121(1):121–131. https://doi.org/10.1007/s10549-010-0788-0
Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, Makhson AN, Cortes J, Lortholary A, Bischoff J, Chan A, Delaloge S, Huang X, Kern KA, Giorgetti C (2012) First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol 30(9):921–929. https://doi.org/10.1200/jco.2011.35.7376
Crown JP, Diéras V, Staroslawska E, Yardley DA, Bachelot T, Davidson N, Wildiers H, Fasching PA, Capitain O, Ramos M, Greil R, Cognetti F, Fountzilas G, Blasinska-Morawiec M, Liedtke C, Kreienberg R Jr, Tassell V, Huang X, Paolini J, Kern KA, Romieu G (2013) Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol 31(23):2870–2878. https://doi.org/10.1200/jco.2012.43.3391
Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, Brufsky AM, Minton SE, Giguere JK, Smith JW 2nd, Richards PD, Gernhardt D, Huang X, Liau KF, Kern KA, Davis J (2011) Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer 11(2):82–92. https://doi.org/10.1016/j.clbc.2011.03.005
Kerbel RS (2011) Reappraising antiangiogenic therapy for breast cancer. Breast (Edinburgh, Scotland) 20(Suppl 3):S56–S60. https://doi.org/10.1016/s0960-9776(11)70295-8
Guerin E, Man S, Xu P, Kerbel RS (2013) A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res 73(9):2743–2748. https://doi.org/10.1158/0008-5472.CAN-12-4183
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15(3):232–239. https://doi.org/10.1016/j.ccr.2009.01.021
Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, Duda DG, Willett CG, Jain RK, Padera TP (2015) Investigation of the lack of angiogenesis in the formation of lymph node metastases. J Natl Cancer Inst 107(9):djv155. https://doi.org/10.1093/jnci/djv155
Colpaert CG, Vermeulen PB, Van Beest P, Soubry A, Goovaerts G, Dirix LY, Harris AL, Van Marck EA (2003) Cutaneous breast cancer deposits show distinct growth patterns with different degrees of angiogenesis, hypoxia and fibrin deposition. Histopathology 42(6):530–540. https://doi.org/10.1046/j.1365-2559.2003.01629.x
Vermeulen PB, Sardari Nia P, Colpaert C, Dirix LY, Van Marck E (2002) Lack of angiogenesis in lymph node metastases of carcinomas is growth pattern-dependent. Histopathology 40(1):105–107. https://doi.org/10.1046/j.1365-2559.2002.1340c.x
Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, Zarba JJ, Gladkov OA, Lee E, Szczylik C, McCann L, Rubin SD, Chen M, Davis ID (2013) A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer (Oxford, England: 1990) 49(6):1287–1296. https://doi.org/10.1016/j.ejca.2012.12.010
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939. https://doi.org/10.1016/S0140-6736(11)61613-9
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, Group TS (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134. https://doi.org/10.1056/NEJMoa060655
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124. https://doi.org/10.1056/NEJMoa065044
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Geczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ, Investigators M (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1814–1823. https://doi.org/10.1056/NEJMoa1510016
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26(33):5422–5428. https://doi.org/10.1200/JCO.2008.16.9847
Rini BI (2009) Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer 115(10 Suppl):2306–2312. https://doi.org/10.1002/cncr.24227
Rini BI, Atkins MB (2009) Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 10(10):992–1000. https://doi.org/10.1016/S1470-2045(09)70240-2
Vasudev NS, Goh V, Juttla JK, Thompson VL, Larkin JM, Gore M, Nathan PD, Reynolds AR (2013) Changes in tumour vessel density upon treatment with anti-angiogenic agents: relationship with response and resistance to therapy. Br J Cancer 109(5):1230–1242. https://doi.org/10.1038/bjc.2013.429
Qian CN (2013) Hijacking the vasculature in ccRCC–co-option, remodelling and angiogenesis. Nat Rev Urol 10(5):300–304. https://doi.org/10.1038/nrurol.2013.26
Fukatsu A, Tsuzuki T, Sassa N, Nishikimi T, Kimura T, Majima T, Yoshino Y, Hattori R, Gotoh M (2013) Growth pattern, an important pathologic prognostic parameter for clear cell renal cell carcinoma. Am J Clin Pathol 140(4):500–505. https://doi.org/10.1309/ajcpimpe6zft8ame
Ronny FM, Sarungbam J, Zhong X, Yusuf Y, Yang X, Zhong M (2014) Glomerular sparing pattern in primary kidney neoplasms: clinical, morphological and immunohistochemical study. Am J Clin Exp Urol 2(1):76–81
Welti JC, Gourlaouen M, Powles T, Kudahetti SC, Wilson P, Berney DM, Reynolds AR (2011) Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib. Oncogene 30(10):1183–1193. https://doi.org/10.1038/onc.2010.503
Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT (2010) Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70(3):1063–1071. https://doi.org/10.1158/0008-5472.CAN-09-3965
Hwang HS, Go H, Park JM, Yoon SY, Lee JL, Jeong SU, Cho YM (2019) Epithelial-mesenchymal transition as a mechanism of resistance to tyrosine kinase inhibitors in clear cell renal cell carcinoma. Lab Invest 99(5):659–670. https://doi.org/10.1038/s41374-019-0188-y
Mizumoto A, Yamamoto K, Nakayama Y, Takara K, Nakagawa T, Hirano T, Hirai M (2015) Induction of epithelial-mesenchymal transition via activation of epidermal growth factor receptor contributes to sunitinib resistance in human renal cell carcinoma cell lines. J Pharmacol Exp Ther 355(2):152–158. https://doi.org/10.1124/jpet.115.226639
Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, Ding Z, Tannir N, Wood CG, Matin SF, Karam JA, Tamboli P, Sircar K, Rao P, Rankin EB, Laird DA, Hoang AG, Walker CL, Giaccia AJ, Jonasch E (2016) Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 35(21):2687–2697. https://doi.org/10.1038/onc.2015.343
Zhai W, Li S, Zhang J, Chen Y, Ma J, Kong W, Gong D, Zheng J, Xue W, Xu Y (2018) Sunitinib-suppressed miR-452-5p facilitates renal cancer cell invasion and metastasis through modulating SMAD4/SMAD7 signals. Mol Cancer 17(1):157. https://doi.org/10.1186/s12943-018-0906-x
Butz H, Ding Q, Nofech-Mozes R, Lichner Z, Ni H, Yousef GM (2018) Elucidating mechanisms of sunitinib resistance in renal cancer: an integrated pathological-molecular analysis. Oncotarget 9(4):4661–4674. https://doi.org/10.18632/oncotarget.23163
Cooke VG, Lebleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21(1):66–81. https://doi.org/10.1016/j.ccr.2011.11.024
Blagoev KB, Wilkerson J, Stein WD, Motzer RJ, Bates SE, Fojo AT (2013) Sunitinib does not accelerate tumor growth in patients with metastatic renal cell carcinoma. Cell Rep 3(2):277–281. https://doi.org/10.1016/j.celrep.2013.01.015
Kats-Urgurlu G (2018) Pulmonary lymphangitis carcinomatosis of clear cell renal cell carcinoma after angiogenesis inhibition. Arch Surg Clin Case Rep. https://doi.org/10.29011/ASCR-107/100007
Chen Z, Zhang J, Zhang Z, Feng Z, Wei J, Lu J, Fang Y, Liang Y, Cen J, Pan Y, Huang Y, Zhou F, Chen W, Luo J (2017) The putative tumor suppressor microRNA-30a-5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Dis 8(6):e2859. https://doi.org/10.1038/cddis.2017.252
Henrion M, Frampton M, Scelo G, Purdue M, Ye Y, Broderick P, Ritchie A, Kaplan R, Meade A, McKay J, Johansson M, Lathrop M, Larkin J, Rothman N, Wang Z, Chow WH, Stevens VL, Ryan Diver W, Gapstur SM, Albanes D, Virtamo J, Wu X, Brennan P, Chanock S, Eisen T, Houlston RS (2013) Common variation at 2q22.3 (ZEB2) influences the risk of renal cancer. Hum Mol Genet 22(4):825–831. https://doi.org/10.1093/hmg/dds489
Fang Y, Wei J, Cao J, Zhao H, Liao B, Qiu S, Wang D, Luo J, Chen W (2013) Protein expression of ZEB2 in renal cell carcinoma and its prognostic significance in patient survival. PLoS ONE 8(5):e62558. https://doi.org/10.1371/journal.pone.0062558
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Horsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364(6):501–513. https://doi.org/10.1056/NEJMoa1003825
Casanovas O, Hicklin DJ, Bergers G, Hanahan D (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8(4):299–309. https://doi.org/10.1016/j.ccr.2005.09.005
Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, Atkins MB, Leming P, Kirkwood JM (2013) Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 31(3):373–379. https://doi.org/10.1200/jco.2012.42.1529
Lugassy C, Zadran S, Bentolila LA, Wadehra M, Prakash R, Carmichael ST, Kleinman HK, Peault B, Larue L, Barnhill RL (2014) Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron 7(3):139–152. https://doi.org/10.1007/s12307-014-0156-4
Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370(8):734–743. https://doi.org/10.1056/NEJMoa1309748
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, Group RS (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235. https://doi.org/10.1016/s1470-2045(14)70420-6
Schlumberger M, Elisei R, Muller S, Schoffski P, Brose M, Shah M, Licitra L, Krajewska J, Kreissl MC, Niederle B, Cohen EEW, Wirth L, Ali H, Clary DO, Yaron Y, Mangeshkar M, Ball D, Nelkin B, Sherman S (2017) Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol 28(11):2813–2819. https://doi.org/10.1093/annonc/mdx479
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX, Gynecologic Oncology G (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365(26):2473–2483. https://doi.org/10.1056/NEJMoa1104390
Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, Vogelzang NJ, Small EJ (2012) Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 30(13):1534–1540. https://doi.org/10.1200/jco.2011.39.4767
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28(22):3617–3622. https://doi.org/10.1200/jco.2010.28.1386
Bruix J, Takayama T, Mazzaferro V, Chau G-Y, Yang J, Kudo M, Cai J, Poon RT, Han K-H, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre M-AL, Meinhardt G, Llovet JM (2015) Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 16(13):1344–1354. https://doi.org/10.1016/S1470-2045(15)00198-9
Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, Chang Y-H, Escudier B, Donskov F, Magheli A, Carteni G, Laguerre B, Tomczak P, Breza J, Gerletti P, Lechuga M, Lin X, Martini J-F, Ramaswamy K, Casey M, Staehler M, Patard J-J (2016) Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 375(23):2246–2254. https://doi.org/10.1056/NEJMoa1611406
Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, Jewett M, Dutcher JP, Atkins MB, Pins M, Wilding G, Cella D, Wagner L, Matin S, Kuzel TM, Sexton WJ, Wong YN, Choueiri TK, Pili R, Puzanov I, Kohli M, Stadler W, Carducci M, Coomes R, DiPaola RS (2016) Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387(10032):2008–2016. https://doi.org/10.1016/s0140-6736(16)00559-6
Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Lopa SH, Wolmark N (2013) Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 31(3):359–364. https://doi.org/10.1200/jco.2012.44.4711
de Gramont A, Van Cutsem E, Schmoll H-J, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im S-A, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, André T, Hoff PM (2012) Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 13(12):1225–1233. https://doi.org/10.1016/S1470-2045(12)70509-0
Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, Lee J-L, Melichar B, Rini BI, Choueiri TK, Zemanova M, Wood LA, Fahlenkamp D, Reaume MN, Stenzl A, Bao W, Aimone P, Doehn C, Russo P, Sternberg CN (2017) Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT). J Clin Oncol 35(15_suppl):4507. https://doi.org/10.1200/JCO.2017.35.15_suppl.4507
Miller KD, O’Neill A, Gradishar W, Hobday TJ, Goldstein LJ, Mayer IA, Bloom S, Brufsky AM, Tevaarwerk AJ, Sparano JA, Le-Lindqwister NA, Hendricks CB, Northfelt DW, Dang CT, Sledge GW Jr (2018) Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103). J Clin Oncol 36(25):2621–2629. https://doi.org/10.1200/JCO.2018.79.2028
Wakelee HA, Dahlberg SE, Keller SM, Tester WJ, Gandara DR, Graziano SL, Adjei AA, Leighl NB, Aisner SC, Rothman JM, Patel JD, Sborov MD, McDermott SR, Perez-Soler R, Traynor AM, Butts C, Evans T, Shafqat A, Chapman AE, Kasbari SS, Horn L, Ramalingam SS, Schiller JH, Ecog A (2017) Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 18(12):1610–1623. https://doi.org/10.1016/S1470-2045(17)30691-5
Corrie PG, Marshall A, Nathan PD, Lorigan P, Gore M, Tahir S, Faust G, Kelly CG, Marples M, Danson SJ, Marshall E, Houston SJ, Board RE, Waterston AM, Nobes JP, Harries M, Kumar S, Goodman A, Dalgleish A, Martin-Clavijo A, Westwell S, Casasola R, Chao D, Maraveyas A, Patel PM, Ottensmeier CH, Farrugia D, Humphreys A, Eccles B, Young G, Barker EO, Harman C, Weiss M, Myers KA, Chhabra A, Rodwell SH, Dunn JA, Middleton MR, Investigators A-M (2018) Adjuvant bevacizumab for melanoma patients at high risk of recurrence: survival analysis of the AVAST-M trial. Ann Oncol 29(8):1843–1852. https://doi.org/10.1093/annonc/mdy229
Kojiro M (2006) Angioarchitecture of hepatocellular carcinoma. Pathol Hepatocell Carcinoma 1:63–75
Nakashima O, Sugihara S, Kage M, Kojiro M (1995) Pathomorphologic characteristics of small hepatocellular carcinoma: a special reference to small hepatocellular carcinoma with indistinct margins. Hepatology (Baltimore, MD) 22(1):101–105. https://doi.org/10.1002/hep.1840220116
Naresh KN, Nerurkar AY, Borges AM (2001) Angiogenesis is redundant for tumour growth in lymph node metastases. Histopathology 38(5):466–470. https://doi.org/10.1046/j.1365-2559.2001.01061.x
Rana P, Pritchard KI, Kerbel R (2017) Plasma vascular endothelial growth factor as a predictive biomarker: door closed? Eur J Cancer 70:143–145. https://doi.org/10.1016/j.ejca.2016.11.002
O’Connor JP, Aboagye EO, Adams JE, Aerts HJ, Barrington SF, Beer AJ, Boellaard R, Bohndiek SE, Brady M, Brown G, Buckley DL, Chenevert TL, Clarke LP, Collette S, Cook GJ, deSouza NM, Dickson JC, Dive C, Evelhoch JL, Faivre-Finn C, Gallagher FA, Gilbert FJ, Gillies RJ, Goh V, Griffiths JR, Groves AM, Halligan S, Harris AL, Hawkes DJ, Hoekstra OS, Huang EP, Hutton BF, Jackson EF, Jayson GC, Jones A, Koh DM, Lacombe D, Lambin P, Lassau N, Leach MO, Lee TY, Leen EL, Lewis JS, Liu Y, Lythgoe MF, Manoharan P, Maxwell RJ, Miles KA, Morgan B, Morris S, Ng T, Padhani AR, Parker GJ, Partridge M, Pathak AP, Peet AC, Punwani S, Reynolds AR, Robinson SP, Shankar LK, Sharma RA, Soloviev D, Stroobants S, Sullivan DC, Taylor SA, Tofts PS, Tozer GM, van Herk M, Walker-Samuel S, Wason J, Williams KJ, Workman P, Yankeelov TE, Brindle KM, McShane LM, Jackson A, Waterton JC (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14(3):169–186. https://doi.org/10.1038/nrclinonc.2016.162
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue R, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Cheng J, Wei J, Tong T, Sheng W, Zhang Y, Han Y, Gu D, Hong N, Ye Y, Tian J, Wang Y (2019) Prediction of histopathologic growth patterns of colorectal liver metastases with a noninvasive imaging method. Ann Surg Oncol. https://doi.org/10.1245/s10434-019-07910-x
Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DT, McDonald DM (2012) Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2(3):270–287. https://doi.org/10.1158/2159-8290.CD-11-0240
Depner C, Zum Buttel H, Bogurcu N, Cuesta AM, Aburto MR, Seidel S, Finkelmeier F, Foss F, Hofmann J, Kaulich K, Barbus S, Segarra M, Reifenberger G, Garvalov BK, Acker T, Acker-Palmer A (2016) EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat Commun 7:12329. https://doi.org/10.1038/ncomms12329
Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G (2008) HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13(3):206–220. https://doi.org/10.1016/j.ccr.2008.01.034
Lamszus K, Brockmann MA, Eckerich C, Bohlen P, May C, Mangold U, Fillbrandt R, Westphal M (2005) Inhibition of glioblastoma angiogenesis and invasion by combined treatments directed against vascular endothelial growth factor receptor-2, epidermal growth factor receptor, and vascular endothelial-cadherin. Clin Cancer Res 11(13):4934–4940. https://doi.org/10.1158/1078-0432.CCR-04-2270
Carbonell WS, DeLay M, Jahangiri A, Park CC, Aghi MK (2013) beta1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res 73(10):3145–3154. https://doi.org/10.1158/0008-5472.CAN-13-0011
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, Ridge SM, Jablonski EM, Therrien J, Tannheimer S, McCall CM, Chenn A, Sipkins DA (2018) Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 560(7716):55–60. https://doi.org/10.1038/s41586-018-0342-5
Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, Giancotti FG, Schachner M, Malladi S, Massague J (2018) Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol 20(8):966–978. https://doi.org/10.1038/s41556-018-0138-8
Jahangiri A, Aghi MK, Carbonell WS (2014) beta1 integrin: critical path to antiangiogenic therapy resistance and beyond. Cancer Res 74(1):3–7. https://doi.org/10.1158/0008-5472.CAN-13-1742
Sharma P, Wagner K, Wolchok JD, Allison JP (2011) Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 11(11):805–812. https://doi.org/10.1038/nrc3153
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086. https://doi.org/10.1158/2159-8290.CD-18-0367
van Dam PJ, Daelemans S, Ross E, Waumans Y, Van Laere S, Latacz E, Van Steen R, De Pooter C, Kockx M, Dirix L, Vermeulen PB (2018) Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin Cancer Biol 52(Pt 2):86–93. https://doi.org/10.1016/j.semcancer.2018.01.009
Arroyo AG, Iruela-Arispe ML (2010) Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res 86(2):226–235. https://doi.org/10.1093/cvr/cvq049
Al-Soudi A, Kaaij MH, Tas SW (2017) Endothelial cells: from innocent bystanders to active participants in immune responses. Autoimmun Rev 16(9):951–962. https://doi.org/10.1016/j.autrev.2017.07.008
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168(4):707–723. https://doi.org/10.1016/j.cell.2017.01.017
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1103–1115. https://doi.org/10.1056/NEJMoa1816047
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T, Investigators K- (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127. https://doi.org/10.1056/NEJMoa1816714
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, Group IMS (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/nejmoa1716948
Khan KA, Kerbel RS (2018) Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 15(5):310–324. https://doi.org/10.1038/nrclinonc.2018.9
Munn LL, Jain RK (2019) Vascular regulation of antitumor immunity. Science 365(6453):544–545. https://doi.org/10.1126/science.aaw7875
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15(5):325–340. https://doi.org/10.1038/nrclinonc.2018.29
Vasan N, Baselga J, Hyman DM (2019) A view on drug resistance in cancer. Nature 575(7782):299–309. https://doi.org/10.1038/s41586-019-1730-1
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuczynski, E.A., Reynolds, A.R. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis 23, 55–74 (2020). https://doi.org/10.1007/s10456-019-09698-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-019-09698-6