Abstract
Burkholderia sp. F24, originally isolated from soil, was capable of growth on xylose and removed organic inhibitors present in a hemicellulosic hydrolysate and simultaneously produced poly-3-hydroxybutyrate (P3HB). Using non-detoxified hydrolysate, Burkholderia sp. F24 reached a cell dry weight (CDW) of 6.8 g L−1, containing 48 % of P3HB and exhibited a volumetric productivity (PP3HB) of 0.10 g L−1 h−1. Poly-3-hydroxybutyrate-co-3-hydroxyvalerate copolymers (P3HB-co-3HV) were produced using xylose and levulinic acid (LA) as carbon sources. In shake flask cultures, the 3HV content in the copolymer increased from 9 to 43 mol% by adding LA from 1.0 to 5.0 g L−1. In high cell density cultivation using concentrated hemicellulosic hydrolysate F24 reached 25.04 g L−1 of CDW containing 49 % of P3HB and PP3HB of 0.28 g L−1 h−1. Based on these findings, second-generation ethanol and bioplastics from sugarcane bagasse is proposed.
Similar content being viewed by others
Introduction
Polyhydroxyalkanoates (PHA) are polyesters synthesized by bacteria as energy and carbon storage materials. Approximately 150 hydroxyalkanoates have been detected as constituents of PHA in various bacteria [40, 41] and poly-3-hydroxybutyrate (P3HB) is the most studied polymer within the PHA family. P3HB shows mechanical properties similar to those of polypropylene, with the advantage that they are biodegradable, biocompatible, and can be produced from renewable carbon sources [16] such as lignocellulosic feedstocks.
The biotechnological use of P3HB was first exploited in the 1980s by Imperial Chemical Industries (ICI) [1]. ICI set up bulk production of P3HB using a polymer-accumulating bacterium, Ralstonia eutropha. Due to P3HB brittleness and poor mechanical properties, a bacterial process was developed allowing the production of copolymers of 3HB and 3HV (3-hydroxyvalerate) with considerably improved material properties [17]. Afterwards, a number of other enterprises developed processes searching for PHA various monomer compositions leading to improved properties and applications: The Tianen Corp. (China) sells a poly-3-hydroxybutyrate-co-3-hydroxyvalerate copolymer (P3HB-co-3HV) known as Enmat, produced by R. eutropha [6]; Metabolix (USA) developed a process to produce poly-3-hydroxybutyrate-co-4-hydroxybutyrate (P3HB-co-4-HB) copolymer named Mirel. Different production scales have been developed worldwide to produce PHA aiming at various applications [7].
Several groups estimated the cost of PHA production [8, 11, 22, 23, 30] and showed that it is heavily dependent on sugar prices. Nonato et al. [30] estimated the P3HB production cost of an industrial plant integrated in a sugar and ethanol mill in Brazil and, even in a system with low sugar production costs, the carbon source accounted for nearly one-third of the final cost. The high cost of production makes PHA substantially more expensive than synthetic plastics [40]. Exploring PHA production from locally available and renewable carbon sources, such as agricultural waste, would be of economic interest considering the environmental gains that would result from this process. In the biorefinery context, sugarcane bagasse is an inexpensive carbon source available in large amounts that can be used as a feedstock for the production of a wide variety of bioproducts [5, 19]. Dilute acid hydrolysis is usually performed to break the hemicellulose layer and to release sugars, mostly xylose, that can be used to produce P3HB [19, 24, 33, 38]. A drawback of acid hydrolysis pretreatment is the formation of inhibitory byproducts, which can negatively affect bacterial performance using hydrolysates [33, 44]. Therefore, it is desirable to reduce the formation of inhibitors during hydrolysis [26, 44] and/or obtain species of microorganisms resistant to inhibitors [15]. This work investigates a simple dilute acid pretreatment to produce a xylose-rich hydrolysate and its microbial conversion to PHA with simultaneous consumption of inhibitors.
Materials and methods
Isolation procedure
Approximately 5 g of soil samples suspended in 50 mL saline (0.85 % NaCl) was incubated in a rotary shaker (200 rev min−1, 30 °C) for 1 h. Samples of different serial dilutions were spread on mineral salts medium (MM) [36] plates containing cycloheximide (0.06 g L−1) and xylose (15 g L−1). All isolates were transferred to MM plates containing xylose (20 g L−1) and Nile Red (0.2 µg mL−1) and incubated for 72 h (30 °C). Under UV irradiation the fluorescent colonies were considered positive for PHA production [39].
Strain identification
Genomic DNA was extracted using DNeasy Blood and Tissue kit (Qiagen Group). The primers used for 16S rDNA gene amplification (27f and 1401r), PCR reactions and procedures of sequencing were described by Lopes et al. [24]. The software Molecular Evolutionary Genetics Analysis, Version 3.1 [21] was used for sequence alignment and phylogram design. The API 20NE system (BioMérieux) was used to determine nitrate reduction, hydrolysis of gelatin and esculin, urease activity, glucose fermentation and arginine dihydrolase activity and, carbon substrate assimilation and oxidation tests. All tests were duplicated at 30 °C.
Bacterial strains and culture conditions
Bacterial isolates were inoculated into nutrient broth (30 °C, 24 h, 150 rev min−1). This culture was inoculated (10 % v/v) to growth and PHA production experiments, which were performed in MM (30 °C, 150 rev min−1) with the appropriate carbon source. Different buffer strengths were tested by changing the concentrations of KH2PO4 and Na2HPO4 in the culture media. The concentration of KH2PO4 and Na2HPO4 (g L−1) were, respectively: 3.5 and 2 (buffer 1), 4.0 and 2.0 (buffer 2), 6.0 and 2.0 (buffer 3), 8 and 2 (buffer 4), 10 and 2 (buffer 5).
For long-term adaptation in furfural, the isolated strain was cultivated in MM supplemented with xylose (10 g L−1) and furfural (0.5 g L−1). In subsequent cultivations, the xylose concentration was decreased by 1 g L−1 per subculture.
Inocula for bioreactor experiments were obtained by a previous cultivation on nutrient broth, transferred to MM with 10 g xylose per liter (both 10 % v/v, cultivated at 30 °C, 24 h, 150 rev min−1).This MM culture was used as inoculum (20 % v/v) to a two-step fed-batch cultivation performed (cell growth and PHA accumulation) in a 5-L bench scale bioreactor Biostat B (B. Braun Biotech International) at 30 °C controlling the dissolved oxygen concentration above 20 % of air saturation. The pH was automatically controlled at 7.0 by adding 0.5 N H2SO4 or 0.5 N NaOH. The composition of the culture media used in the bioreactor experiments is described in the caption of the figures illustrating the respective assays.
Bagasse hydrolysis
Sugarcane bagasse (30 g) was treated in 300 mL of sulfuric acid solution, ranging from 0.5 to 4 % of H2SO4, at 120 °C and 1 atm for 2 h as described by Yu and Stahl [44]. The pH was adjusted to 7 with Ca(OH)2 at room temperature. For high cell density experiments, a modified hydrolysis was used: 100 g of bagasse was treated with 600 mL of 1 % sulfuric acid solution at 60 °C overnight. The excess liquid (300 mL) was separated before autoclaving at 120 °C and 1 atm for 40 min. The pH was adjusted to 10 with Ca(OH)2 at room temperature and then neutralized with H2SO4. The precipitated gypsum was removed using Whatman filter paper # 1. The medium was filtered through a pre-sterilized membrane (0.2 µm) before inoculation. In high cell density experiments the hydrolysate was previously evaporated at 85 °C.
Analytical methods
Bacterial growth was evaluated spectrophotometrically (OD610). Cell dry weight (CDW) was determined gravimetrically; carbohydrates were determined by HPLC as described by Silva et al. [37]; PHA amount and composition were determined by gas chromatography of propyl-esters [14].
Results and discussion
Selection of PHA-producing bacteria resistant to inhibitors present in hemicellulosic hydrolysate
A total of 2,627 isolates were analyzed in mineral medium plates containing xylose and Nile Red. Four isolates showed promising results of PHA accumulation (data not shown) and were analyzed in PHA production experiments in liquid MM supplemented with xylose. They accumulated large P3HB contents ranging from 52 to 62 % of the CDW, confirming the Nile Red results (Table 1).
According to Olsson and Hahn-Hägerdal [31], toxic compounds in hemicellulosic or cellulosic hydrolysates can be divided into (1) sugar degradation products, such as furfural from pentoses, and hydroxymethylfurfural (HMF) from hexoses; (2) compounds derived from lignocellulose structure (formic and acetic acids) and (3) lignin degradation products. A large variety of compounds (aromatic, polyaromatic, phenolic, and aldehydic) are released at low concentration from lignin during hydrolysis of lignocellulosic materials [28]. To study the inhibitory effects on cell growth, the four isolates selected were tested in mineral medium with the addition of each typical inhibitor in concentrations similar to those usually found in hydrolysates (Fig A.1, Supplementary Material).
The isolates were able to grow aerobically in acetic or formic acids as sole carbon sources in concentrations bellow 2.5 and 1.25 g L−1, respectively. In those cultures the specific growth rates were similar (0.023 h−1). As expected, the lag phase increased under higher concentrations of inhibitors. Some growth was observed in furfural and HMF until 2 h, after that the cell concentration declined faster in comparison with the control culture that had no carbon source (Fig A.1, Supplementary Material). Since all the isolates showed similar growth profiles, F24 was chosen, due to its higher P3HB content (Table 1), for additional experiments using xylose combined with other compounds (Fig. 1).
Growth experiment with the isolate F24 in mineral media with xylose (10 g L−1) and individual compounds: (filled square) acetic acid, 2.5 g L−1 (filled triangle) formic acid, 1.25 g L−1 (filled diamond) control experiment only with xylose, (filled circle) HMF, 0.5 g L−1 and (cross) furfural 0.5 g L−1
In growth experiments, acetic or formic acids were consumed within 12 h (data not shown) and promoted better growth when compared to the control culture (supplied only with xylose). In those experiments xylose was completely consumed after 24 h (data not shown). Previous work has shown that appropriate concentrations of volatile organic acids can enhance growth and PHA production [38, 42].
Furfural and HMF inhibited growth of F24 when compared to growth on xylose only. HPLC analysis of the medium, however, suggests that the actual inhibitors could not be furfural or HMF per se, but their metabolic products. As shown in Fig. 2, after 4 h culture, furfural (retention time around 27.5 min) and HMF (retention time 19.2 min) had already been converted into other compounds with the retention times of, respectively, 19.6 and 14.2 min (Fig. 2). Usually, during the degradation pathway of those furanic aldehydes, they are converted to the respective furanic acids: HMF is converted to hydroxymethyl-furoic acid (intermediate 1) and furfural to 2-furoic acid (intermediate 2) [43]. The accumulation of these metabolic products in the culture medium may inhibit cell growth, resulting in high concentrations of residual xylose.
Bioconversion of HMF and furfural into different intermediates. The relative concentrations of the components in the mineral solution are represented with peak area of HPLC. Based on the literature HMF is converted to hydroxymethyl-furoic acid (intermediate 1) and furfural to 2-furoic acid (intermediate 2) [25]
The adverse effect of these metabolic inhibitors on PHA production by strain F24 was also observed. With xylose as the sole carbon source (20 g L−1), the CDW of F24 reached 6.8 g L−1 with 43 % of PHA in 36 h. When xylose was supplemented with HMF (0.5 g L−1), the CDW declined to 3.7 g L−1 with P3HB content of 35 %. When supplying xylose and furfural (0.5 g L−1), the CDW further declined to 1.93 g L−1 and no PHA accumulation was observed. These results indicate that furfural and HMF (or their products) inhibit not only the main metabolic pathways of growth but also PHA formation in the cells of Burkholderia sp. F24 and furfural exhibited greater inhibition than HMF. Similar inhibitory effects of furfural and HMF have been reported in the literature for ethanol production [12, 32] and also for PHA production by Burkholderia cepacia ATCC 17759 [33]. However, in hemicellulosic hydrolysates, HMF is found in lower concentrations and it is usually considered less toxic than furfural (Table 2) [28].
Attempts at long-term adaptation of Burkholderia sp. F24 or even selection of UV mutants capable of reaching higher cell density in furfural were unsuccessful (data not shown). However, it was observed that in cultures with furfural and HMF the pH decreased bellow 3.8, corroborating the hypothesis that furanic aldehydes were being converted to the acid form. To test if low pH inhibits carbon source consumption, experiments with furfural and HMF were performed in mineral media with different buffering strengths.
Effect of pH on HMF and furfural consumption
The pH of shake flask cultures containing xylose and HMF decreased to 4 in the first 8 h and reached 3.8 after 16 h. When grown only on xylose, the pH declined to 5 after 24 h culture (see pH profile in Fig. A.2, Supplementary Material). The metabolic products derived from HMF probably acidified the culture medium, inhibiting cell growth and PHA formation. To verify if the low pH caused the poor cell growth and PHA accumulation, Burkholderia sp. F24 was cultivated in mineral media under different buffering strengths by controlling the phosphate concentration.
In cultures with xylose and HMF, high phosphate concentrations prevented media acidification (Fig. 3) and also resulted in higher cell concentrations. Moreover, the intermediate of HMF metabolism (RT 14.2 min) was fully consumed in the presence of buffer 5.
Effect of different buffer strengths in growth of Burkholderia sp. F24 in mineral media with xylose (10 g L−1) and HMF (0.5 g L−1) after 24 h of cultivation. F24 cultures with only xylose (10 g L−1) or only HMF (0.5 g L−1) as carbon source were used as controls. The initial inocula were around OD610 0.3 for all cultures
The effects of furfural on culture pH were similar to those observed in HMF experiments (see pH profile in Fig. A.3, Supplementary Material). Higher buffering strength allowed the cultures to achieve cell densities similar to the control experiment with xylose as a sole carbon source (see Fig. A.4, Supplementary Material). However, the intermediate of furfural metabolism was not fully consumed even in the presence of higher buffering strengths (data not shown).
Due to the acidification of the culture media in the presence of the furfural intermediate metabolite, this compound is probably an acid considering that the bacterial metabolism of furfural can sometimes generate furonic acid [2]. Moreover, using HPLC analysis, this compound demonstrated a retention time identical to that of pure furonic acid (Sigma, data not shown). The presented data shows that furfural, HMF and their intermediate metabolites are not inhibitors to Burkholderia sp. F24. Moreover, the growth inhibition due to low pH can be easily overcome in a bioreactor by automatically controlling the pH.
Biosynthesis of P3HB-co-3HV
Poly-3-hydroxybutrate was the major biopolyester produced by Burkholderia sp. F24 on the bagasse hydrolysates. However, in the presence of short-chain organic acids with odd carbon numbers, such as levulinic acid, a copolymer, P3HB-co-3HV, is formed (Table 3). Levulinic acid can be produced cost-effectively from a wide-variety of cellulose-containing forest and agricultural waste residues [3, 20], including the residual bagasse of the hemicellulose acid pretreatment. In addition to its low cost and the fact that it can be produced from renewable resources [3, 19], the 3HV yield from levulinc acid is usually higher than those reported for other short-chain organic acids with odd carbon numbers, such as propionic acid [9, 14, 25]. The utilization of low-value agricultural residues as principal carbon sources, associated to co-substrates, offers potential for significant reduction in the raw material component of P3HB and P3HB-co-3HV production costs.
Improvements on cell growth and PHA productivities were observed with increasing concentrations of levulinic acid from 3 to 5 g L−1. Cell and PHA productivities increased about 30 % compared to experiments with xylose as the sole carbon source. In shake flask cultures, the 3HV molar fraction in the copolyester increased from 9 to 43 mol% by adding levulinc acid (1.0 to 5.0 g L−1). The 3HV content in copolymers affects, to some extent, the material properties of bioplastics, especially elongation to break [17].
Description of Burkholderia sp. F24
Burkholderia sp. F24 cells are Gram-negative, non-sporulating rods and grow between 25 and 37 °C with an optimum temperature of 28–30 °C. Strain F24 is catalase and oxidase-positive, produces β-galactosidase, urease, arginine dihydrolase and hydrolyzes aesculin but does not produce indole or gelatinase, and it does not ferment glucose. The following substrates are assimilated as carbon sources: d-glucose, l-arabinose, d-mannose, d-mannitol, N-acetyl-glucosamine, d-maltose, gluconate, capric acid, adipic acid, malic acid, citrate and phenylacetic acid. According to the API 20NE system, these characteristics indicate that F24 belongs to the Burkholderia group.
The 16S rRNA sequence of strain F24 (GenBank accession number JX649146) was compared with other Burkholderia sp. sequences present in GenBank using CLUSTAL W. The phylogenetic analysis (Fig. 4) obtained by the neighbor-joining method showed ≥99 % identity with the 16S rRNA of Burkholderia cenocepacia indicating that this species is taxonomically very close to the isolate. Due to the phylogenetic and biochemical analyses, the isolate was named Burkholderia sp. F24. Burkholderia species have already been described as PHA-producing bacteria when utilizing xylose as a carbon source [19, 24, 38].
Sugarcane hydrolysate production and utilization
Hemicellulosic hydrolysis of sugarcane bagasse was performed at 120 °C and 1 atm using different concentrations of acid solutions (Table 2). The hemicellulose fraction was hydrolyzed as indicated by the high content of xylose, arabinose and acetic acid in the hydrolysates. The highest sugar concentration was obtained at 2 % H2SO4 (22.89 g L−1) and decreased as the acid concentration increased. The concentration of potential toxic compounds such as formic acid, acetic acid, furfural and HMF increased with the augmentation of acid concentrations. An initial test with Burkholderia sp. F24 showed that harsh conditions of hydrolysis generate byproducts that can inhibit growth (Table 2).
In further experiments using the 2 % H2SO4 hydrolysate, the positive effect of a higher initial density on cell growth was observed (Table 4). An initial cell density higher than 1.5 g L−1 was capable of overcoming the unfavorable conditions and decreased the concentrations of inhibitor compounds (Table 4). Similar results were obtained by other authors who have considered that since the cells would be inhibited due to accumulation of hydrolysate, inside of them or in their membranes, thus both the use of high cell density and/or the use of diluted hydrolysates would be effective to circumvent this problem [44].
Balanced nutrients are needed for cell growth and usually one limiting nutrient will promote PHA accumulation. Since sugarcane bagasse hydrolysate is a complex feedstock, it could supply other nutrients besides carbon sources. Therefore, it is important to identify nutrient limitations in hydrolysates and to adjust their concentrations in order to control cell growth and PHA accumulation. Different nutrient sources were supplemented to the hydrolysate: trace elements solution, ammonium ferric citrate (NH4)2SO4, NH4Cl, KH2PO4, Na2PO4, MgSO4, MgCl2 (see Table A.1, Supplementary Material). Balanced nutrients are needed for cell growth and usually one limiting nutrient will promote PHA accumulation. Since sugarcane bagasse hydrolysate is a complex feedstock, it could supply other nutrients besides carbon sources. Therefore, it is important to identify nutrient limitations in hydrolysates and to adjust their concentrations in order to control cell growth and PHA accumulation. Different nutrient sources were supplemented to the hydrolysate: trace elements solution, ammonium ferric citrate, (NH4)2SO4, NH4Cl, KH2PO4, Na2PO4, MgSO4, MgCl2 (see Table A.1, Supplementary Material).The hydrolysate has enough nitrogen in its composition to produce 1 g L−1 of residual biomass (residual biomass = total biomass − PHA biomass) (see Fig. A.5, Supplementary Material), however, nitrogen appears to be a growth-limiting factor in the hydrolysate. For that reason, the supply of different concentrations of ammonium sulphate was analyzed.
Table 5 shows that ammonium sulphate is a limiting nutrient to obtain a residual biomass of 4.19 g L−1 using hydrolysate as the only source of carbon and nutrients. Moreover, the highest PHA content was obtained when the C/N was 20. Low amounts of (NH4)2SO4, ranging from 0 to 1 g L−1, will not increase the formation of residual biomass (Table 5). For that reason, the supply of different concentrations of ammonium sulphate was analyzed. Table 5 shows that ammonium sulphate is a limiting nutrient to obtain a residual biomass of 4.19 g L−1 using hydrolysate as the only source of carbon and nutrients. Moreover, the highest PHA content was obtained when the C/N was 20. Low amounts of (NH4)2SO4, ranging from 0 to 1 g L−1, will not increase the formation of residual biomass (Table 5). Interestingly, it was observed that precipitates formed when the hydrolysate was supplemented with different nutrients, which was probably due to the interaction of these nutrients with the high amounts of Ca(OH)2 used to neutralize the hydrolysate. Based on this observation we speculate that (NH4)2SO4 is probably not available for bacterial metabolism due to an interaction with hydrolysate compounds.
A 2-L culture bioreactor (400 mL inoculum) was performed using 2 % H2SO4 hydrolysate supplemented with (NH4)2SO4 3 g L−1. After 8 h, 0.5 L of hydrolysate was added without any supplementation and after 20 h, another 0.5 L of hydrolysate was added (Fig. 5). After 33 h, the cell density reached 6.8 g L−1, the PHA content was 48.6 % of CDW, corresponding to a volumetric productivity (PP3HB) of 0.10 g L−1 h−1. The cells were recovered and the PHA composition analysis indicated the presence of only 3-hydroxybutyrate monomers.
Bioreactor experiment with hydrolysate using Burkholderia sp. F24 to produce P3HB. Experiments consisted in an initial 2-L batch of hydrolysate (with 400 mL of inocula) plus ammonium sulphate (3 g L−1) with pulses of hydrolysate during the experiment (1 L of hydrolysate without any supplementation). Feeding was based on dissolved oxygen. When DO values suddenly increased, indicating exhaustion of carbon source, a new feed was made. P3HB and CDW are presented
Increasing cell density
From the economic standpoint, high volumetric productivities are essential for bioprocesses featuring high initial investment costs as is the case of PHA production. High cell densities are a prerequisite for high volumetric productivities [35]. To increase cell density, a hydrolysate was produced using less harsh conditions (1 % H2SO4, 40 min) at 120 °C in comparison to the first method (2 % H2SO4, 2 h at 120 °C). The detoxification method consisted in increasing pH from 0.9 to 10 using 136 mmol Ca(OH)2. This method, together with less harsh conditions, resulted in a 20 % higher productivity in comparison with the previous method. Overliming is an inexpensive and simple detoxification method. The amount of Ca(OH)2 needed to adjust the hydrolysate to pH 7 was 127 mM, and the additional amount required do increase the pH to 10 was only 9 mM, due to the low buffering capacity of the hydrolysate.
Pan et al. [33] compared several wood hydrolysate detoxification methods for PHA production using B. cepacia ATCC 17759. The methods included overliming, activated charcoal, anion exchange resins and cation exchange resins. Overliming associated to low-temperature sterilization exhibited the greatest removal of phenolic compounds (65 %), while other treatments removed no more than 10 % of total phenolics from the membrane-treated wood hydrolysate. Martinez et al. [27] had previously showed that overliming also removed a significant amount of furfural and HMF from a hemicellulosic hydrolysate of sugar cane bagasse. Ammonium Fiber Expansion (AFEX) has also been used before enzymatic hydrolysis of lignocellulosic residues to reduce the formation of toxic compounds [4].
Bioreactor experiments were carried out using non-concentrated and threefold concentrated hydrolysate (Fig. 6). Using non-concentrated hydrolysate, F24 reached, after 39 h, 9.74 g L−1 of CDW containing 48 % P3HB, corresponding to a PP3HB of 0.12 g L−1 h−1. Using threefold concentrated hydrolysate (considering volume after evaporation), strain F24 reached 25.04 g L−1 of dry biomass containing 49 % of P3HB after 44 h, corresponding to a PP3HB of 0.28 g L−1 h−1. Therefore, by concentrating the hydrolysate it was possible to increase productivity 2.3-fold. Similar PHA content (51.4 %) was obtained by Pan et al. [33], however reaching 16.9 g L−1 of CDW in 96. Moreover the strain B. cepacia ATCC 17759 tested was unable to grow directly in non-detoxified hydrolysate, obtained from wood hydrolysate processed by overliming combined with low-temperature sterilization.
Effect of hydrolysate concentration on cell dry weight, CDW and PHA production (PHA). White symbols represent the experiment using the hemicellulosic hydrolysate without concentration and black symbols represent the utilization of the hemicellulosic hydrolysate concentrated threefold. Experiments consisted of fed-batch cultivations with pulses of hydrolysate during the experiment (1 L of hydrolysate without any supplementation). Feeding was based on dissolved oxygen. When DO values suddenly increased, indicating exhaustion of carbon source, a new feed was made. An initial 2 L culture (400 mL inoculum) with 2 % H2SO4 hydrolysate media supplemented with 0.5 g L−1 KH2PO4, 3 g L−1 (NH4)2SO4, 0.3 g L−1 MgSO4·7H2O, 0.18 g L−1 CaCl2·2H2O, 0.03 g L−1 ferric ammonium citrate, 1 mL L−1 trace element solution [12]. For the assay with the concentrated hydrolysated the medium contained 0.1 g L−1 KH2PO4, 8 g L−1 (NH4)2SO4, 1.3 g L−1 MgSO4·7H2O, 0.18 g L−1 CaCl2·2H2O, 0.03 g L−1 ferric ammonium citrate, 1 mL L−1 trace element solution [13]
Other important issue is that hydrolysate concentration by evaporation represents a physical detoxification method to reduce the contents of volatile compounds such as acetic acid, furfural and HMF. However, this method also increases the concentration of non-volatile compounds, such as lignin derivatives [29]. A positive effect of concentration, the increase in specific growth rate (0.09 to 0.12 h−1), was probably due to the lower concentration of furfural and HMF. Interestingly, positive and negative effects of hydrolysate evaporation in xylitol production have been previously described [10, 34, 37]. Concentrated hydrolysates are essential to achieve high cell densities. Furthermore, this work has demonstrated that concentration by evaporation was an efficient method of detoxification, enabling the hydrolysate use to produce P3HB by Burkholderia sp. F24.
In this work cell densities were increased when hydrolysate was used and compared to values described in the literature (Table 6). Higher cell densities were attained with B. sacchari, however, under optimized conditions and using hydrolysates containing less toxic compounds [4]. For this strain F24 resistant to toxic compounds however, higher values of productivity and PHA accumulation are still required for a more competitive process.
Integrating production of second-generation ethanol and xylose-based PHA
A developed biorefinery is not only able to produce a variety of chemicals fuels and intermediates or end products but can also use various types of feedstocks [18]. The production of PHA integrated into a sugar and ethanol mill relies mostly on the capacity of the system to supply the necessary energy for running the processes, the availability of raw materials, and the ability of the system to process and use effluents without adverse impact on the environment [30].
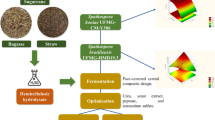
The biorefinery proposal presented in this work is depicted in Fig. 7.
Master Flow Diagram of an integrated production of sugar, first- and second-generation ethanol and bioplastics based on the utilization sugarcane bagasse. After dilute acid hydrolysis two streams are produced: stream 1 is a liquid xylose rich stream that could be used to produce PHA and stream 2 is a residual cellulose fraction can be subjected to enzymatic hydrolysis to produce other chemicals, such as second-generation ethanol or levulinic acid. The fermentation of glucose from cellulose hydrolysis (stream 3) and the sucrose from the juice treatment could be simultaneously fermented by yeast
Considering a traditional mill, producing ethanol and first-generation ethanol, sugarcane bagasse could be used for steam for power generation or for the process involving hydrolysis to release second-generation sugars. Dilute acid treatment is usually a pretreatment of lignocellulose to separate the hemicellulose and lignin fraction for later breakdown of cellulose. Therefore, after dilute acid hydrolysis two streams can be produced (1) a liquid xylose rich stream that could be used to produce PHA and (2) a residual cellulose fraction that could be subjected to enzymatic hydrolysis to produce other chemicals, such as second-generation ethanol or levulinic acid. The latter stream is particularly interesting since Saccharomyces cerevisiae is unable to naturally consume pentoses and is not tolerant to the inhibitors generated during hydrolysis [15]. However, since yeasts are capable of fermenting glucose, the latter stream could be fed combined with sucrose obtained from the milling of sugarcane to produce ethanol.
Dias et al. [13] performed an economic risk analysis for the integrated first- and second-generation ethanol production process using cellulose from sugarcane. It was concluded that to be more economically competitive, decreasing the initial investment, improving yields and developing pentose fermentation to ethanol are needed.
Conclusions
In this study we selected bacteria capable of resisting and consume inhibitory compounds present in lignocellulosic hydrolysates. 16S rDNA analysis indicated that the strain belongs to the genus Burkholderia, being denominated Burkholderia sp. F24. Different conditions for hydrolysis of sugarcane bagasse were tested, and the best condition for an optimal release of sugars, better growth and PHA production by Burkholderia sp. F24 used 2 % H2SO4, 120 °C, 40 min, 1 atm. A positive effect on the use of the hydrolysates was observed when increasing inocula (up to 3.2 g L−1) were used. The assessment of nutrient limitation of the hydrolysate indicated that there was NH4 + limitation. Cultivations in bioreactor allowed to reach values of biomass of 25 g L−1, with a content of 49 % PHB in 44 h, corresponding to a volumetric productivity 0.28 g L−1 h−1. This is a relevant result since productivity and PHA content have strong influences on the capital cost and downstream processing, respectively. Supplementation of levulinic acid in mineral medium with xylose allowed the production and control of monomer composition of the copolymer P3HB-co-3HV F24 Burkholderia sp.
It is proposed that a new process to produce biodegradable polymers from the hemicellulosic fraction of lignocellulosic raw materials can be integrated with second-generation ethanol production in order to enhance the feasibility of the process. The cellulosic fraction can also be used as a substrate for levulinc acid production. Metabolic engineering of Burkholderia sp. F24 is currently in progress in our laboratory to increase performance on the use of hemicelullosic hydrolysates.
References
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhyroxyalkanoates. Microbiol Rev 54:450–472
Boopathy R, Bokang H, Daniels L (1996) Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J Ind Microbiol Biotechnol 11(3):147–150
Bozell JJ, Moens L, Elliott DC, Wang Y, Neuenscwander G, Fitzpatrick SW et al (2000) Production of levulinic acid and use as a platform chemical for derived products. Resour Conserv Recycl 28:227–239
Cesário MT, Raposo RS, Almeida MCND, van Keulen F, Ferreira BS, Fonseca MMR (2014) Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol 31(1):104–113
Chandrakant P, Bisaria VS (1998) Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit Rev Biotechnol 18(4):295–331
Chanprateep S (2010) Current trends in biodegradable Polyhydroxyalkanoates. J Biosci Bioeng 110(6):621–632
Chen G (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Choi J, Lee SY (1997) Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng 17:335–342
Chung SH, Choi GG, Kim HW, Rhee YH (2001) Effect of levulinic acid on the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Ralstonia eutropha KHB-8862. J Microbiol 39:79–82
Converti A, Domınguez JM, Perego P, Silva SS, Zilli M (2000) Wood hydrolysis and hydrolyzate detoxification for subsequent xylitol production. Chem Eng Technol 23:1013–1020
De Koning GJM, Kellerhals M, Van Meurs C, Witholt B (1997) A process for the recovery of poly(3-hydroxyalkanoates) from Pseudomonas 2. Process development and economic evaluation. Bioprocess Eng 17:15–21
Delgenes JP, Moletta R, Navarro JM (1996) Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb Technol 19:220–225
Dias MOS, Cunha MP, Maciel Filho R, Bonomi A, Jesus CDF, Rossell CEV (2011) Simulation of integrated first and second-generation bioethanol production from sugarcane: comparison between different biomass pretreatment methods. J Ind Microbiol Biotechnol 38:955–966
Gomez JGC, Rodrigues MFA, Alli RCP, Torres BB, Bueno Netto CL, Oliveira MS et al (1996) Evaluation of soil gram negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic acid. Appl Microbiol Biotechnol 45:785–791
Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953
Harding KG, Dennis JS, Blottnitz HV, Harrison STL (2007) Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically-based poly-3-hydroxybutyric acid using life cycle analysis. J Biotechnol 130:57–66
Holmes PA (1985) Applications of PHB—a microbially produced biodegradable thermoplastic. Phys Technol 16:32–36
Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145
Keenan TM, Nakas JP, Tanenbaum SW (2006) Polyhydroxyalkanoate copolymers from forest biomass. J Ind Microbiol Biotechnol 33:616–621
Keenan TM, Tanenbaum SW, Stipanovic AJ, Nakas JP (2004) Production and characterization of poly-beta-hydroxyalkanoate copolymers from Burkholderia cepacia utilizing xylose and levulinic acid. Biotechnol Prog 20:1697–1704
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinf 5:150–163
Lee SY, Choi JI (2001) Production of microbial polyester by fermentation of recombinant microorganisms. Adv Biochem Eng Biotechnol 71:183–207
Lee SY (1996) Bacterial Polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Lopes MSG, Rocha RCS, Zanotto SP, Gomez JGC, Silva LFD (2009) Screening of bacteria to produce polyhydroxyalkanoates from xylose. World J Microbiol Biotechnol 25:1751–1756
Marangoni C, Furigo A Jr, Aragão GMF (2000) Oleic acid improves poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Ralstonia eutropha in inverted sugar and propionic acid. Biotechnol Lett 22:1635–1638
Martin C, Galbe M, Wahlbom FC, Hahn-Hägerdal B, Jonsson LJ (2002) Ethanol production from enzymatic hydrolyzates of sugar cane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb Technol 31:274–282
Martinez A, Rodriguez ME, York SW, Preston JF, Ingram LO (2000) Effects of Ca(OH)2 treatments (“overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol Bioeng 69(5):526–536
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of dilute-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93:1–10
Mussatto SI, Santos JC, Roberto IC (2004) Effect of pH and activated charcoal adsorption on hemicellulosic hydrolysate detoxification for xylitol production. J Chem Technol Biotechnol 79:590–596
Nonato RV, Mantelatto PE, Rossell CEV (2001) Integrated production of biodegradable plastic, sugar and ethanol. Appl Microbiol 57:1–5
Olsson L, Hahn-Hägerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol 18:312–331
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates I: inhibition and detoxification. Bioresour Technol 74:17–24
Pan W, Perrota JA, Stipanovic AJ, Nomura CT, Nakas JP (2012) Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J Ind Microbiol Biotechnol 39:459–469
Parajó JC, Domınguez H, Domınguez JM (1997) Improved xylitol production with Debaryomyces hansenii Y-7426 from raw or detoxified wood hydrolysates. Enzyme Microb Technol 21:18–24
Riesenberg D, Guthke R (1999) High-cell-density cultivation of microorganisms. Appl Microbiol Biotechnol 51:422–430
Rocha RCS, Silva LF, Taciro MK, Pradella JGC (2008) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) P(3H-co-3HV) with a broad range of 3HV content at high yields by Burkholderia sacchari IPT 189. World J Microbiol Biotechnol 24:427–431
Silva CJSM, Roberto IC (1999) Statistical screening method for selection of important variables on xylitol biosynthesis from rice straw hydrolysate by Candida guilliermondii FTI 20037. Biotechnol Tech 13:743–747
Silva LF, Taciro MK, Michelin-Ramos ME, Carter JM, Pradella JGC, Gomez JGC (2004) Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J Ind Microb Biotechnol 31:245–254
Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbüchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol 171:73–80
Steinbüchel A, Fuchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Steinbüchel A (1991) Polyhydroxyalkanoic acids. In: Byrom D (ed) Biomaterials: novel materials from biological sources. Stockton, New York, pp 124–213
Van-Thuoc D, Quillaguama J, Mamo G, Mattiasson B (2007) Utilization of agricultural residues for poly(3-hydroxybutyrate) production by Halomonas boliviensis LC1. J Appl Microbiol 104:420–428
Wierckx N, Koopman F, Ruijssenaars HJ, Winde JH (2011) Microbial degradation of furanic compounds: biochemistry, genetics, and impact. Appl Microbiol Biotechnol 92(6):1095–1105
Yu J, Stahl H (2008) Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour Technol 99:8042–8048
Acknowledgments
The authors would like to thank Dr. Jian Yu from the Hawaii Natural Energy Institute of the University of Hawaii for helping with some of the experiments and to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes, M.S.G., Gomez, J.G.C., Taciro, M.K. et al. Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp.. J Ind Microbiol Biotechnol 41, 1353–1363 (2014). https://doi.org/10.1007/s10295-014-1485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1485-5