Abstract
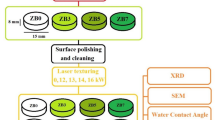
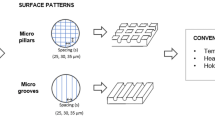
3 mol% yttria-stabilized zirconia ceramics have been gaining attention as promising restorative materials that are extensively used in dental implant applications. However, implant failure due to bacterial infection and its bioinert surface slow osseointegration in vivo, which are significant issues in clinical applications. In this work, surface modification was achieved using a continuous wave carbon dioxide laser at a wavelength of 10.6 µm in an air atmosphere. Changes in the surface characteristics were evaluated using X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), atomic force microscopy (AFM), and 2D roughness and hardness tests. The bioactivity of the laser-treated samples was studied by examining their behavior when immersed in the SBF solution. The formation of the hydroxyapatite phase on the laser-treated sample was much more uniform than that of its untreated counterparts. The antibacterial properties of surface-treated zirconia ceramics against Streptococcus mutans and Escherichia coli bacteria were rigorously examined. These results indicate that the laser-induced nanoscale grooves significantly improved antibacterial activity by creating hydrophobic surfaces. The cellular response was evaluated for 7 days on microtextures on the zirconia surfaces and an untreated sample with MC3T3-E1 pre-osteoblast cell line cultured under basal conditions. Surface topography was revealed to improve the cellular response with increased metabolic activity compared to the untreated sample and showed modulation of cell morphology for the entire time. These results suggest that laser modification can be an appropriate non-contact method for designing nanoscale microtextures to improve the biological response and antibacterial behavior of zirconia ceramics in restorative dentistry.














Similar content being viewed by others
Data availibility
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Schünemann FH, Galárraga-Vinueza ME, Magini R, Fredel M, Silva F, Souza JC, et al. Zirconia surface modifications for implant dentistry. Mater Sci Eng. 2019;98:1294–305.
Liu X, Zou B, Xing H, Huang C. The preparation of ZrO2-Al2O3 composite ceramic by SLA-3D printing and sintering processing. Ceram Int. 2020;46(1):937–44.
Hanawa T. Zirconia versus titanium in dentistry: a review. Dent Mater J. 2020;39(1):24–36.
Pettersson M, Pettersson J, Johansson A, Molin TM. Titanium release in peri-implantitis. J Oral Rehabil. 2019;46(2):179–88.
Corazza P, Duan Y, Kimpara E, Griggs J, Della BA. Lifetime comparison of Y-TZP/porcelain crowns under different loading conditions. J Dent. 2015;43(4):450–7.
Abrahamsson L, Berglundh T, Lindhe J. Soft tissue response to plaque formation at different implant systems. A comparative study in the dog. Clin Oral Implants Res. 1998;9(2):73–9.
Xu J, Ji M, Li L, Wu Y, Yu Q, Chen M. Improving wettability, antibacterial and tribological behaviors of zirconia ceramics through surface texturing. Ceram Int. 2022;48(3):3702–10.
Wassmann T, Kreis S, Behr M, Buergers R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int J Implant Dent. 2017;3(1):1–11.
Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003;29(1):8–12.
Akagawa Y, Hosokawa R, Sato Y, Kamayama K. Comparison between freestanding and tooth-connected partially stabilized zirconia implants after two years’ function in monkeys: a clinical and histologic study. J Prosthet Dent. 1998;80(5):551–8.
Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000;15(5):675.
Triplett RG, Frohberg U, Sykaras N, Woody RD. Implant materials, design, and surface topographies: their influence on osseointegration of dental implants. J Long Term Eff Med Implants. 2003;13(6):485.
Wenz HJ, Bartsch J, Wolfart S, Kern M. Osseointegration and clinical success of zirconia dental implants: a systematic review. Int J Prosthodont. 2008;21(1):27.
Möller B, Terheyden H, Açil Y, Purcz N, Hertrampf K, Tabakov A, et al. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study. Int J Oral Maxillofac Surg. 2012;41(5):638–45.
Gahlert M, Röhling S, Wieland M, Sprecher C, Kniha H, Milz S. Osseointegration of zirconia and titanium dental implants: a histological and histomorphometrical study in the maxilla of pigs. Clin Oral Implant Res. 2009;20(11):1247–53.
Gahlert M, Roehling S, Sprecher C, Kniha H, Milz S, Bormann K. In vivo performance of zirconia and titanium implants: a histomorphometric study in mini pig maxillae. Clin Oral Implant Res. 2012;23(3):281–6.
Rohr N, Hoda B, Fischer J. Surface structure of zirconia implants: an integrative review comparing clinical results with preclinical and in vitro data. Materials. 2022;15(10):3664.
Gaharwar AK, Schexnailder PJ, Jin Q, Wu C-J, Schmidt G. Addition of chitosan to silicate cross-linked PEO for tuning osteoblast cell adhesion and mineralization. ACS Appl Mater Interfaces. 2010;2(11):3119–27.
Kurella A, Dahotre NB. Surface modification for bioimplants: the role of laser surface engineering. J Biomater Appl. 2005;20(1):5–50.
Soon G, Pingguan-Murphy B, Lai KW, Akbar SA. Review of zirconia-based bioceramic: surface modification and cellular response. Ceram Int. 2016;42(11):12543–55.
Camposilvan E, Flamant Q, Anglada M. Surface roughened zirconia: towards hydrothermal stability. J Mech Behav Biomed Mater. 2015;47:95–106.
Ramsden JJ, Allen DM, Stephenson DJ, Alcock JR, Peggs G, Fuller G, et al. The design and manufacture of biomedical surfaces. CIRP Ann. 2007;56(2):687–711.
Gaggl A, Schultes G, Müller W, Kärcher H. Scanning electron microscopical analysis of laser-treated titanium implant surfaces—a comparative study. Biomaterials. 2000;21(10):1067–73.
Zhao Z, Jia G, Liu Y, Zhang Q, Zhou Y, Chang K. Carbonized bark by laser treatment for efficient solar-driven interface evaporation. ACS Omega. 2020;5(23):13482–8.
Ferraris S, Bobbio A, Miola M, Spriano S. Micro-and nano-textured, hydrophilic and bioactive titanium dental implants. Surf Coat Technol. 2015;276:374–83.
Kita J, Dziedzic A, Golonka LJ, Zawada T. Laser treatment of LTCC for 3D structures and elements fabrication. Microelectron Int. 2002;19:14.
Asl SM, Ganjali M, Karimi M. Surface modification of 316L stainless steel by laser-treated HA-PLA nanocomposite films toward enhanced biocompatibility and corrosion-resistance in vitro. Surf Coat Technol. 2019;363:236–43.
Yeo I-S, Kim H-Y, Lim KS, Han J-S. Implant surface factors and bacterial adhesion: a review of the literature. Int J Artif Organs. 2012;35(10):762–72.
Yang K, Shi J, Wang L, Chen Y, Liang C, Yang L, et al. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: a review. J Mater Sci Technol. 2022;99:82–100.
Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implant Res. 2006;17(S2):68–81.
Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 2015;94(8):1027–34.
Cheng Y, Feng G, Moraru CI. Micro-and nanotopography sensitive bacterial attachment mechanisms: a review. Front Microbiol. 2019;10:191.
Harvey AG, Hill EW, Bayat A. Designing implant surface topography for improved biocompatibility. Expert Rev Med Devices. 2013;10(2):257–67.
Nicolas-Silvente AI, Velasco-Ortega E, Ortiz-Garcia I, Monsalve-Guil L, Gil J, Jimenez-Guerra A. Influence of the titanium implant surface treatment on the surface roughness and chemical composition. Materials. 2020;13(2):314.
Ganjali M, Mousavi S, Nikzamir S, Milan PB, Mozafari M. Effect of laser cladded co-doped strontium fluorapatite nanopowder coating on the antibacterial and cell attachment of Ti-6Al-4V implants for bone applications. Mater Technol. 2021;37:1–13.
Shaikh S, Singh D, Subramanian M, Kedia S, Singh AK, Singh K, et al. Femtosecond laser induced surface modification for prevention of bacterial adhesion on 45S5 bioactive glass. J Non-Cryst Solids. 2018;482:63–72.
Hizal F, Rungraeng N, Lee J, Jun S, Busscher HJ, van der Mei HC, et al. Nanoengineered superhydrophobic surfaces of aluminum with extremely low bacterial adhesivity. ACS Appl Mater Interfaces. 2017;9(13):12118–29.
Tesler AB, Kim P, Kolle S, Howell C, Ahanotu O, Aizenberg J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat Commun. 2015;6(1):1–10.
Carvalho A, Grenho L, Fernandes MH, Daskalova A, Trifonov A, Buchvarov I, et al. Femtosecond laser microstructuring of alumina toughened zirconia for surface functionalization of dental implants. Ceram Int. 2020;46(2):1383–9.
Kostov KG, Nishime TMC, Castro AHR, Toth A, Hein LRdO. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl Surf Sci. 2014;314:367–75.
Stalder AF, Melchior T, Müller M, Sage D, Blu T, Unser M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Coll Surf A. 2010;364(1–3):72–81.
Stalder AF, Kulik G, Sage D, Barbieri L, Hoffmann P. A snake-based approach to accurate determination of both contact points and contact angles. Coll Surf A. 2006;286(1–3):92–103.
Chicot D, Mercier D, Roudet F, Silva K, Staia M, Lesage J. Comparison of instrumented Knoop and Vickers hardness measurements on various soft materials and hard ceramics. J Eur Ceram Soc. 2007;27(4):1905–11.
Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–15.
Wang H, Ren D. Controlling Streptococcus mutans and Staphylococcus aureus biofilms with direct current and chlorhexidine. AMB Express. 2017;7(1):1–9.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–9.
Cullity BD. Elements of X-ray diffraction. Boston: Addison-Wesley Publishing; 1956.
Naeem S, Mehmood T, Wu KM, Khan BS, Majid A, Siraj K, et al. Laser surface hardening of gun metal alloys. Materials. 2019;12(16):2632.
Khaleeq-ur-Rahman M, Butt M, Samuel A, Siraj K. Investigation of laser irradiation effects on the hardness of Al 5086 alloy under different conditions. Vacuum. 2010;85(3):474–9.
Bushroa AR, Rahbari R, Masjuki HH, Muhamad MR. Approximation of crystallite size and microstrain via XRD line broadening analysis in TiSiN thin films. Vacuum. 2012;86(8):1107–12.
Chan C-W, Carson L, Smith GC, Morelli A, Lee S. Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl Surf Sci. 2017;404:67–81.
Alagiriswamy G, Krishnan CS, Ramakrishnan H, Jayakrishnakumar SK, Mahadevan V, Azhagarasan NS. Surface characteristics and bioactivity of zirconia (Y-TZP) with different surface treatments. J Pharm Bioallied Sci. 2020;12(Suppl 1):S114.
Riveiro A, Maçon AL, del Val J, Comesaña R, Pou J. Laser surface texturing of polymers for biomedical applications. Front Phys. 2018;6:16.
Chen X, Nouri A, Li Y, Lin J, Hodgson PD, Ce W. Effect of surface roughness of Ti, Zr, and TiZr on apatite precipitation from simulated body fluid. Biotechnol Bioeng. 2008;101(2):378–87.
Baino F, Yamaguchi S. The use of simulated body fluid (SBF) for assessing materials bioactivity in the context of tissue engineering: review and challenges. Biomimetics. 2020;5(4):57.
Lee SY, Regnault W, Antonucci J, Skrtic D. Effect of particle size of an amorphous calcium phosphate filler on the mechanical strength and ion release of polymeric composites. J Biomed Mater Res Part B. 2007;80(1):11–7.
Carvalho A, Cangueiro L, Oliveira V, Vilar R, Fernandes MH, Monteiro FJ. Femtosecond laser microstructured alumina toughened zirconia: a new strategy to improve osteogenic differentiation of hMSCs. Appl Surf Sci. 2018;435:1237–45.
Hwang K-J, Shin M, Lee M-H, Lee H, Oh MY, Shin TH. Investigation on the phase stability of yttria-stabilized zirconia electrolytes for high-temperature electrochemical application. Ceram Int. 2019;45(7):9462–7.
Rab A, Siraj K, Irshad M, Latif A, Naz S, Bashir S, et al. Laser irradiation effects on structural, morphological and mechanical properties of ZirCAD dental ceramic. Dig J Nanomater Biostructures. 2021;16:677–84.
Aivazi M, Nejatidanesh F, Mortazavi V, HashemiBeni B, Matinlinna JP, Savabi O. The evaluation of prepared microgroove pattern by femtosecond laser on alumina-zirconia nano-composite for endosseous dental implant application. Lasers Med Sci. 2016;31(9):1837–43.
Soltaninejad F, Moezizadeh M, Khatiri M, Razaghi H, Namdari M, Nojehdehian H. Effect of Nd: YAG laser energy density on bond strength and phase transformation of zirconia. Biomed Res (Aligarh). 2017;28(17):7341–7.
Carvalho A, Pelaez-Vargas A, Hansford DJ, Fernandes MH, Monteiro FJ. Effects of line and pillar array microengineered SiO2 thin films on the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Langmuir. 2016;32(4):1091–100.
Abi-Rached FO, Martins SB, Campos JA, Fonseca RG. Evaluation of roughness, wettability, and morphology of an yttria-stabilized tetragonal zirconia polycrystal ceramic after different airborne-particle abrasion protocols. J Prosthet Dent. 2014;112(6):1385–91.
Milne A, Amirfazli A. The Cassie equation: how it is meant to be used. Adv Coll Interface Sci. 2012;170(1–2):48–55.
Liu Y, Lin W, Lin Z, Xiu Y, Wong C. A combined etching process toward robust superhydrophobic SiC surfaces. Nanotechnology. 2012;23(25): 255703.
Rab A, Siraj K, Irshad M, Naz S, Latif A. Evaluation of morphology, hardness and structure of laser exposed dental porcelain. RadiaT Eff Defects Solids. 2022;177:1–9. https://doi.org/10.1080/10420150.2022.2073871.
Roitero E, Anglada M, Mücklich F, Jiménez-Piqué E. Mechanical reliability of dental grade zirconia after laser patterning. J Mech Behav Biomed Mater. 2018;86:257–63.
Gottenbos B, Busscher H, Van Der Mei H, Nieuwenhuis P. Pathogenesis and prevention of biomaterial centered infections. J Mater Sci Mater Med. 2002;13(8):717–22.
Dunne WM Jr. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15(2):155–66.
Katsikogianni M, Missirlis Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8(3):37–57.
Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, Jin X, et al. Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol. 2020;11:928.
Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4:112.
Dahlstrand Rudin A, Khamzeh A, Venkatakrishnan V, Persson T, Gabl M, Savolainen O, et al. Porphyromonas gingivalis produce neutrophil specific chemoattractants including short chain fatty acids. Front Cell Infect Microbiol. 2021;10: 620681.
Hamadi F, Latrache H, Zahir H, Elghmari A, Timinouni M, Ellouali M. The relation between Escherichia coli surface functional groups’ composition and their physicochemical properties. Braz J Microbiol. 2008;39:10–5.
Mitik-Dineva N, Wang J, Truong VK, Stoddart P, Malherbe F, Crawford RJ, et al. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol. 2009;58(3):268–73.
Ermis M, Antmen E, Hasirci V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: a review from the tissue engineering perspective. Bioactive Mater. 2018;3(3):355–69.
Taniguchi Y, Kakura K, Yamamoto K, Kido H, Yamazaki J. Accelerated osteogenic differentiation and bone formation on zirconia with surface grooves created with fiber laser irradiation. Clin Implant Dent Relat Res. 2016;18(5):883–94.
Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro-and nano-structured surfaces on cell behavior. Biomaterials. 1999;20(6):573–88.
Barbucci R, Pasqui D, Wirsen A, Affrossman S, Curtis A, Tetta C. Micro and nano-structured surfaces. J Mater Sci Mater Med. 2003;14(8):721–5.
Wang T, Wan Y, Liu Z. Effects of superimposed micro/nano-structured titanium alloy surface on cellular behaviors in vitro. Adv Eng Mater. 2016;18(7):1259–66.
Xie Y, Zheng X, Huang L, Ding C. Influence of hierarchical hybrid micro/nano-structured surface on biological performance of titanium coating. J Mater Sci. 2012;47(3):1411–7.
Mesquita-Guimarães J, Detsch R, Souza AC, Henriques B, Silva FS, Boccaccini AR, et al. Cell adhesion evaluation of laser-sintered HAp and 45S5 bioactive glass coatings on micro-textured zirconia surfaces using MC3T3-E1 osteoblast-like cells. Mater Sci Eng C. 2020;109: 110492. https://doi.org/10.1016/j.msec.2019.110492.
Sun Y, Sun J, Wu X, Li Y, Li X, Li R, et al. Mechanism of zirconia microgroove surface structure for osseointegration. Mater Today Adv. 2021;12: 100159.
Han A, Tsoi JK, Lung CY, Matinlinna JP. An introduction of biological performance of zirconia with different surface characteristics: a review. Dent Mater J. 2020;39(4):523–30.
Lumetti S, Manfredi E, Ferraris S, Spriano S, Passeri G, Ghiacci G, et al. The response of osteoblastic MC3T3-E1 cells to micro-and nano-textured, hydrophilic and bioactive titanium surfaces. J Mater Sci Mater Med. 2016;27(4):1–9.
Hao L, Lawrence J, Chian K. Osteoblast cell adhesion on a laser modified zirconia based bioceramic. J Mater Sci Mater Med. 2005;16(8):719–26.
Acknowledgements
Financial support for this study was provided by a Grant No. 371399055 from the Materials and Energy Research Center (MERC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghalandarzadeh, A., Javadpour, J., Majidian, H. et al. The evaluation of prepared microstructure pattern by carbon-dioxide laser on zirconia-based ceramics for dental implant application: an in vitro study. Odontology 111, 580–599 (2023). https://doi.org/10.1007/s10266-022-00781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-022-00781-x