Abstract
Age-related alterations of skeletal muscle are numerous and present inconsistently, and the effect of their interaction on contractile performance can be nonintuitive. Hill-type muscle models predict muscle force according to well-characterised contractile phenomena. Coupled with simple, yet reasonably realistic activation dynamics, such models consist of parameters that are meaningfully linked to fundamental aspects of muscle excitation and contraction. We aimed to illustrate the utility of a muscle model for elucidating relevant mechanisms and predicting changes in output by simulating the individual and combined effects on isometric force of several known ageing-related adaptations. Simulating literature-informed reductions in free Ca2+ concentration and Ca2+ sensitivity generated predictions at odds qualitatively with the characteristic slowing of contraction speed. Conversely, incorporating slower Ca2+ removal or a fractional increase in type I fibre area emulated expected changes; the former was required to simulate slowing of the twitch measured experimentally. Slower Ca2+ removal more than compensated for force loss arising from a large reduction in Ca2+ sensitivity or moderate reduction in Ca2+ release, producing realistic age-related shifts in the force-frequency relationship. Consistent with empirical data, reductions in free Ca2+ concentration and Ca2+ sensitivity reduced maximum tetanic force only slightly, even when acting in concert, suggesting a modest contribution to lower specific force. Lower tendon stiffness and slower intrinsic shortening speed slowed and prolonged force development in a compliance-dependent manner without affecting force decay. This work demonstrates the advantages of muscle modelling for exploring sources of variation and identifying mechanisms underpinning the altered contractile properties of aged muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Evidence has mounted in favour of the view that the intrinsic contractile properties of skeletal muscle are altered in advanced age. Age-related deficits in single fibre specific tension and maximum velocity of shortening have been found for type I and type II fibres from aged muscles of both rodents (Degens et al. 1998; Thompson et al. 1998; González et al. 2000, 2003; Zhong et al. 2006; Kim and Thompson 2013) and humans (Larsson et al. 1997; Frontera et al. 2000; Krivickas et al. 2001; D’Antona et al. 2003; Ochala et al. 2007; Yu et al. 2007; Lamboley et al. 2015; Power et al. 2016; Brocca et al. 2017) and can manifest despite long-term training (Korhonen et al. 2006; Power et al. 2016). Such findings imply that the broad decline in contractile performance with ageing isn’t the sole product of reductions in muscle fibre number and size. Reinforcing this notion are a more limited number of studies demonstrating that fundamental processes involved in activation and contraction are prone to impairment in old muscle, including the kinetics of cross-bridge cycling (Höök et al. 2001; D’Antona et al. 2003; Miller et al. 2013), mechanics of myosin (Lowe et al. 2001) and Ca2+ sensitivity of force (Brooks and Faulkner 1994; Lowe et al. 2002; Lamboley et al. 2015; Straight et al. 2018; Mazara et al. 2021), and handling of Ca2+ by the sarcoplasmic reticulum [SR (Larsson and Salviati 1989; Delbono et al. 1995; Narayanan et al. 1996; Wang et al. 2000; Jiménez-Moreno et al. 2008; Andersson et al. 2011; Umanskaya et al. 2014)].
Impaired intrinsic contractile performance, however, is not universally observed for old muscle (Trappe et al. 2003; Hvid et al. 2011; Sundberg et al. 2018; Teigen et al. 2020; Mazara et al. 2021). The same is true of disturbances to cellular level contractile processes, such as Ca2+ sensitivity of force (Eddinger et al. 1986; Plant and Lynch 2001; Lamboley et al. 2015; Teigen et al. 2020) and SR Ca2+ uptake (Fitts et al. 1984; Narayanan et al. 1996; Thomas et al. 2010). When human single fibre data published within the last decade are considered (Claflin et al. 2011; Hvid et al. 2011, 2017; Miller et al. 2013; Sundberg et al. 2018; Straight et al. 2018; Gries et al. 2019; Teigen et al. 2020; Grosicki et al. 2021; Mazara et al. 2021), a compelling argument could be made that neither type I nor type II fibres show an appreciable decline in specific force or shortening speed. Yet, age-related deficits in joint-level contraction speed and mass-specific mechanical power arise in the absence of impaired single fibre function (Reid et al. 2012; Sundberg et al. 2018). Prolonged or slowed force rise and decay and elevated force generation at submaximal stimulation frequencies (i.e. leftward-shifted force-frequency relationship) are also among the most commonly observed features of whole muscle in advanced age (Fitts et al. 1984; Davies et al. 1986; Larsson and Edström 1986; Vandervoort and McComas 1986; Brooks and Faulkner 1988; Alway 1995; Roos et al. 1999; Dow et al. 2005; McNeil et al. 2007; Tevald et al. 2009). A plausible explanation may be that an age-related elevation of the fractional area occupied by type I fibres or of the myosin heavy chain (MHC) I fibre content (Larsson et al. 1978; Coggan et al. 1992; Hunter et al. 1999; Short et al. 2005; Cui et al. 2008; Nilwik et al. 2013; Sonjak et al. 2019) is sufficient to produce a slower contractile phenotype (Ranatunga and Thomas 1990; Harridge et al. 1996).
Predicting altered mechanical output and identifying the underlying determinants remains challenging because contractile properties present inconsistently in advanced age, which may be reconciled with the myriad alterations that aged muscle can exhibit. Reports of the effect of age on single fibre or whole muscle contractile performance can be conflicting or show variation across taxa (Ballak et al. 2014), rodent strains (Rice et al. 2005), muscles (Brooks and Faulkner 1988; Brown and Hasser 1996; Narayanan et al. 1996; Hill et al. 2020), and as a function of activity level or training status (Fitts et al. 1984; Klitgaard et al. 1989; D’Antona et al. 2007), sex (Degens et al. 1998; Krivickas et al. 2001; Hill et al. 2020) and fibre type (Yu et al. 2007; Kim and Thompson 2013; Lamboley et al. 2015). Even for a given muscle of a model organism, the effect of ageing on contractile behaviour can vary (Brooks and Faulkner 1988; Moran et al. 2005). In addition to the aforementioned adaptations of cellular level function (e.g. slower cross-bridge kinetics), and a relative increase in type I fibre content, aged muscle may exhibit structural adaptations, such as altered intramuscular and extramuscular connective tissue properties (Gao et al. 2008; Wood et al. 2011; Stenroth et al. 2012; Danos et al. 2016; Holt et al. 2016). Compared to a loss of muscle mass, it is less clear how these adaptations (and others) impact contractile performance, especially when acting in concert, and to what extent these adaptations must present to be meaningful.
Determining the impact of age-related changes in muscle structure and function on mechanical output isn’t always feasible. For example, experimental approaches to quantifying excitation-SR Ca2+ release coupling and SR Ca2+ uptake dynamics may preclude myosin-actin interaction or be performed without simultaneous measurement of contractile force (Larsson and Salviati 1989; Delbono et al. 1995; Narayanan et al. 1996; Wang et al. 2000). In this context, it is also worth noting that crude homogenates of muscle frequently used to study Ca2+ release and Ca2+ uptake in advanced age (Fitts et al. 1984; Hunter et al. 1999; Thomas et al. 2010; Russ et al. 2011) may also be sensitive to an age-related increase in type I fibre content. The multifaceted and diverse nature of muscle deterioration and remodelling in response to ageing places importance on the interaction of adaptations. Interaction effects may be nonintuitive and may not always result in obvious impairment. Whereas an age-related reduction in the Ca2+ sensitivity of force may compound a reduction in SR Ca2+ release, an age-related slowing of SR Ca2+ uptake may offer a buffering effect. Linking any single adaptation to impaired contractile performance may be difficult when the scope of the study from an explanatory point of view is narrow and the broader extent of senescence is uncertain.
Establishing the likelihood that an altered property would appreciably impair contractile performance, in isolation and when acting in concert, might aid our understanding of altered contractile performance in advanced age from mechanistic and predictive perspectives. Muscle models are useful tools for exploring the effects of muscle design and adaptation on contractile performance (Wisdom et al. 2015). Several common traits of aged muscle contractile performance have been accurately simulated by adjusting model parameters to reflect known changes in activation and contraction dynamics and muscle–tendon morphology (Thelen 2003; Hasson and Caldwell 2012). Hill-type models simulate contractile behaviour according to well-established intrinsic mechanical phenomena (Curtin et al. 1998; Williams et al. 1998; Wakeling and Johnston 1999) and can be integrated with relatively simple, yet physiologically-grounded activation dynamics (Lichtwark and Wilson 2005a). Because many of the parameters used in Hill-type models can be related to muscle–tendon structure and intrinsic muscle properties, such models have the potential to elucidate the predominant mechanisms of altered force output and help explain unexpected observations and variance reported in the literature.
In this study, we implement a three-element Hill-type muscle model to examine how known changes in muscle function and structure in advanced age affect the contractile properties of muscle during fixed-end contractions. Drawing upon published literature, we simulate the individual and combined effects of impaired Ca2+ release, slower Ca2+ uptake, lower Ca2+ sensitivity of force, slowed intrinsic shortening speed, altered series elastic compliance, and a greater fractional content of type I fibres. Specifically, we evaluate the effects of these adaptations on isometric force during a twitch, brief tetanic contraction, and sustained contractions at submaximal and maximal stimulation frequencies. We then discuss the use of the model to explain how these known adaptations might affect muscle force, consider the adaptations most consistent with the contractile properties of aged muscle observed experimentally and reported by others in the literature, and identify certain conditions that may result in non-intuitive outcomes.
2 Methods
2.1 Experimental twitch data
Plantar flexion twitch torque was measured in 10 young (mean ± SD; age: 28 ± 3 years; body mass: 78 ± 11 kg; height: 179 ± 6 cm) and 18 older (age: 72 ± 5 years; body mass: 76 ± 10 kg; height: 174 ± 5 cm) healthy human adult males. An analysis of the experimental data obtained from young adults and a detailed description of the experimental protocol used for both young and older adults have been published previously (Mayfield et al. 2015). In brief, participants sat with their knee extended and right foot securely fixed to a non-compliant rotational footplate. The ankle was set to a neutral position (foot 90° relative to tibia). Two custom-built strain gauges positioned directly under the footplate measured isometric plantar flexion force evoked by percutaneous electrical stimulation of the tibial nerve. Single supramaximal square-wave pulses were delivered to elicit unpotentiated twitches, which were evaluated for peak torque, contraction time and half-relaxation time. Unpaired Student’s t-tests or Welch’s t-tests (unequal variance) were performed to test the effect of age. Statistical significance was set at P < 0.05 and effect sizes were calculated as eta squared (η2).
2.2 Hill-type muscle model
We implemented an adapted Hill-type muscle model previously shown to successfully predict the time course of muscle force during contractions involving either ramp shortening or lengthening, or sinusoidal length changes (Curtin et al. 1998; Lichtwark and Wilson 2005a). The model (Mayfield and Lichtwark 2022), developed in Simulink (MathWorks, Natick, MA), consists of a contractile element (CE) and parallel elastic element (PEE) arranged in-series with an elastic element (SEE). The active force output of the CE depends on the interaction of CE activation, length, and velocity dynamics.
2.2.1 Activation
Activation of the CE is regulated by the concentration of an activator, which we consider to be calcium, in a single compartment. Calcium ions (Ca2+) are released transiently at a constant rate in response to each stimulus and subsequently removed at a rate dependent on the Ca2+ concentration (Fig. 1a). Ca2+ release occurs over a defined pulse width according to the following equation:
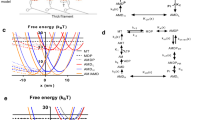
Muscle model properties. a Activator concentration (lower trace) and activation level during a twitch (dashed) and 1 s stimulation train at 10 Hz. b Activation-activator relationship. c Activation-pCa relationship. d CE and PEE force–length relationships. Shaded region represents range of optimal CE lengths. e CE force–velocity relationship. f SEE force-strain relationship
Otherwise, Ca2+ is removed according to the following equation:
where a is the concentration of activator (Ca2+) and τ1 and τ2 are the time constants for the rise and fall of Ca2+, respectively (Lichtwark and Wilson 2005a). Ca2+ release for a second stimulus is attenuated relative to the first for brief interstimulus intervals (Caputo et al. 2004; Barclay 2012). Inactivation of Ca2+ release was incorporated into the model by reducing pulse width according to a single exponential equation describing the recovery of Ca2+ release with respect to interstimulus interval:
where A is the minimum relative Ca2+ release (i.e. maximum inactivation), isi is the interstimulus interval and r is the time constant for the recovery of relative Ca2+ release (Barclay 2012). A and r were set at 20% and 350 ms, respectively, such that the force-frequency relationship was comparable to empirical observations for predominantly slow muscle [e.g. rat soleus (Ranatunga 1982; Larsson and Edström 1986)] or several muscles with varying fibre type compositions crossing the same joint (Marsh et al. 1981; Sale et al. 1982).
The relationship between Ca2+ and activation (Fig. 1b) is given by a sigmoidal function of the form:
where Act is thin filament activation and represents the fraction of cross-bridge binding sites available for cycling, nH is the Hill coefficient, and a50 is the activator concentration required for half-maximal cross-bridge activation (Curtin et al. 1998). nH and a50 (i.e. pCa50) are indices of cooperative activation and the Ca2+ sensitivity of force, respectively (Walker et al. 2010).
To relate the activation-activator relationship in the model to the force-pCa relationship of permeabilised single fibres (Hellam and Podolsky 1969; Stephenson and Williams 1982), we assumed that the activator concentration achieving saturation was equivalent to a calcium concentration of 10 μM or pCa 5 (Fig. 1c); pCa is the negative log of the theoretical calcium concentration. A form of the Hill equation was then used to describe the relationship between calcium concentration and force:
where pCa is the negative log of the activator concentration, and pCa50 is the negative log equivalent of a50 (Martyn and Gordon 2001).
2.2.2 CE force–length & force–velocity relationships
Active force generated by the CE was modelled according to classic force–length (Gordon et al. 1966) and force–velocity (Hill 1938) relationships (Fig. 1d, e). The speed at which the CE shortens with respect to force, which is scaled by CE activation and length, was modelled according to a normalised form of the Hill equation:
where P is force, P0 is the maximum isometric force, K represents a/P0 and indicates the curvature of the rectangular hyperbola describing the concentric force–velocity relationship, V is the velocity of CE shortening and Vmax is the maximum velocity of CE shortening (Seow 2013). The speed at which the CE lengthens with respect to an externally applied force (Fig. 1e) was modelled according to the following equation:
where c is a constant indicating the maximum eccentric force expressed relative to P0, k is a constant relating to the y-intercept, whereby \(c-k=1\) to meet the condition of \(P/{P}_{0}=1\) for a lengthening velocity of 0, and q is a constant describing the curvature of the eccentric force–velocity relationship (Otten 1987; Azizi and Roberts 2014). c, k, and q were assigned values of 1.8, 0.8 and 7, respectively.
2.2.3 PEE force–length relationship
The PEE was assigned an exponential passive force–length relationship (Fig. 1d) according to the following exponential equation:
where kPEE is an exponential shape factor, LCE is the relative CE length, Lslack is the slack length of the PEE, and εPEE is the passive strain of the CE when an external load equal to P0 is applied (Thelen 2003). When Lslack is not equal to 1.0, the CE length at εPEE is equal to Lslack plus εPEE. kPEE, Lslack and εPEE were assigned values of 4, 0.98 and 0.57, respectively. These values are similar to those used previously for human plantar flexors (Thelen 2003) and generate a passive-force length relationship generally consistent with experimental data (Winters et al. 2011; Rubenson et al. 2012; Moo et al. 2020).
2.2.4 SEE force–length relationship
The SEE was assigned a non-linear load-deformation relationship consistent with experimental data for tendon and aponeurosis (Lieber et al. 1991; Trestik and Lieber 1993; Zuurbier et al. 1994; Loren and Lieber 1995; Cui et al. 2009). The general relationship was derived from a non-linear least squares fit of force and deformation data reported for mammalian tendon (Bennett et al. 1986) using the following equation:
where a and b are regression constants and x is tendon deformation. SEE strain at P0 (εSEE, Fig. 1f) was set to 0.05 (Muramatsu et al. 2001; Arampatzis et al. 2005; Karamanidis and Arampatzis 2006). SEE stiffness was defined as the maximum deformation of the SEE normalised to P0 and the optimum length of the CE (L0), giving a normalised stiffness [kSEE (Lichtwark and Wilson 2005b)]. SEE length (LSEE) and L0 were set at 300 (Arampatzis et al. 2005; Karamanidis and Arampatzis 2005) and 50 mm (see Hessel et al. 2021 main text and supplementary data), respectively, giving a kSEE of 3.33 P0·L0−1. The inverse of normalised SEE stiffness—normalised SEE compliance (i.e. 30%)—relates closely to the fixed-end compliance, which represents CE strain against the stretch of the SEE during a maximum tetanic contraction (Roberts 2002). We have instead defined fixed-end compliance as CE shortening expressed relative to L0, as to allow normalised SEE compliance and fixed-end compliance to be equal. Because there is considerable passive tension at the optimal MTU length in the model (initial CE length of ~ 1.23 L0), consistent with experimental observations for the human plantar flexors (see Hessel et al. 2021 supplementary data), SEE deformation and CE shortening during maximum force development are ~ 23%, rather than 30% of L0. This value generally agrees with experimental data for the human plantar flexors (see Hessel et al. 2021 supplementary data) after considering the overestimation of fascicle shortening against the stretch of tendon and aponeurosis owing to inevitable ankle rotation (Karamanidis et al. 2005).
2.3 Model optimisation to simulate plantar flexion twitch of young men
Model parameters for the initial state or control condition were optimised to minimise the combined error in contraction time and half-relaxation time between simulated and experimental twitches. An additional requirement was that the relative amplitude of the simulated twitch be ~ 0.2 P0. The simulated twitch was for an initial CE length of 1.0 L0. The experimental twitch represented the waveform average for young men determined from twitches recorded with the ankle at 0° and the knee extended. Initially, τ1, τ2, [a]50, nH and Vmax parameters were included in the optimization process. Where appropriate, physiological upper and lower limits were imposed. Subsequently, nH and Vmax were constrained at values of 3 and 6, respectively, and the optimization process was repeated.
Although reported values of nH are wide-ranging, a value of 3 is generally intermediate between values reported for type I and II fibres or similar to values reported for the former (Stephenson and Williams 1981; Fink et al. 1986, 1990; Lynch et al. 1991; Hvid et al. 2011, 2013). The Vmax value of 6 L0·s−1 agrees closely with the value of 6.2 FL·s−1 (fibre lengths per second) measured for human medial gastrocnemius fascicles in vivo (Hauraix et al. 2015), and is comparable to values reported for rat soleus [6–7.3 FL·s−1 (Ranatunga and Thomas 1990; Ranatunga 1998)] and mouse soleus [4.5 and 8.6 FL·s−1 (Luff 1981; Lichtwark and Barclay 2010)] muscles at physiological temperatures; the type I fibre composition of these muscles is approximately 73 and 67%, respectively (Asmussen and Maréchal 1989). We arrived at a slightly lower estimate of 60% for the MHC I fibre content for the triceps surae [see section ‘2.3.10 Type I fibre fractional area (i.e., MHC I fibre content)’].
2.4 Simulating ageing-related adaptations
2.4.1 Free Ca2+ concentration
There is evidence that both SR Ca2+ release and SR Ca2+ uptake are impaired in advanced age (Larsson and Salviati 1989; Delbono et al. 1995; Narayanan et al. 1996; Hunter et al. 1999; Wang et al. 2000; Jiménez-Moreno et al. 2008), and that aged single fibres can exhibit a deficit in peak free Ca2+ concentration (González et al. 2003; Andersson et al. 2011; Umanskaya et al. 2014). To our knowledge, the effect of slowed SR Ca2+ uptake on steady-state free Ca2+ concentration and free Ca2+ decay in intact fibres has not been studied in advanced age. It is unclear whether both adaptations can coexist (Russ et al. 2011, 2014) and to what extent each alteration influences free Ca2+ concentration. Accordingly, we simulated the independent and concomitant effects of impaired Ca2+ release and slowed Ca2+ uptake. For simplicity, we assume that the reductions in peak free Ca2+ concentration reported in the literature reflect impaired Ca2+ release without a concomitant slowing of SR Ca2+ uptake. This simplification allows experimental values of the deficit in Ca2+ concentration in intact fibres to be emulated by scaling down the instantaneous Ca2+ availability in the model, rather than increasing the time constant of Ca2+ release, τ1. Importantly, the fractional deficit in peak free Ca2+ concentration in intact fibres associated with ageing appears to be similar for maximal and submaximal contractions (González et al. 2003; Eshima et al. 2020).
To our knowledge, there exists only one study of the effect of age on SR Ca2+ release in human single fibres (Delbono et al. 1995), whereas several studies have been performed on rodent single fibres. In these studies, intact fibres were isolated exclusively from fast-twitch muscles without fibre type identification (Wang et al. 2000, 2002; González et al. 2003; Jiménez-Moreno et al. 2008; Andersson et al. 2011; Umanskaya et al. 2014; Fodor et al. 2020; Eshima et al. 2020). Each of these studies, including the study on human type II fibres, demonstrated an age-related deficit in SR Ca2+ release rate or peak intracellular Ca2+ concentration. Collectively, impairment typically ranged from ~ 30–50%. Accordingly, we incorporated a 30 or 50% reduction in peak Ca2+ concentration by applying a scaling factor to the instantaneous Ca2+ concentration of 0.7 or 0.5, respectively. We described the qualitative and quantitative effect (% change) of lower free Ca2+ availability on the time course (i.e., contraction time, half-relaxation time) and amplitude of the twitch, submaximal force and the force-frequency relationship, and maximum force.
2.4.2 SR Ca2+ uptake (τ 2)
The rate of SR Ca2+ uptake may be lower in advanced age, but it is not a universal observation. The effect of age on SR Ca2+ uptake rate has been predominantly studied in rodent muscle and using a variety of muscle preparations. To our knowledge, only a single study has been performed on human muscle, specifically, crude homogenates form the vastus lateralis muscle (Hunter et al. 1999). In many regards, the findings are inconsistent. Slowing has been demonstrated for skinned type II fibres but not skinned type I fibres (Larsson and Salviati 1989), SR vesicles isolated from slow-twitch muscle but not fast-twitch muscle (Narayanan et al. 1996; Russ et al. 2014), and muscle homogenates from fast-twitch (Russ et al. 2011), slow-twitch (Narayanan et al. 1996), and mixed-fibre type (Hunter et al. 1999) muscles but not in every instance (Fitts et al. 1984; Narayanan et al. 1996; Thomas et al. 2010); measurements from muscle homogenates may be susceptible to confoundment by a shift in MHC isoform composition. For those studies supporting an age-related reduction in Ca2+ uptake rate, the size of the slowing effect ranged from ~ 20–52% (Larsson and Salviati 1989; Narayanan et al. 1996; Hunter et al. 1999; Russ et al. 2011). Accordingly, we incorporated a 30 or 50% reduction in the rate constant for Ca2+ removal. The rate constant for Ca2+ removal is the reciprocal of the time constant, τ2. Therefore, τ2 was increased by 43 and 100%. We described the qualitative and quantitative effect of slower Ca2+ uptake on the time course and amplitude of the twitch, and submaximal force and the force-frequency relationship.
2.4.3 Ca2+ sensitivity (pCa50)
Lower Ca2+ sensitivity of force has been found in advanced age, but it is not a universal observation. Studies showing no effect of age (Plant and Lynch 2001; Hvid et al. 2011, 2013, 2017; Lamboley et al. 2015; Teigen et al. 2020; Mazara et al. 2021) are similar in number to those showing an age-related deficit. Reduced Ca2+ sensitivity in advanced age has been demonstrated by several studies for type II fibres from human (Lamboley et al. 2015; Straight et al. 2018; Mazara et al. 2021) and rodent (Brooks and Faulkner 1994; Lowe et al. 2002) muscles. In contrast, only a single study has shown Ca2+ sensitivity to be lower for type I fibres (Straight et al. 2018). The deficit in pCa50 reported by these studies ranges from 0.05 to 0.15 pCa units but is typically ~ 0.10 pCa units. Three studies reported a similar difference between means (0.08–0.10 pCa units) without detecting a significant age effect (Hvid et al. 2011, 2013; Mazara et al. 2021). For two of those studies, which sampled from just 11–15 aged type II fibres, we could deduce that the deficit in pCa50 was of a moderate effect size (Cohen's d = 0.38–0.49). Accordingly, we incorporated a reduction in pCa50 of 0.05 or 0.10 pCa units and described the qualitative and quantitative effect on the time course and amplitude of a twitch, submaximal force and the force-frequency relationship, maximum force, and relative force summation.
2.4.4 Cooperativity of activation (n H)
The weight of evidence from human and rodent studies indicates that the slope of the force-pCa relationship (i.e. cooperativity)—represented by the Hill coefficient, nH—is unaltered in advanced age (Eddinger et al. 1986; Brooks and Faulkner 1994; Hvid et al. 2011, 2013; Lamboley et al. 2015; Straight et al. 2018; Teigen et al. 2020). Challenging this view is one study on human muscle that found nH to be elevated for type II fibres in advanced age (Straight et al. 2018) and one study on rat muscle that found nH (> 0.5 P0) to be lower for type II fibres (Lowe et al. 2002). Because there are generally pronounced differences in nH between fibre types (Fink et al. 1986, 1990; Gardetto et al. 1989; Danieli-Betto et al. 1990; Gregorevic et al. 2004; Hvid et al. 2011, 2013), and stronger evidence that nH is affected by disuse (Gardetto et al. 1989; Widrick et al. 1998; Hvid et al. 2011, 2013; Monti et al. 2021), we thought it was important to illustrate the effect of this parameter on force generation. We performed simulations in which the reference value of nH was increased and decreased by 1.0. We described the qualitative and quantitative effect on the time course and amplitude of a twitch, submaximal force and the force-frequency relationship, and relative force summation.
2.4.5 Lower free Ca2+ concentration & lower Ca2+ sensitivity (pCa50) in concert
Because the force-pCa relationship is sigmoidal in form, force at near-maximal Ca2+ concentrations is practically insensitive to shifts in Ca2+ sensitivity. A deficit in maximum force generation may only arise when Ca2+ concentration and Ca2+ sensitivity decrease concomitantly. Accordingly, we reduced the instantaneous Ca2+ concentration by 30 or 50% whilst lowering pCa50 by 0.05 or 0.10 pCa units, consistent with our previous manipulations of Ca2+ concentration and Ca2+ sensitivity. We described the qualitative effect of concurrent reductions in Ca2+ concentration and Ca2+ sensitivity on submaximal force and the force-frequency relationship, and quantified the effect on maximal force.
2.4.6 Lower Ca2+ release & slower Ca2+ uptake (τ 2) in concert
It is unclear from recordings of intracellular Ca2+ transients whether impaired Ca2+ release and slowed Ca2+ uptake occur in parallel and the extent to which they may offset one another (González et al. 2003; Andersson et al. 2011; Eshima et al. 2020). Accordingly, we reduced Ca2+ release by 30% and the rate constant of Ca2+ uptake by 40 (67% increase in τ2) in concert. Reductions of equal amount would not alter steady-state Ca2+ in the model relative to the control condition. We described the qualitative effect of lower Ca2+ release and slower Ca2+ uptake on submaximal force and the force-frequency relationship.
2.4.7 Lower Ca2+ sensitivity (pCa50) and slower Ca2+ uptake (τ 2) in concert
On the basis that slower Ca2+ uptake will increase free Ca2+ concentration, thereby increasing force, whereas lower Ca2+ sensitivity will act to decrease force, we examined the effect of these two adaptations acting concomitantly. Specifically, we incorporated both a 30% reduction in the rate constant for Ca2+ removal (43% increase in τ2) and a 0.1 pCa unit reduction in Ca2+ sensitivity (pCa50) and described the qualitative effect on the force-frequency relationship.
2.4.8 Maximum velocity of shortening (V max)
Comparatively, the intrinsic speed of shortening has been extensively studied in young and old muscle fibres from humans and rodents. For both groups, and for both fibre types, the effect of age is inconsistent. Several studies have demonstrated slowing of either or both type I and type II human fibres (Krivickas et al. 2001; D’Antona et al. 2003; Ochala et al. 2007; Yu et al. 2007; Power et al. 2016; Brocca et al. 2017), but just as many studies have found no age effect (Trappe et al. 2003; Claflin et al. 2011; Sundberg et al. 2018; Teigen et al. 2020; Grosicki et al. 2021; Mazara et al. 2021). Similarly, a lower maximal shortening velocity has been demonstrated for aged type I and type II fibres from rodents (Degens et al. 1998; Thompson and Brown 1999; Kim and Thompson 2013), but not in every instance (Eddinger et al. 1986; Brooks and Faulkner 1994; Zhong et al. 2006; Kim and Thompson 2013). Muscle inactivity in advanced age may minimize or negate the effect of age on Vmax (Thompson et al. 1998; D’Antona et al. 2003; Kim and Thompson 2013), although there is evidence to the contrary (Grosicki et al. 2021). The deficit in maximal shortening velocity reported for human muscle fibres ranges from ~ 7–46% (Larsson et al. 1997; Krivickas et al. 2001; Claflin et al. 2011; Power et al. 2016) but is most often ~ 15–25% (Larsson et al. 1997; Krivickas et al. 2001; D’Antona et al. 2003; Ochala et al. 2007; Yu et al. 2007; Brocca et al. 2017). The slowing of intrinsic shortening speed tends to be more pronounced for rodent muscle fibres; three of the four identified instances of slowing represent a deficit of 32–50% (Degens et al. 1998; Thompson and Brown 1999; Kim and Thompson 2013). Accordingly, we incorporated reductions in intrinsic shortening speed of 30 or 50% and described the qualitative and quantitative effect on the time course and amplitude of a twitch and brief tetanic contraction.
The curvature of the force–velocity relationship (i.e. a/P0) does not appear to be altered in aged fibres that do not exhibit a reduction in Vmax (Brooks and Faulkner 1994; Trappe et al. 2003). To our knowledge, a/P0 has not been quantified for aged fibres exhibiting a reduction in Vmax. However, because a/P0 differs markedly between fibre types—human type IIa fibres compared to type I fibres exhibit a two-fold greater value of a/P0—(Bottinelli et al. 1996; Widrick et al. 1996; Gilliver et al. 2009)—we deemed it important to illustrate the effect of this parameter on force development. We performed simulations in which the reference value of a/P0 was increased by 0.05 (50%). We described the qualitative effect on the time course and amplitude of a twitch and brief tetanic contraction. We did not incorporate reductions in a/P0 because the model was unable to simulate the twitch measured experimentally with a more realistic value for muscle at physiological temperatures [model: 0.10; mouse soleus: 0.18 (Luff 1981); rat soleus: 0.22–0.26 (Ranatunga and Thomas 1990; Ranatunga 1998)].
2.4.9 SEE stiffness
The general effect of age on tendon stiffness appears to be distinct for humans compared to certain animal models of ageing. The weight of evidence from in vivo human studies favours an age-associated reduction in tendon stiffness and elastic modulus (see McCrum et al., 2018). Though some studies have found tendon loading behaviour to be unchanged (Carroll et al. 2008; Couppé et al. 2012), no human studies appear to have reported an age-related increase in tendon stiffness. A recent review reported median reductions in stiffness and elastic modulus of 20% and 28%, respectively (McCrum et al. 2018). However, age-related deficits may be as high as 30–55% (Karamanidis and Arampatzis 2006; Onambele et al. 2006; Stenroth et al. 2012; Csapo et al. 2014). Moreover, relative to old adults (> 65 years), very old adults (> 83 years) can exhibit marked reductions (35–40%) in tendon stiffness and elastic modulus (Eriksen et al. 2018).
In contrast to humans, hindlimb tendons of rodents in advanced age regularly exhibit higher stiffness or elastic modulus (Wood et al. 2011; Danos et al. 2016; Wood and Brooks 2016; Leahy et al. 2022). The magnitude of the increase is typically close to 50%. A number of studies have also reported no ageing effect (Nakagawa et al. 1996; Pardes et al. 2017); fewer have reported an age-related reduction (LaCroix et al. 2013). The variable nature of tendon mechanics in advanced age may be partially explained by methodological approach, age at measurement, ageing-associated inactivity, species, and muscle function (Svensson et al. 2016; McCrum et al. 2018).
We incorporated both a reduction in normalised SEE stiffness of 30% and an increase in normalised stiffness of 50%. We described the qualitative and quantitative effect of SEE stiffness on the time course and amplitude of a twitch and brief tetanic contraction.
2.4.10 Type I fibre fractional area (i.e., MHC I fibre content)
Several human and rodent studies have reported an ageing-related increase of 0.10–0.20, or greater (Brocca et al. 2017), for fractional MHC I content or fractional area occupied by type I fibres (Larsson et al. 1978; Klitgaard et al. 1990b; Kadhiresan et al. 1996; Short et al. 2005; Cui et al. 2008; Nilwik et al. 2013; Sonjak et al. 2019; Soendenbroe et al. 2022). Smaller shifts have also been reported (Sullivan et al. 1995; Hunter et al. 1999). We approximated the effect of this adaptation by considering fibre type-related differences in Ca2+ removal rate, Ca2+ sensitivity and cooperativity, and Vmax, and by adjusting composite parameter values according to an increase in type I fibre fractional area. Fibre type-specific values and composite relationships were derived from the whole muscle control values by assigning weightings based on the fractional cross-sectional area of type I and II fibres and assigning fibre type-related differences in each property (Wakeling et al. 2012). Fibre type-specific and composite parameter values were determined according to the following expression:
where x is the fibre type-specific parameter value, p is relative fibre content, xwhole is the whole muscle parameter value, and y is the fibre type-related difference or offset. Some empirical observations support this approach. Single fast and slow fibres arranged in parallel exhibit an intermediate force-pCa relationship generally consistent with a theoretical composite relationship based on fractional fibre type content (Lynch et al. 1995). In contrast to some methods (Zajac 1989; Claflin and Faulkner 1989; Ranatunga and Thomas 1990), our approach assumes some degree of attenuation of shortening speed at loads where whole muscle velocity exceeds the Vmax assigned to type I fibres, which is generally consistent with observations that inactive muscle depresses the speed of shortening (Hatcher and Luff 1987; Holt et al. 2014).
The total MHC isoform content or fractional area occupied by type I fibres is approximately 50 and 65% for the gastrocnemius and soleus muscles, respectively (Edström and Nyström 1969; Green et al. 1981; Coggan et al. 1992; Harridge et al. 1996, 1998). Thus, given that the combined physiological cross-sectional area (PCSA) of the lateral and medial gastrocnemius muscles represents ~ 38% of the total PCSA of the triceps surae (Morse et al. 2005; Albracht et al. 2008; Crouzier et al. 2018), it is estimated that the total type I fibre area of the triceps surae is ~ 60% (i.e. 0.60). Type I fibres, compared to type II fibres, were assumed to exhibit the following differences: Ca2+ removal rate constant 50% slower (Carroll et al. 1997; Liu et al. 1997; Baylor and Hollingworth 2003; Calderón et al. 2010); Ca2+ sensitivity (pCa50) 0.15 pCa units greater and nH 40% lower (Stephenson and Williams 1981; Fink et al. 1986, 1990; Ruff 1989; Laszewski-Williams et al. 1989; Gardetto et al. 1989; Ruff and Whittlesey 1991; Plant and Lynch 2001; Gregorevic et al. 2004; Hvid et al. 2013; Xu et al. 2017; Lamboley et al. 2020); Vmax 70% slower (Larsson and Moss 1993; Bottinelli et al. 1996; Harridge et al. 1996; Widrick et al. 2002; Trappe et al. 2003; Yu et al. 2007; Luden et al. 2008; Sundberg et al. 2018; Teigen et al. 2020). Note that much variability exists for the force-pCa relationships of type I and type II fibres.
After increasing the fractional area of type I fibres by 0.10 and 0.20, from 0.60 to 0.70 and 0.80, respectively, and incorporating adjusted values of τ2, pCa50, nH and Vmax, we described the qualitative and quantitative effect on the contraction time and half-relaxation time of the twitch, and on submaximal force and the force-frequency relationship.
2.5 Model optimisation to simulate plantar flexion twitch of old men
The parameters of the model were adjusted to simulate the plantar flexion twitch of old men. The optimisation approach was similar to that used initially, except parameter limits were imposed consistent with the directionality of impairment. We also incorporated a force-generating capacity (FGC) parameter—analogous to muscle PCSA—to allow twitch force to be lower in advanced age, which we observed experimentally. We assumed that the deficit in twitch force is owing, at least in part, to muscle atrophy (Narici et al. 2003; Morse et al. 2005; Thom et al. 2007). The magnitude of a given parameter adjustment was compared to the parameter change incorporated to simulate age-related adaptation reported in the literature.
3 Results
3.1 Model optimisation to simulate plantar flexion twitch of young men
Force during simulated and experimental twitches are shown in Fig. 2. The time course of force rise and decay are matched well, with both the contraction time and half-relaxation time of the simulated twitch being identical to the values measured experimentally for young men (inset Fig. 2). All model parameters used in simulations of young muscle (i.e., control condition) are reported in Table 1. Unless specified otherwise, all simulated fixed-end contractions were performed at an initial CE length of 1.23 L0 and force displayed in figures represents active CE force. This initial length for a maximal tetanic contraction resulted in a final CE length—after shortening against the stretch of the SEE—on the plateau of the force–length relationship.
Hill-type muscle model optimisation. Experimental plantar flexion twitch torque and simulated twitch force for young (Y) adult humans. Inset, twitch contraction time (CT) and half-relaxation time (HRT) for experimental (filled) and simulated (open) data were identical following parameter optimization (CT: 125 ms; HRT: 102.5 ms). Agreement was achieved between twitch torque measured with the ankle at 0° and the knee extended and twitch force simulated at an initial CE length of 1.0 L0
3.2 Ca2+ handling and thin filament activation
3.2.1 Instantaneous Ca2+ concentration
Reducing the instantaneous free Ca2+ concentration by either 30 or 50% dramatically lowered twitch force and abbreviated the rise and decay of twitch force (Fig. 3b, inset). Substantial force loss was also evident during sustained stimulation at submaximal frequencies, as shown by the marked rightward shift of the force-frequency relationship (Fig. 3d, e). There was a pronounced deficit in force for frequencies yielding calcium concentrations situated on the steep region of the force-pCa relationship (Fig. 3e, f). In contrast, maximum tetanic force was only modestly affected by the imposed reductions in free Ca2+ concentration (Fig. 3e, f). During 200 Hz stimulation, the 50% reduction in Ca2+ availability only lowered cross-bridge activation to 97%. Because submaximal force was disproportionately affected, reduced Ca2+ availability lowered the ratio of twitch-to-tetanic force.
Effect of calcium concentration on submaximal and maximal force generation. a Activator concentration and b active force during a twitch at 1.23 L0 with varying levels of instantaneous Ca2+ concentration (con.). Instantaneous Ca2+ concentration was reduced by 30 and 50% relative to the control condition. Inset, twitch contraction time and half-relaxation time. c Activator concentration and d active force during sustained 10 Hz stimulation at 1.23 L0. e Force-frequency relationships at 1.23 L0. Active force expressed relative to P0 of control condition. f Activation-pCa relationships. In e and f, solid squares denote 10 Hz stimulation and small and large solid circles denote 100 and 200 Hz stimulation, respectively. In f, shaded regions represent the Ca2+ concentration range between 100 and 200 Hz
3.2.2 Ca2+ uptake
Decreasing the rate constant of Ca2+ decay by 30% to slow the removal of Ca2+ increased twitch force by 12% (elevating the ratio of twitch-to-tetanic force) and prolonged the contraction time and half-relaxation time by 25% and 38%, respectively (Fig. 4b, inset). Slowing Ca2+ uptake by 50% increased the size of these effects. Slower Ca2+ removal during sustained stimulation resulted in greater calcium accumulation (Fig. 4c), which caused force at submaximal frequencies to increase dramatically (Fig. 4d), as illustrated by the leftward-shifted force-frequency relationships (Fig. 4e).
Effect of calcium uptake rate on force generation at submaximal calcium. a Activator concentration and b active force during a twitch at 1.23 L0 with varying rates of Ca2+ removal (rem.). The rate constant of Ca2+ removal was reduced by 30 and 50% relative to the control condition, representing increases in the time constant of Ca2+ removal, τ2, of 43 and 100%, respectively. Inset, twitch contraction time and half-relaxation time. c Activator concentration and d active force during sustained 10 Hz stimulation at 1.23 L0. e Force-frequency relationships at 1.23 L0. Active force expressed relative to P0 of control condition. Inset, activation-pCa relationship. Solid squares denote the activator concentration and activation level during sustained stimulation at 10 Hz
3.2.3 Calcium sensitivity (pCa50)
Reducing the Ca2+ sensitivity of force by shifting the force-pCa relationship rightward 0.05 pCa units reduced twitch force by 16% and abbreviated the twitch contraction time and half-relaxation time by 11 and 5%, respectively (Fig. 5b, inset). Lowering Ca2+ sensitivity by 0.10 pCa units resulted in additional force attenuation and an even briefer contraction. Reduced Ca2+ sensitivity also decreased force during stimulation at submaximal stimulation frequencies (< 50 Hz) such that the force-frequency relationship was shifted rightward (Fig. 5c). Force loss was greatest for frequencies that encompassed the steep region of the force-pCa relationship and increased in proportion to the reduction in Ca2+ sensitivity. Maximum tetanic force was unaffected by the imposed reductions in Ca2+ sensitivity; no force loss was evident for 100 or 200 Hz stimulation. Relative force summation—illustrated as the force during a brief tetanic contraction (50 ms, 100 Hz) expressed relative to twitch force (Fig. 5d, inset)—was higher following the reduction in Ca2+ sensitivity, though there remained a deficit in tetanic force.
Effect of calcium sensitivity and cooperativity on force at submaximal and maximal calcium concentrations. a Activation-pCa relationships with varying Ca2+ sensitivities and e cooperativities. Ca2+ sensitivity of force was reduced by 0.05 and 0.10 pCa units relative to the control condition. Cooperativity was decreased from 3 to 2 and increased to 4. a Inset, corresponding activation-activator relationships. Small and large black circles denote 100 and 200 Hz stimulation, respectively. b, f Twitch force at 1.23 L0. Inset, twitch contraction time and half-relaxation time. c, g Force-frequency relationships at 1.23 L0. Active force expressed relative to P0 of control condition. d, h Active force plotted as a function of activator concentration for a 50 ms, 100 Hz train (i.e., 6 pulses) at 1.23 L0. Inset, force summation; the ratio of tetanic (50 ms, 100 Hz) force-to-twitch force
3.2.4 Cooperativity of activation (n H)
Decreasing nH, or cooperativity, from 3 to 2 to reduce the slope of the force-pCa relationship increased twitch force by 11% and prolonged the twitch contraction time and half-relaxation time by 26 and 44%, respectively (Fig. 5f, inset). Lowering cooperativity also reduced the slope of the force-frequency relationship such that force was slightly greater at low frequencies but considerably lower at moderate and high frequencies of stimulation (Fig. 5g); increasing cooperativity had the opposite effect. Again, maximum tetanic force was largely unaffected by altering cooperativity. A small deficit (2%) in maximum cross-bridge activation level arose when cooperativity was lowered. As such, reducing cooperativity increased the ratio of twitch-to-tetanic force, whereas increasing cooperativity reduced this ratio. Similarly, lowering cooperativity also reduced relative force summation (Fig. 5h, inset). Despite twitch force being 20% (0.04 P0) greater for a nH of 2 compared to a nH of 4, peak force during the brief tetanic contraction (50 ms, 100 Hz) was 12% (0.06 P0) lower for the former compared to the latter (Fig. 5h).
3.2.5 Lower Ca2+ concentration & lower Ca2+ sensitivity in concert
Reducing Ca2+ concentration and Ca2+ sensitivity in concert lowered force dramatically at submaximal stimulation frequencies but only slightly reduced maximum tetanic force (Fig. 6b, c). Reducing Ca2+ availability by 30% and lowering pCa50 by 0.05 pCa units merely reduced the cross-bridge activation level to 98% during 200 Hz stimulation (Fig. 6b). Even when calcium concentration and calcium sensitivity were concurrently reduced by 50% and 0.10 pCa units, respectively, cross-bridge activation (Act) still exceeded 92% during 200 Hz stimulation (Fig. 6c). For 100 Hz stimulation, the steady-state Ca2+ concentration was 29% lower compared to 200 Hz. As such, there was a more significant reduction in cross-bridge activation level during 100 Hz stimulation when a 50% reduction in Ca2+ concentration was imposed and Ca2+ sensitivity was concurrently reduced by 0.05 (Act = 87%) or 0.10 pCa (Act = 82%) units (Fig. 6c). Figure 6d illustrates how the sigmoidal form of the force-pCa relationship limits the effect of an imposed reduction in Ca2+ concentration on maximum tetanic force, even when the Ca2+ sensitivity of force is reduced by 0.1 pCa units.
Effect of reducing calcium concentration and Ca2+ sensitivity in concert on maximal force generation. a Activation-pCa relationships with varying Ca2+ sensitivities. Ca2+ sensitivity was reduced by 0.05 and 0.10 pCa units relative to the control condition (5.99). b, c Force-frequency relationships at 1.23 L0 for concomitant reduction in Ca2+ concentration and Ca2+ sensitivity. Force expressed relative to P0 of control condition. Instantaneous activator concentration was reduced by 30% in b and 50% in c. Inset, activator-frequency relationships for control and reduced Ca2+ concentration (dashed) conditions. d Activation-pCa relationships illustrating Ca2+ concentration and activation level for 100 (small circle) and 200 Hz (large circle) stimulation. Data are shown for a 50% reduction in instantaneous activator concentration. The two overlapping shaded regions represent the Ca2+ concentration range between the control and 50% reduction conditions for 100 and 200 Hz stimulation. Inset, activator-frequency relationships
3.2.6 Slower Ca2+ uptake and lower Ca2+ release or lower Ca2+ sensitivity in concert
The pronounced deficit in steady-state force at low and moderate stimulation frequencies that resulted from a 30% reduction in Ca2+ release (Fig. 3e) was more than balanced by concurrently decreasing the rate constant for Ca2+ uptake by 40% (Fig. 7b, c). The reduction in Ca2+ release caused force to be lower at the beginning of the contraction, as is evident when twitch force is compared (Fig. 7b); however, the slower rate of Ca2+ removal allowed Ca2+ to accumulate to a higher steady-state concentration (Fig. 7a). A similar compensation effect occurred when Ca2+ uptake rate was reduced by 30% whilst Ca2+ sensitivity was lowered by 0.10 pCa units. During a twitch and at very low stimulation frequencies, lower Ca2+ sensitivity reduced force (Fig. 7e, f). At faster frequencies, the increase in Ca2+ concentration more than compensated for the reduction in Ca2+ sensitivity, shifting the force-frequency relationship leftward (Fig. 7e, f).
Effect of reducing Ca2+ release and Ca2+ uptake rate, or Ca2+ sensitivity and Ca2+ uptake rate in concert. a, d Activator concentration and b, e force during a twitch and sustained 10 Hz stimulation at 1.23 L0. In a, Ca2+ release was reduced by 30%, but the rate constant of Ca2+ uptake was also reduced by 40% (67% increase in τ2) relative to the control condition. In d, the rate constant of Ca2+ uptake was reduced by 30% (43% increase in τ2), but the Ca2+ sensitivity of force (e inset) was reduced by 0.10 pCa units relative to the control condition. c, f Force-frequency relationships at 1.23 L0. Force expressed relative to P0 of control condition
3.3 CE-SEE interaction
3.3.1 Maximum velocity of shortening (V max)
Because the CE shortens against the stretch of the SEE during force development (see Fig. 8c inset), reducing Vmax by 30% reduced twitch force by 12% and increased twitch contraction time by 13%; twitch half-relaxation time was practically unaltered (Fig. 8b, inset). A similar effect was observed for a brief tetanic contraction (50 ms, 100 Hz) following a 30% reduction in Vmax (Fig. 8c). Lowering Vmax by 50% increased the loss of force and further prolonged the rise of force. Brief tetanic contractions were performed at an initial CE length of 1.0 L0, illustrating that the reductions in peak force with decreasing Vmax arise despite more favourable final CE lengths—greater force arises from greater shortening against the stretch of the SEE (Fig. 8c inset). Increasing a/P0 produced qualitatively similar results for twitch and tetanic contractions as increasing Vmax (Fig. 8).
Effect of intrinsic shortening speed and SEE stiffness on force development. a Force–velocity relationships with varying values of Vmax and a/P0. Vmax was reduced by 30 and 50% relative to the control condition, and a/P0 was increased by 0.05 for illustrative purposes only. d SEE force–length relationships with varying stiffnesses. Deformation expressed as percentage of CE L0. Average SEE stiffness normalised to P0 and CE L0 (kSEE, inset) was reduced by 30% and increased by 50% relative to the control condition. Dotted line indicates passive force at optimal initial CE length of 1.23 L0. b, e Twitch force at an initial CE length of 1.0 and 1.23 L0, respectively. Inset, twitch contraction time and half-relaxation time. c, f Force during a brief tetanic contraction (50 ms, 100 Hz) at an initial CE length of 1.0 and 1.23 L0, respectively. Inset, CE length as a function of time in c and force in f; the dotted lines indicate total force. Solid bar underneath force and CE length traces at contraction onset represents duration of stimulation
3.3.2 SEE stiffness (k SEE)
Reducing SEE stiffness by 30% prolonged twitch contraction time by 13% and attenuated twitch force by 9%. Conversely, increasing SEE stiffness by 50% abbreviated twitch rise time by 14% and increased twitch force by 9% (Fig. 8e). The attenuation or improvement of the rate of force development and peak force during a brief contraction was not the result of less or more favourable CE operating lengths, which were restricted to the descending limb of the force–length relationship. For brief tetanic contractions performed at an initial CE length of 1.23 L0, peak force was greatest for the stiffer SEE condition, even though the average operating length of the CE was less favourable (Fig. 8f, inset).
3.3.3 V max & SEE stiffness interaction
Decreasing Vmax by 50%, from 6 to 3 L0·s−1, had a more modest effect on peak twitch force when SEE stiffness was adjusted to give normalised SEE deformations of less than 8% (compare Fig. 9b and 8b). At 3.33% (i.e., 30 P0·L0−1), twitch force was 13% (0.05 P0) lower (Fig. 9b). In contrast, reducing Vmax by 50% lowered twitch force (at a comparable initial CE length) by 26% (0.06 P0) when normalised SEE deformation was 30% [i.e., 3.33 P0·L0−1 (Fig. 8b). The greater effect of intrinsic shortening speed on force development with decreasing SEE stiffness was independent of differences in force-generating potential related to CE length (Fig. 9c).
Effect of SEE stiffness on modulation of force development by Vmax. a CE force–velocity relationships for control condition and 50% reduction in Vmax (dashed). Inset, SEE force–length relationships with varying stiffnesses. Normalised stiffness (kSEE) was reduced by 30% (14 P0·L0−1) and increased by 50% (30 P0·L0−1) relative to a reference kSEE of 20 P0·L0−1, which gives a normalised SEE deformation value of 5%. b Twitch force at an initial CE length of 1.02 L0. c Force as a function of instantaneous CE length during a twitch. CE length was constrained to the plateau region of the CE force–length relationship
3.4 Type I fibre fractional area
Adjusting the rate constant of Ca2+ decay, the force-pCa relationship, and Vmax to reflect a fractional increase in type I fibre area resulted in elevated twitch force and prolonged force rise and decay during a twitch (Fig. 10). Specifically, increasing the type I fibre area from 0.60 to 0.70 increased the twitch contraction time and half-relaxation time by 17 and 15%, respectively (Fig. 10d, inset). The slowing effect increased to 37 and 33% when type I fibre area was increased to 0.80. Both adjustments of type I fibre fractional area increased force at submaximal stimulation frequencies (Fig. 10e), with the latter causing the greatest leftward shift of the force-frequency relationship (Fig. 10f).
Effect of type I fibre fractional area. a Activator concentration during a twitch, b activation-pCa relationship, and c force–velocity relationship for varying fractions of muscle cross-sectional area (CSA) occupied by type I fibres (i.e., type I MHC content). Control values for Ca2+ removal (rem.) rate, pCa50 and nH, and Vmax were adjusted after back-calculation of the fibre type specific value from a defined fibre type-related difference and a weighting factor proportional to the fractional area of type I fibres. The fractional area of type I fibres was increased by 0.10 and 0.20 from a control value of 0.60 (i.e., 60%). d Twitch force at an initial CE length of 1.23 L0. Inset, twitch contraction time and half-relaxation time. e Force during sustained 10 Hz stimulation at 1.23 L0. f Force-frequency relationship at 1.23 L0. Active force expressed relative to P0 of control condition. Solid squares denote the force for 10 Hz stimulation
3.5 Model optimisation to simulate plantar flexor twitch of old men
The plantar flexor twitch of older men was of a lower amplitude (18%) and exhibited a prolonged contraction time (25%) and prolonged half-relaxation time (29%) compared to young adult men (Table 2, Fig. 11a). The weaker, slower twitch in advanced age was well-simulated by the model with ageing-realistic adjustments to a few parameters (Fig. 11b, c). To emulate the time course and relative amplitude of the twitch exhibited by older men, which was possible with multiple parameter combinations, required significant slowing of Ca2+ uptake rate (~ 25%, Table 3). Because slowing the uptake of Ca2+ prolonged the duration of the Ca2+ transient, which increased twitch force, it was possible for the reduction in force-generating capacity (23%) to exceed that of the reduction in twitch force (18%) despite additional force loss from reductions in Vmax and SEE stiffness (Table 3). A smaller reduction in force-generating capacity (15%) was possible with the addition of modest reductions in Ca2+ release and Ca2+ sensitivity, which had to be balanced by further slowing of Ca2+ uptake. Incorporating slower Ca2+ uptake produced a leftward shift of the force-frequency relationship (Fig. 11c inset).
Model optimization to simulate time course and relative amplitude of plantar flexion twitch of old men. a Measured plantar flexion twitch torque of young (Y) and older (O) men. b Plantar flexion twitch torque of older men and simulated twitch force after parameter optimisation. Twitch contraction time and half-relaxation time for experimental and simulated data were identical following optimization (CT: 154 ms; HRT: 134.5 ms). c Simulated twitch force for young and old muscle. The relative deficit in twitch amplitude owing to ageing was 18% for simulated and experimental conditions. Inset, force-frequency relationships, where force is expressed relative to the respective P0 of each muscle
4 Discussion
Ca2+ transportation and calcium-activated force are perturbed in advanced age. Model simulations in the current work show that imposing literature-informed deficits in free Ca2+ concentration or Ca2+ sensitivity of force results in a substantial loss of submaximal force and a slow-to-fast shift in several indices of contraction speed. Their combined effect is especially dramatic. Imposing slowed Ca2+ reuptake had the opposite effect on contractile performance, increasing twitch force and the ratio of twitch-to-tetanic force, prolonging the duration of contraction, and shifting the force-frequency leftward. Simulations estimating the effect of a fractional increase in type I fibre area produced the same outcomes, although to a lesser extent. It is difficult to find support from human or animal studies for a slow-to-fast shift in contraction speed mediated by ageing in either single muscle fibres or whole muscle. Rather, the contractile properties of muscle in advanced age are understood to be defined by slowing (reviewed by Hunter et al., 1998, 2016; Larsson et al., 2018)—twitch contraction time and half-relaxation time are longer (e.g. Vandervoort and McComas, 1986), tetanic force decay is slower (e.g. Tevald et al., 2009), and the force-frequency relationship is shifted to lower frequencies (e.g. Brooks and Faulkner, 1988). As such, incorporating slower Ca2+ removal, or a combination of slower Ca2+ removal, greater Ca2+ sensitivity, lower cooperativity, and slower intrinsic shortening speed—to reflect an increase in type I fibre content—emulated many aspects of contractile performance frequently reported in advanced age.
4.1 Ca2+ uptake
The extent to which the rise and decay of twitch force were prolonged by slowing Ca2+ removal was consistent with experimental observations of slowed twitch speed in advanced age (Vandervoort and McComas 1986; Brooks and Faulkner 1988; Larsson and Salviati 1989; Hicks et al. 1991; Alway 1995; Connelly et al. 1999). In fact, the predictions were comparable to experimental data of the association between slowed SR Ca2+ uptake activity and twitch speed (Narayanan et al. 1996). For the soleus muscle of old rats, a 52% deficit in SR Ca2+ uptake activity was accompanied by a 28 and 48% increase in twitch contraction time and half-relaxation time, respectively (Narayanan et al. 1996). When we imposed a 30% decrease in the rate constant for Ca2+ uptake, twitch contraction time and half-relaxation time increased by 25 and 38%. At 50%, slowing of the twitch, and the associated increase in submaximal force, far exceeded typical ageing-related slowing. To our knowledge, intracellular Ca2+ transients during twitches in young and old muscle have been compared in terms of amplitude but not half-width or rate of decay (González et al. 2003; Eshima et al. 2020). It would be advantageous for the model to incorporate the extent of slowing observed for the decay phase of the Ca2+ transient in a contracting fibre rather than the reduction in Ca2+ uptake rate demonstrated for an isolated SR vesicle or muscle homogenate.
Slower Ca2+ removal increased twitch force and, therefore, increased the ratio of twitch-to-tetanic force. Consistent with the model simulations, twitch force and twitch rise time are inversely related to the decay rate constant of the intracellular Ca2+ transient in single fibres (Sun and Edman 1996). Maintenance of twitch force despite a considerable deficit in maximum force or a higher ratio of twitch-to-tetanic force are commonly reported in advanced age (Carlsen and Walsh 1987; Pettigrew and Gardiner 1987; Hicks et al. 1991; van Schaik et al. 1994; Brown and Hasser 1996; Connelly et al. 1999; Klass et al. 2005; Moran et al. 2005). Slower Ca2+ removal, by prolonging the duration for which the contractile apparatus is exposed to Ca2+ during a twitch, may partially offset or completely compensate for intrinsic processes that facilitate force loss, such as lower free Ca2+ concentration and lower Ca2+ sensitivity.
For contractions at submaximal stimulation frequencies, slower Ca2+ removal led to greater steady-state Ca2+ availability, which resulted in higher forces and a leftward shift of the force-frequency relationship. An age-related shift of the force-frequency relationship toward lower frequencies is a common observation for both human (Narici et al. 1991; Roos et al. 1999; Allman and Rice 2004; Tevald et al. 2009) and animal skeletal muscle (Larsson and Edström 1986; Brooks and Faulkner 1988; Alway 1995; González et al. 2000; Moran et al. 2005). Generally, elevated force generation at submaximal frequencies is accompanied by an increase in twitch contraction time, half-relaxation time, or both. Because altered activation dynamics causing prolonged force rise and decay result in a slower fusion frequency, it is not surprising that aged muscles exhibiting normal twitch speed tend not to exhibit elevated relative force at submaximal frequencies (Walters et al. 1990; González et al. 2000; Dalton et al. 2010a; Elliott et al. 2016). Simulations incorporating a large reduction in Ca2+ sensitivity or moderate impairment of Ca2+ release in concert with slower Ca2+ uptake indicate that an ageing-appropriate increase in submaximal force may still be possible if these alterations coexisted. It seems less likely that a leftward shift of the force-frequency relationship would arise if SR Ca2+ release was greatly impaired, especially if the impairment occurred in concert with lower Ca2+ sensitivity or was only balanced by a modest slowing of SR Ca2+ uptake.
A limited number of studies have recorded intracellular Ca2+ transients in contracting fibres from young and old muscles, fewer have examined a twitch or employed a range of submaximal stimulation frequencies, and none appear to have studied slow twitch fibres or examined the decay of the intracellular Ca2+ transient (González et al. 2003; Andersson et al. 2011; Umanskaya et al. 2014; Eshima et al. 2020). Nonetheless, these studies support the view that impaired SR Ca2+ leads to lower free Ca2+ concentrations during both submaximal and maximal contractions. Therefore, because the free Ca2+ concentration reflects the net effect of Ca2+ release and removal processes, these observations suggest that impaired SR Ca2+ uptake is not a universal outcome, presents at a more advanced age with respect to impaired SR Ca2+ release, or is only capable of minimising the deficit in free Ca2+ concentration caused by impaired Ca2+ release. According to the model predictions, for slower contraction speed to arise in the presence of a lower free Ca2+ concentration, there would need to be considerable involvement from an alternative mechanism. Future work should be directed at establishing whether impaired SR Ca2+ release and slower SR Ca2+ uptake coexist, how they interact, or why submaximal force in advanced age isn’t disproportionately lower given the large deficit in free Ca2+ concentration and possible exacerbation by lower Ca2+ sensitivity.
4.2 Type I fibre fractional area
Simulating an elevated fractional area of type I fibres also produced an appropriate level of slowing. For simulations incorporating a fractional increase of 0.1 or 0.2, the relative increases in twitch contraction time and twitch half-relaxation time, and of normalised force at submaximal stimulation frequencies, were similar to the age effect reported by some studies (Fitts et al. 1984; Davies et al. 1986; Roos et al. 1999; Connelly et al. 1999). Greater age-related prolonging of the contraction time and/or half-relaxation time, a more pronounced shift in the force-frequency relationship, or both (Vandervoort and McComas 1986; Brooks and Faulkner 1988; Alway 1995; Narayanan et al. 1996; Baudry et al. 2005; McNeil et al. 2005; Dow et al. 2005), may indicate that our weighting approach was not entirely effective or that the imposed fibre type differences were too conservative. Alternatively, the greater magnitude of slowing demonstrated by these studies may implicate an additive effect or the sole involvement of slower Ca2+ uptake (Narayanan et al. 1996); simulations of the latter produced larger effects.
Slowed SR Ca2+ uptake rate has not been consistently demonstrated in advanced age, at least not for rat muscles (Fitts et al. 1984; Larsson and Salviati 1989; Narayanan et al. 1996; Thomas et al. 2010; Russ et al. 2014). Comparatively, greater evidence can be found to support an elevated fractional area of type I fibres (Coggan et al. 1992; Kadhiresan et al. 1996; Cui et al. 2008; Elliott et al. 2016), especially for the human vastus lateralis muscle (Larsson et al. 1978; Klitgaard et al. 1990a; Hunter et al. 1999; Short et al. 2005; Korhonen et al. 2006; Nilwik et al. 2013; Lamboley et al. 2015; Brocca et al. 2017; Sonjak et al. 2019; Soendenbroe et al. 2022). In some instances, the fast-to-slow shift in MHC isoform content may manifest as a reduction in MHC IIb content and an increase in MHC IIa or hybrid MHC isoforms (Hepple et al. 2004; Cui et al. 2008). Myosin isoform composition correlates strongly with whole muscle performance (Ranatunga and Thomas 1990; Harridge et al. 1996). Slower contractile properties in advanced age have been associated with a greater fractional content of MHC I (Klitgaard et al. 1990a; Korhonen et al. 2006), demonstrated in the absence of slowed SR Ca2+ uptake (Larsson and Salviati 1989), and observed without slower Ca2+ removal being the rate-limiting process (Hunter et al. 1999). Our simulations add weight to this body of evidence—fibre-type related differences in contraction speed appear sufficient for a moderate-to-large age-related increase in type I fibre content to account for empirical observations of slowed whole muscle contraction speed.
4.3 Ca2+ sensitivity and free Ca2+ concentration
Lower Ca2+ sensitivity of force and excitation-SR Ca2+ release decoupling are thought to play a role in the age-related decline of muscle specific force (Delbono et al. 1995; González et al. 2003; Andersson et al. 2011; Lamboley et al. 2015). The model predictions suggest that the deficits in free Ca2+ concentration and Ca2+ sensitivity reported in the literature, despite dramatically reducing force at submaximal stimulation frequencies, are insufficient to appreciably lower maximum tetanic force (< 5%). Our findings are supported by experimental observations from studies of dantrolene exposure (Krarup 1981; Macintosh et al. 2011) and low-frequency fatigue (Westerblad et al. 1993; Chin and Allen 1996; Glass et al. 2018; Olsson et al. 2020). For example, dantrolene partially inhibits SR Ca2+ release (Desmedt and Hainaut 1977), inducing moderate reductions in twitch force (27–53%) or shifting the force-frequency relationship rightward without appreciable, if any, tetanic force loss [0–6% (Krarup 1981; Macintosh et al. 2011)].
Of course, a greater deficit in maximum force would arise if the designation of thin filament activation (i.e., cross-bridge activation) during maximum tetanic stimulation was greatly overestimated in the model. This assertion would imply that maximum tetanic stimulation does not induce saturating Ca2+. However, our designation seems appropriate because tetanic force plateaus with increasing stimulation frequency despite an increasing free Ca2+ concentration (Westerblad and Allen 1993; Glass et al. 2018, 2020). Similarly, several studies demonstrate that tetanic forces with and without caffeine—which potentiates SR Ca2+ release—can be virtually identical, if not equal (Lannergren and Westerblad 1991; Westerblad and Allen 1991; Glass et al. 2018; Olsson et al. 2020).
Thin filament activation is also worth considering from the perspective of voluntary muscle excitation. Motor unit discharge rates during a maximal voluntary contraction are considerably lower than the stimulation rate required for muscle maximal tetanic force (Roos et al. 1999; Dalton et al. 2010a; Kirk and Rice 2016). Asynchronous stimulation, by minimising the oscillation of fibre length against series elasticity (Sandercock 2006), can elevate force at low and intermediate stimulation frequencies without reducing the frequency required for maximal tetanic force (Rack and Westbury 1969). As such, the discrepancy could infer submaximal thin filament activation during a volitional effort. Voluntary muscle activation isn’t easily quantified (Horstman 2009), and raising single fibre force from just 0.95 to 1.0 P0 can require a near two-fold increase in stimulation frequency and free Ca2+ concentration (Glass et al. 2018). If voluntary activation were submaximal, even only slightly, maximal voluntary contraction force would be lowered dramatically by a reduction in free Ca2+ concentration or Ca2+ sensitivity.
This notion must be viewed with caution, however, because Lind and Petrofsky (1978) found that the entire force-frequency relationship could indeed be shifted to lower frequencies through asynchronous stimulation. Thus, the differences between the two modes of excitation appear to be more complex than appreciated. Nonetheless, the force-frequency relationship of the model, where force is 0.95 P0 at 50 Hz, is generally consistent with relationships established for human muscle groups in vivo (Marsh et al. 1981; Davies et al. 1982; Roos et al. 1999; Allman and Rice 2004) and animal studies of predominantly slow muscle at physiological temperatures (Ranatunga 1982; Larsson and Edström 1986).
A more significant deficit in maximum tetanic force (8%) arose when free Ca2+ concentration and Ca2+ sensitivity were lowered in concert by 50% and 0.10 pCa units, respectively. However, these modifications represent the upper limit of the age effect for Ca2+ sensitivity and Ca2+ availability reported in the literature. It’s possible that moderate ageing-related reductions in Ca2+ availability or Ca2+ sensitivity may compromise tetanic specific force when Ca2+ sensitivity is lowered further by reducing fibre length (Stephenson and Williams 1982; Martyn and Gordon 1988; Balnave and Allen 1996), decreasing muscle temperature (Maughan et al. 1995; Debold et al. 2006; Nelson and Fitts 2014) or inducing fatigue, which also impairs SR Ca2+ release (Westerblad and Allen 1991, 1993).
Although there are concurrent measurements of free Ca2+ and force from intact single fibres (González et al. 2003), as well as combined measurements from single fibres and whole muscle (Andersson et al. 2011; Umanskaya et al. 2014), respectively, that implicate impaired SR Ca2+ release as an important determinant of the age-related deficit in specific force, it is unlikely that this mechanism is wholly responsible. Specific force remains lower for old compared to young intact single fibres after caffeine administration, which mitigates the age-related deficit in free Ca2+ concentration (González et al. 2003). Corroborating this finding are numerous studies using skinned fibres from young and old muscle (Lowe et al. 2002; D’Antona et al. 2003; Zhong et al. 2006; Yu et al. 2007; Kim and Thompson 2013; Hvid et al. 2013; Lamboley et al. 2015), some of which demonstrated a deficit in specific force of 25% or more (Thompson and Brown 1999; Frontera et al. 2000; Lowe et al. 2001; Ochala et al. 2007; Power et al. 2016; Brocca et al. 2017). These bodies of work, along with our findings, suggest that a substantial proportion of the deficit in tetanic specific force exhibited by intact single fibres, as well as whole muscle, is independent of lower tetanic free Ca2+ concentration. Although, as we have illustrated, the latter may exert a more considerable effect when accompanied by a moderate-to-large reduction in Ca2+ sensitivity.
4.4 CE-SEE interaction
In addition to activation dynamics, force development is regulated by the intrinsic speed of shortening and the stiffness of the SEE being acted upon by the CE (Hill 1938; Edman and Josephson 2007). Incorporating ageing-related reductions in Vmax and SEE stiffness slowed and prolonged the rise of force. During a twitch or brief tetanic contraction, both modifications also attenuated peak force. The effect on force development of a given reduction in intrinsic shortening speed depended on SEE stiffness, being more modest for low normalised SEE compliances (< 8%), suggesting that force rise during a twitch of a single fibre may not be appreciably affected by a slower Vmax. The current work supports the involvement of slower intrinsic shortening speed and higher SEE compliance as factors contributing to prolonged and slower force rise in advanced age, although consideration may need to be given to the muscle preparation. Elevated tendon stiffness, conversely, would likely act to offset factors causing force development to be slowed or twitch force to be attenuated.
The simulations with altered SEE stiffness are generally consistent with experimental work with added compliance (Hill 1951; Brown and Matthews 1960; Bawa et al. 1976; Mayfield et al.2016b) and where active shortening has been limited by means of a small muscle stretch (Hill 1949; Griffiths 1991; Sawicki and Roberts 2009; Mayfield et al. 2016a). Our findings also appear to be quantitively appropriate, although there is limited information to draw upon. We found that 33 (5 vs. 3.33 P0·L0−1) and 53% (5 vs. 2.33 P0·L0−1) reductions in normalised SEE stiffness reduced twitch force by 16 and 29%, respectively. Cat soleus twitch force was reduced by 35–40% (Bawa et al. 1976) following the addition of a spring that we estimate reduced the in-series stiffness by ~ 87% [isometric twitch force: ~ 5 N; spring stiffness: ~ 1.52 N·mm-1; tendon stiffness from spindle null method at 5 N: ~ 10 N·mm-1 (Rack and Westbury 1984)].
We found that twitch force and contraction time were similarly affected by increased SEE compliance. However, empirical measurements show that the reduction in force mediated by added compliance is more pronounced compared to the associated delay in peak force, and that the latter may not increase in proportion to the former (Hill 1951; Bawa et al. 1976; Mayfield et al. 2016b). Modest delays in peak force in response to a large amount of added compliance may relate to the effects of length or active shortening on factors such as cross-bridge kinetics (Fenwick et al. 2021), Ca2+ sensitivity of force (Stephenson and Williams 1982; Martyn and Gordon 1988), and force depression (Joumaa et al. 2012). With this observation in mind and the fact that imposing lower SEE stiffness prolonged the twitch contraction time by just 13%, it appears that this adaptation may not be an important determinant of twitch rise time.
Importantly, neither adaptation produced other facets of slowed contractile speed, such as slower force decay or elevated force at submaximal stimulation frequencies. Whilst the effect of added compliance on force decay is inconsistent and only modest (Bawa et al. 1976; Mayfield et al. 2016b), slower force decay should accompany a slower intrinsic shortening speed, especially at intermediate loads (Jones et al. 2006). Ignoring a dramatic change in SEE stiffness, which would bring force–length effects and possibly length-dependent Ca2+ sensitivity into play, a shift in the force-frequency relationship must arise from factors affecting Ca2+ concentration and calcium-activated force. Greater compliance and slower intrinsic shortening may minimise force oscillations or increase the apparent degree of fusion without affecting average force.
4.5 Model simulation of age effect observed for experimental twitch data
The age-related prolonging of force rise and decay during a twitch observed for the plantar flexors in this study was within the range of slowing reported by others for the same muscle group (Davies et al. 1986; Vandervoort and McComas 1986; Simoneau et al. 2005; Dalton et al. 2009, 2010b). Simulating this age-related shift in twitch contraction speed was possible with parameter adjustments that fell within the ranges of age-related adaptation obtained from empirical observations used to inform earlier simulations. Specifically, the slower, weaker twitch was achieved with reductions in force-generating capacity (23%), Ca2+ uptake rate (24%), Vmax (20%), and SEE stiffness (6%). Multiple solutions were possible, and it is likely that the slowing of twitch speed could have been emulated with parameter adjustments more consistent with an increase in type I fibre content. An increase in Ca2+ sensitivity and reduction in cooperativity would likely lessen the required reduction in Ca2+ uptake rate. We speculate because we imposed parameter limits that were consistent with the directionality of impairment reported in the literature.
5 Summary
Age-related reductions in Ca2+ sensitivity and Ca2+ release abbreviated the twitch and dramatically lowered force during submaximal contractions (e.g., twitch, unfused tetanic contraction) without greatly influencing maximum tetanic force, even when acting in concert (< 10%). These predictions are at odds with experimental observations of the effect of age on indices of isometric contraction speed (i.e., twitch contraction time and half-relaxation time, force-frequency relationship), and suggest that reduced Ca2+ sensitivity and impaired SR Ca2+ release may contribute only modestly to the reduction in specific force in advanced age (depending on Ca2+ saturation during tetanic stimulation). Conversely, simulations that incorporated slowed Ca2+ removal or a greater fractional area of type I fibres prolonged the rise and decay of twitch force and shifted the force-frequency relationship leftward. These predictions are consistent with the characteristic fast-to-slow shift in contractile performance associated with ageing. Slowed and prolonged force development, and lower twitch force, also resulted from imposing a slower intrinsic shortening speed and lower SEE stiffness but occurred without a concomitant slowing of force decay or elevation of submaximal force. As such, these properties alone did not produce the characteristic slowing of contraction speed. The effect of Vmax depended on SEE stiffness, and empirical observations do not always support a pronounced delay in peak force from added compliance, possibly because of additional factors related to active shortening not captured in the model. Simulating the slower, weaker twitch observed experimentally for the plantar flexors of older men required significant slowing of Ca2+ uptake (~ 25%) and could be coupled with an appreciable reduction in force-generating capacity (i.e., the reduction in force-generating capacity exceeded that of the deficit in twitch force). Slowed Ca2+ removal, when acting in concert, negated the depressive effects of moderate and large reductions in Ca2+ release and Ca2+ sensitivity, respectively.
6 Conclusion
Whole muscle contractile performance in advanced age is characterised by slowed isometric contraction speed. This work provides support for the involvement of multiple mechanisms, although these adaptations do not necessarily affect the same aspects of contraction speed. As such, identifying the most important adaptations should be aided by characterising an array of isometric contractile properties in advanced age. Both slower Ca2+ uptake and a greater fractional area of type I fibres seem to be suitable mechanisms for explaining the slower isometric contraction speed exhibited by aged muscle. Simulations incorporating these adaptations with a degree of impairment reported in the literature generated realistic age-related changes in contractile behaviour. Ageing-appropriate adjustments to these parameters also emulated the age-related slowing of twitch speed observed experimentally. In general, the model simulations were well-supported by empirical observations. We propose that this model or similar models might be effective in determining a meaningful impairment threshold or identifying the factors contributing to altered contractile properties.
Adaptation of muscle function and structure in advanced age is inconsistent and wide-ranging, thus, careful consideration should be given to the strength of evidence implicating the presence of a particular adaptation (e.g., fibre type, species, activity level, age). With the multifaced nature of impairment in mind, we adjusted multiple model parameters concurrently and have illustrated the importance of considering interaction effects. Inconsistent reports regarding the effect of age on contractile performance may relate to variation in the disruption of function and structure. We believe this work underscores the utility of simple, yet physiologically-grounded and parameter rich Hill-type muscle models for studying conditions that involve a multitude of adaptations (e.g. ageing, disuse, training). Such models also hold great value in being able to predict functional performance (e.g. walking, standing from a chair) when used in musculoskeletal simulations (e.g. Song and Geyer 2018; Ong et al. 2019).
Data availability
The Hill-type muscle model developed in Simulink and a MATLAB application that can be used to run simulations are accessible as open source (under the MIT license) from GitHub [https://github.com/dlmayfield/Muscle-Model (Mayfield and Lichtwark 2022)]. Experimental twitch data from young and older human adults are accessible from this repository.
References
Albracht K, Arampatzis A, Baltzopoulos V (2008) Assessment of muscle volume and physiological cross-sectional area of the human triceps surae muscle in vivo. J Biomech 41:2211–2218. https://doi.org/10.1016/j.jbiomech.2008.04.020
Allman BL, Rice CL (2004) An age-related shift in the force-frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol 96:1026–1032. https://doi.org/10.1152/japplphysiol.00991.2003
Alway SE (1995) Slowing of contractile properties in quail skeletal muscle with aging. J Gerontol A Biol Sci Med Sci 50A:B26–B33. https://doi.org/10.1093/gerona/50A.1.B26
Andersson DC, Betzenhauser MJ, Reiken S et al (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14:196–207. https://doi.org/10.1016/j.cmet.2011.05.014
Arampatzis A, Stafilidis S, DeMonte G et al (2005) Strain and elongation of the human gastrocnemius tendon and aponeurosis during maximal plantarflexion effort. J Biomech 38:833–841. https://doi.org/10.1016/j.jbiomech.2004.04.031
Asmussen G, Maréchal G (1989) Maximal shortening velocities, isomyosins and fibre types in soleus muscle of mice, rats and guinea-pigs. J Physiol 416:245–254. https://doi.org/10.1113/jphysiol.1989.sp017758
Azizi E, Roberts TJ (2014) Geared up to stretch: pennate muscle behavior during active lengthening. J Exp Biol 217:376–381. https://doi.org/10.1242/jeb.094383
Ballak SB, Degens H, de Haan A, Jaspers RT (2014) Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev 14:43–55. https://doi.org/10.1016/j.arr.2014.01.005
Balnave CD, Allen DG (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J Physiol 492:705–713. https://doi.org/10.1113/jphysiol.1996.sp021339
Barclay CJ (2012) Quantifying Ca2+ release and inactivation of Ca2+ release in fast- and slow-twitch muscles. J Physiol 590:6199–6212. https://doi.org/10.1113/jphysiol.2012.242073
Baudry S, Klass M, Duchateau J (2005) Postactivation potentiation influences differently the nonlinear summation of contractions in young and elderly adults. J Appl Physiol 98:1243–1250. https://doi.org/10.1152/japplphysiol.00735.2004
Bawa P, Mannard A, Stein RB (1976) Effects of elastic loads on the contractions of cat muscles. Biol Cybern 22:129–137. https://doi.org/10.1007/BF00365523
Baylor SM, Hollingworth S (2003) Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol 551:125–138. https://doi.org/10.1113/jphysiol.2003.041608
Bennett MB, Ker RF, Imery NJ, Alexander RMN (1986) Mechanical properties of various mammalian tendons. J Zool 209:537–548. https://doi.org/10.1111/j.1469-7998.1986.tb03609.x
Bottinelli R, Canepari M, Pellegrino MA, Reggiani C (1996) Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495:573–586. https://doi.org/10.1113/jphysiol.1996.sp021617
Brocca L, McPhee JS, Longa E et al (2017) Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595:4823–4844. https://doi.org/10.1113/JP274148
Brooks SV, Faulkner JA (1988) Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404:71–82. https://doi.org/10.1113/jphysiol.1988.sp017279
Brooks SV, Faulkner JA (1994) Isometric, shortening, and lengthening contractions of muscle fiber segments from adult and old mice. Am J Physiol Cell Physiol 267:C507–C513. https://doi.org/10.1152/ajpcell.1994.267.2.C507
Brown, M.C., Matthews, P.B., 1960. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J. Physiol. 151, 436–457. https://doi.org/10.1113/jphysiol.1960.sp006450
Brown M, Hasser EM (1996) Complexity of age-related change in skeletal muscle. J Gerontol A Biol Sci Med Sci 51:B117–B123. https://doi.org/10.1093/gerona/51a.2.b117
Calderón JC, Bolaños P, Caputo C (2010) Myosin heavy chain isoform composition and Ca2+ transients in fibres from enzymatically dissociated murine soleus and extensor digitorum longus muscles. J Physiol 588:267–279. https://doi.org/10.1113/jphysiol.2009.180893
Caputo C, Bolaños P, Gonzalez A (2004) Inactivation of Ca2+ transients in amphibian and mammalian muscle fibres. J Muscle Res Cell Motil 25:315–328. https://doi.org/10.1007/s10974-004-4071-z
Carlsen RC, Walsh DA (1987) Decrease in force potentiation and appearance of α-adrenergic mediated contracture in aging rat skeletal muscle. Pflügers Arch 408:224–230. https://doi.org/10.1007/BF02181463
Carroll SL, Klein MG, Schneider MF (1997) Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. J Physiol 501:573–588. https://doi.org/10.1111/j.1469-7793.1997.573bm.x
Carroll CC, Dickinson JM, Haus JM et al (2008) Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105:1907–1915. https://doi.org/10.1152/japplphysiol.00059.2008
Chin ER, Allen DG (1996) The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol 491:813–824. https://doi.org/10.1113/jphysiol.1996.sp021259
Claflin DR, Faulkner JA (1989) The force-velocity relationship at high shortening velocities in the soleus muscle of the rat. J Physiol 411:627–637. https://doi.org/10.1113/jphysiol.1989.sp017595
Claflin DR, Larkin LM, Cederna PS et al (2011) Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. J Appl Physiol 111:1021–1030. https://doi.org/10.1152/japplphysiol.01119.2010
Coggan AR, Spina RJ, King DS et al (1992) Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47:71–76. https://doi.org/10.1093/geronj/47.3.B71
Connelly DM, Rice CL, Roos MR, Vandervoort AA (1999) Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87:843–852. https://doi.org/10.1152/jappl.1999.87.2.843
Couppé C, Suetta C, Kongsgaard M et al (2012) The effects of immobilization on the mechanical properties of the patellar tendon in younger and older men. Clin Biomech 27:949–954. https://doi.org/10.1016/j.clinbiomech.2012.06.003
Crouzier M, Lacourpaille L, Nordez A et al (2018) Neuromechanical coupling within the human triceps surae and its consequence on individual force-sharing strategies. J Exp Biol 221:jeb187260. https://doi.org/10.1242/jeb.187260
Csapo R, Malis V, Hodgson J, Sinha S (2014) Age-related greater achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. J Appl Physiol 116:961–969. https://doi.org/10.1152/japplphysiol.01337.2013
Cui L, Ju Y, Ding L et al (2008) Arteriolar and venular capillary distribution in skeletal muscles of old rats. J Gerontol A Biol Sci Med Sci 63:928–935. https://doi.org/10.1093/gerona/63.9.928
Cui L, Maas H, Perreault EJ, Sandercock TG (2009) In situ estimation of tendon material properties: differences between muscles of the feline hindlimb. J Biomech 42:679–685. https://doi.org/10.1016/j.jbiomech.2009.01.022
Curtin NA, Gardner-Medwin AR, Woledge RC (1998) Predictions of the time course of force and power output by dogfish white muscle fibres during brief tetani. J Exp Biol 201:103–114
D’Antona G, Pellegrino MA, Adami R et al (2003) The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552:499–511. https://doi.org/10.1113/jphysiol.2003.046276
D’Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R (2007) Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol 100:603–611. https://doi.org/10.1007/s00421-007-0402-2
Dalton BH, Harwood B, Davidson AW, Rice CL (2009) Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol 107:1781–1788. https://doi.org/10.1152/japplphysiol.00464.2009
Dalton BH, Jakobi JM, Allman BL, Rice CL (2010a) Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol 200:45–55. https://doi.org/10.1111/j.1748-1716.2010.02100.x
Dalton BH, Power GA, Vandervoort AA, Rice CL (2010b) Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol 109:1441–1447. https://doi.org/10.1152/japplphysiol.00335.2010
Danieli-Betto D, Betto R, Midrio M (1990) Calcium sensitivity and myofibrillar protein isoforms of rat skinned skeletal muscle fibres. Pflugers Arch 417:303–308. https://doi.org/10.1007/BF00370996
Danos N, Holt NC, Sawicki GS, Azizi E (2016) Modeling age-related changes in muscle-tendon dynamics during cyclical contractions in the rat gastrocnemius. J Appl Physiol 121:1004–1012. https://doi.org/10.1152/japplphysiol.00396.2016
Davies CTM, Mecrow IK, White MJ (1982) Contractile properties of the human triceps surae with some observations on the effects of temperature and exercise. Eur J Appl Physiol Occup Physiol 49:255–269. https://doi.org/10.1007/BF02334074
Davies CTM, Thomas DO, White MJ (1986) Mechanical properties of young and elderly human muscle. Acta Med Scand 220:219–226. https://doi.org/10.1111/j.0954-6820.1986.tb08954.x
Debold EP, Romatowski J, Fitts RH (2006) The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. Am J Physiol Cell Physiol 290:C1041–C1050. https://doi.org/10.1152/ajpcell.00342.2005
Degens H, Yu F, Li X, Larsson L (1998) Effects of age and gender on shortening velocity and myosin isoforms in single rat muscle fibres. Acta Physiol Scand 163:33–40. https://doi.org/10.1046/j.1365-201x.1998.00329.x
Delbono O, O’Rourke KS, Ettinger WH (1995) Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol 148:211–222. https://doi.org/10.1007/BF00235039
Desmedt JE, Hainaut K (1977) Inhibition of the intracellular release of calcium by dantrolene in barnacle giant muscle fibres. J Physiol 265:565–585. https://doi.org/10.1113/jphysiol.1977.sp011731
Dow DE, Dennis RG, Faulkner JA (2005) Electrical stimulation attenuates denervation and age-related atrophy in extensor digitorum longus muscles of old rats. J Gerontol A Biol Sci Med Sci 60:416–424. https://doi.org/10.1093/gerona/60.4.416
Eddinger TJ, Cassens RG, Moss RL (1986) Mechanical and histochemical characterization of skeletal muscles from senescent rats. Am J Physiol Cell Physiol 251:C421–C430. https://doi.org/10.1152/ajpcell.1986.251.3.C421
Edman KAP, Josephson RK (2007) Determinants of force rise time during isometric contraction of frog muscle fibres. J Physiol 580:1007–1019. https://doi.org/10.1113/jphysiol.2006.119982
Edström L, Nyström BO (1969) Histochemical types and sizes of fibres in normal human muscles. Acta Neurol Scand 45:257–269. https://doi.org/10.1111/j.1600-0404.1969.tb01238.x
Elliott JE, Omar TS, Mantilla CB, Sieck GC (2016) Diaphragm muscle sarcopenia in Fischer 344 and Brown Norway rats. Exp Physiol 101:883–894. https://doi.org/10.1113/EP085703
Eriksen CS, Henkel C, Svensson RB et al (2018) Lower tendon stiffness in very old compared with old individuals is unaffected by short-term resistance training of skeletal muscle. J Appl Physiol 125:205–214. https://doi.org/10.1152/japplphysiol.00028.2018
Eshima H, Tamura Y, Kakehi S et al (2020) A chronic high-fat diet exacerbates contractile dysfunction with impaired intracellular Ca2+ release capacity in the skeletal muscle of aged mice. J Appl Physiol 128:1153–1162. https://doi.org/10.1152/japplphysiol.00530.2019
Fenwick AJ, Lin DC, Tanner BCW (2021) Myosin cross-bridge kinetics slow at longer muscle lengths during isometric contractions in intact soleus from mice. Proc R Soc B 288:20202895. https://doi.org/10.1098/rspb.2020.2895
Fink RH, Stephenson DG, Williams DA (1986) Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J Physiol 373:513–525. https://doi.org/10.1113/jphysiol.1986.sp016060
Fink RH, Stephenson DG, Williams DA (1990) Physiological properties of skinned fibres from normal and dystrophic (Duchenne) human muscle activated by Ca2+ and Sr2+. J Physiol 420:337–353. https://doi.org/10.1113/jphysiol.1990.sp017916
Fitts RH, Troup JP, Witzmann FA, Holloszy JO (1984) The effect of ageing and exercise on skeletal muscle function. Mech Ageing Dev 27:161–172. https://doi.org/10.1016/0047-6374(84)90041-1
Fodor J, Al-Gaadi D, Czirják T et al (2020) Improved calcium homeostasis and force by selenium treatment and training in aged mouse skeletal muscle. Sci Rep 10:1707. https://doi.org/10.1038/s41598-020-58500-x
Frontera WR, Suh D, Krivickas LS et al (2000) Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279:C611–C618. https://doi.org/10.1152/ajpcell.2000.279.3.C611
Gao Y, Kostrominova TY, Faulkner JA, Wineman AS (2008) Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41:465–469. https://doi.org/10.1016/j.jbiomech.2007.09.021
Gardetto PR, Schluter JM, Fitts RH (1989) Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol 66:2739–2749. https://doi.org/10.1152/jappl.1989.66.6.2739
Gilliver SF, Degens H, Rittweger J et al (2009) Variation in the determinants of power of chemically skinned human muscle fibres. Exp Physiol 94:1070–1078. https://doi.org/10.1113/expphysiol.2009.048314
Glass LD, Cheng AJ, MacIntosh BR (2018) Role of Ca2+ in changing active force during intermittent submaximal stimulation in intact, single mouse muscle fibers. Pflügers Arch - Eur J Physiol 470:1243–1254. https://doi.org/10.1007/s00424-018-2143-y
Glass LD, Cheng AJ, MacIntosh BR (2020) Calcium sensitivity during staircase with sequential incompletely fused contractions. J Muscle Res Cell Motil 42:59–65. https://doi.org/10.1007/s10974-019-09572-4
González E, Messi ML, Delbono O (2000) The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178:175–183. https://doi.org/10.1007/s002320010025
González E, Messi ML, Zheng Z, Delbono O (2003) Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol 552:833–844. https://doi.org/10.1113/jphysiol.2003.048165
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192. https://doi.org/10.1113/jphysiol.1966.sp007909
Green HJ, Daub B, Houston ME et al (1981) Human vastus lateralis and gastrocnemius muscles: a comparative histochemical and biochemical analysis. J Neurol Sci. 52:201–210. https://doi.org/10.1016/0022-510X(81)90005-8
Gregorevic P, Plant DR, Stupka N, Lynch GS (2004) Changes in contractile activation characteristics of rat fast and slow skeletal muscle fibres during regeneration. J Physiol 558:549–560. https://doi.org/10.1113/jphysiol.2004.066217
Gries KJ, Minchev K, Raue U et al (2019) Single-muscle fiber contractile properties in lifelong aerobic exercising women. J Appl Physiol 127:1710–1719. https://doi.org/10.1152/japplphysiol.00459.2019
Griffiths, R.I., 1991. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J. Physiol. 436, 219–236. https://doi.org/10.1113/jphysiol.1991.sp018547
Grosicki GJ, Gries KJ, Minchev K et al (2021) Single muscle fibre contractile characteristics with lifelong endurance exercise. J Physiol 599:3549–3565. https://doi.org/10.1113/JP281666
Harridge SDR, Bottinelli R, Canepari M et al (1996) Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch 432:913–920. https://doi.org/10.1007/s004240050215
Harridge SDR, Bottinelli R, Canepari M et al (1998) Sprint training, in vitro and in vivo muscle function, and myosin heavy chain expression. J Appl Physiol 84:442–449. https://doi.org/10.1152/jappl.1998.84.2.442
Hasson CJ, Caldwell GE (2012) Effects of age on mechanical properties of dorsiflexor and plantarflexor muscles. Ann Biomed Eng 40:1088–1101. https://doi.org/10.1007/s10439-011-0481-4
Hatcher DD, Luff AR (1987) Force-velocity properties of fatigue-resistant units in cat fast-twitch muscle after fatigue. J Appl Physiol 63:1511–1518. https://doi.org/10.1152/jappl.1987.63.4.1511
Hauraix H, Nordez A, Guilhem G et al (2015) In vivo maximal fascicle-shortening velocity during plantar flexion in humans. J Appl Physiol 119:1262–1271. https://doi.org/10.1152/japplphysiol.00542.2015
Hellam DC, Podolsky RJ (1969) Force measurements in skinned muscle fibres. J Physiol 200:807–819. https://doi.org/10.1113/jphysiol.1969.sp008723
Hepple RT, Hagen JL, Krause DJ, Baker DJ (2004) Skeletal muscle aging in F344BN F1-hybrid rats: II. improved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol A Biol Sci Med Sci 59:1111–1119. https://doi.org/10.1093/gerona/59.11.1111
Hessel AL, Raiteri BJ, Marsh MJ, Hahn D (2021) Rightward shift of optimal fascicle length with decreasing voluntary activity level in the soleus and lateral gastrocnemius muscles. J Exp Biol 224:jeb235614. https://doi.org/10.1242/jeb.235614
Hicks AL, Cupido CM, Martin J, Dent J (1991) Twitch potentiation during fatiguing exercise in the elderly: the effects of training. Eur J Appl Physiol Occup Physiol 63:278–281. https://doi.org/10.1007/BF00233862
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc B 126:136–195. https://doi.org/10.1098/rspb.1938.0050
Hill AV (1949) The abrupt transition from rest to activity in muscle. Proc. R. Soc. B 136, 399–420. https://doi.org/10.1098/rspb.1949.0033
Hill AV (1951) The effect of series compliance on the tension developed in a muscle twitch. Proc R Soc B 138:325–329. https://doi.org/10.1098/rspb.1951.0025
Hill C, James RS, Cox VM et al (2020) Age-related changes in isolated mouse skeletal muscle function are dependent on sex, muscle, and contractility mode. Am J Physiol Regul Integr Comp Physiol 319:R296–R314. https://doi.org/10.1152/ajpregu.00073.2020
Holt NC, Wakeling JM, Biewener AA (2014) The effect of fast and slow motor unit activation on whole-muscle mechanical performance: The size principle may not pose a mechanical paradox. Proc R Soc B 281:2–7. https://doi.org/10.1098/rspb.2014.0002
Holt NC, Danos N, Roberts TJ, Azizi E (2016) Stuck in gear: age-related loss of variable gearing in skeletal muscle. J Exp Biol 219:998–1003. https://doi.org/10.1242/jeb.133009
Höök P, Sriramoju V, Larsson L (2001) Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol Cell Physiol 280:782–788
Horstman AM (2009) Comments on point: counterpoint: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107:359–366. https://doi.org/10.1152/japplphysiol.00362.2009
Hunter S, White M, Thompson M (1998) Techniques to evaluate elderly human muscle function: a physiological basis. J Gerontol A Biol Sci Med Sci 53:B204–B216. https://doi.org/10.1093/gerona/53a.3.b204
Hunter SK, Thompson MW, Ruell PA et al (1999) Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol 86:1858–1865. https://doi.org/10.1152/jappl.1999.86.6.1858
Hunter SK, Pereira XHM, Keenan KG (2016) The aging neuromuscular system and motor performance. J Appl Physiol 121:982–995. https://doi.org/10.1152/japplphysiol.00475.2016
Hvid LG, Ørtenblad N, Aagaard P et al (2011) Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol 589:4745–4757. https://doi.org/10.1113/jphysiol.2011.215434
Hvid LG, Suetta C, Aagaard P et al (2013) Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp Gerontol 48:154–161. https://doi.org/10.1016/j.exger.2012.11.005
Hvid LG, Brocca L, Ørtenblad N et al (2017) Myosin content of single muscle fibers following short-term disuse and active recovery in young and old healthy men. Exp Gerontol 87:100–107. https://doi.org/10.1016/j.exger.2016.10.009
Jiménez-Moreno R, Wang ZM, Gerring RC, Delbono O (2008) Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J 94:3178–3188. https://doi.org/10.1529/biophysj.107.118786
Jones DA, de Ruiter CJ, de Haan A (2006) Change in contractile properties of human muscle in relationship to the loss of power and slowing of relaxation seen with fatigue. J Physiol 576:913–922. https://doi.org/10.1113/jphysiol.2006.116343
Joumaa V, MacIntosh BR, Herzog W (2012) New insights into force depression in skeletal muscle. J Exp Biol 215:2135–2140. https://doi.org/10.1242/jeb.060863
Kadhiresan VA, Hassett CA, Faulkner JA (1996) Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol 493:543–552. https://doi.org/10.1113/jphysiol.1996.sp021402
Karamanidis K, Arampatzis A (2005) Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol 208:3907–3923. https://doi.org/10.1242/jeb.01830
Karamanidis K, Arampatzis A (2006) Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech 39:406–417. https://doi.org/10.1016/j.jbiomech.2004.12.017
Karamanidis K, Stafilidis S, DeMonte G et al (2005) Inevitable joint angular rotation affects muscle architecture during isometric contraction. J Electromyogr Kinesiol 15:608–616. https://doi.org/10.1016/j.jelekin.2005.02.001
Kim J-H, Thompson LV (2013) Inactivity, age, and exercise: single-muscle fiber power generation. J Appl Physiol 114:90–98. https://doi.org/10.1152/japplphysiol.00525.2012
Kirk EA, Rice CL (2016) Contractile function and motor unit firing rates of the human hamstrings. J Neurophysiol 117:243–250. https://doi.org/10.1152/jn.00620.2016
Klass M, Baudry S, Duchateau J (2005) Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol 99:31–38. https://doi.org/10.1152/japplphysiol.01426.2004
Klitgaard H, Marc R, Brunet A et al (1989) Contractile properties of old rat muscles: effect of increased use. J Appl Physiol 67:1401–1408. https://doi.org/10.1152/jappl.1989.67.4.1401
Klitgaard H, Mantoni M, Schiaffino S et al (1990a) Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140:41–54. https://doi.org/10.1111/j.1748-1716.1990.tb08974.x
Klitgaard H, Zhou M, Schiaffino S et al (1990b) Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140:55–62. https://doi.org/10.1111/j.1748-1716.1990.tb08975.x
Korhonen MT, Cristea A, Alén M et al (2006) Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol 101:906–917. https://doi.org/10.1152/japplphysiol.00299.2006
Krarup C (1981) The effect of dantrolene on the enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol 311:389–400. https://doi.org/10.1113/jphysiol.1981.sp013591
Krivickas LS, Suh D, Wilkins J et al (2001) Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil 80(6):447–455
LaCroix AS, Duenwald-Kuehl SE, Brickson S et al (2013) Effect of age and exercise on the viscoelastic properties of rat tail tendon. Ann Biomed Eng 41:1120–1128. https://doi.org/10.1007/s10439-013-0796-4
Lamboley CR, Wyckelsma VL, Dutka TL et al (2015) Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593:2499–2514. https://doi.org/10.1113/JP270179
Lamboley CR, Rouffet DM, Dutka TL et al (2020) Effects of high-intensity intermittent exercise on the contractile properties of human type I and type II skeletal muscle fibers. J Appl Physiol 128:1207–1216. https://doi.org/10.1152/japplphysiol.00014.2020
Lannergren J, Westerblad H (1991) Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol 434:307–322. https://doi.org/10.1113/jphysiol.1991.sp018471
Larsson L, Edström L (1986) Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci 76:69–89. https://doi.org/10.1016/0022-510X(86)90143-7
Larsson L, Moss RL (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472:595–614. https://doi.org/10.1113/jphysiol.1993.sp019964
Larsson L, Salviati G (1989) Effects of age on calcium transport activity of sarcoplasmic reticulum in fast- and slow-twitch rat muscle fibres. J Physiol 419:253–264. https://doi.org/10.1113/jphysiol.1989.sp017872
Larsson L, Sjödin B, Karlsson J et al (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103:31–39. https://doi.org/10.1111/j.1748-1716.1978.tb06187.x
Larsson L, Li X, Frontera WR (1997) Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272:C638–C649. https://doi.org/10.1152/ajpcell.1997.272.2.C638
Larsson L, Degens H, Li M et al (2018) Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev 99:427–511. https://doi.org/10.1152/physrev.00061.2017
Laszewski-Williams B, Ruff RL, Gordon AM (1989) Influence of fiber type and muscle source on Ca2+ sensitivity of rat fibers. Am J Physiol Cell Physiol 256:C420–C427. https://doi.org/10.1152/ajpcell.1989.256.2.C420
Leahy TP, Nuss CA, Evans MK et al (2022) Achilles tendon ruptures in middle-aged rats heal poorly compared with those in young and old rats. Am J Sports Med 50:170–181. https://doi.org/10.1177/03635465211055476
Lichtwark GA, Barclay CJ (2010) The influence of tendon compliance on muscle power output and efficiency during cyclic contractions. J Exp Biol 213:707–714. https://doi.org/10.1242/jeb.038026
Lichtwark GA, Wilson AM (2005a) A modified Hill muscle model that predicts muscle power output and efficiency during sinusoidal length changes. J Exp Biol 208:2831–2843. https://doi.org/10.1242/jeb.01709
Lichtwark GA, Wilson AM (2005b) Effects of series elasticity and activation conditions on muscle power output and efficiency. J Exp Biol 208:2845–2853. https://doi.org/10.1242/jeb.01710
Lieber RL, Leonard ME, Brown CG, Trestik CL (1991) Frog semitendinosis tendon load-strain and stress-strain properties during passive loading. Am J Physiol Cell Physiol 261:C86-92. https://doi.org/10.1152/ajpcell.1991.261.1.C86
Lind AR, Petrofsky JS (1978) Isometric tension from rotary stimulation of fast and slow cat muscles. Muscle Nerve 1:213–218. https://doi.org/10.1002/mus.880010306
Liu Y, Kranias EG, Schneider MF (1997) Regulation of Ca2+ handling by phosphorylation status in mouse fast- and slow-twitch skeletal muscle fibers. Am J Physiol Cell Physiol 273:C1915–C1924. https://doi.org/10.1152/ajpcell.1997.273.6.C1915
Loren GJ, Lieber RL (1995) Tendon biomechanical properties enhance human wrist muscle specialization. J Biomech 28:791–799. https://doi.org/10.1016/0021-9290(94)00137-S
Lowe DA, Surer JT, Thomas DD et al (2001) Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol 280:540–547. https://doi.org/10.1152/ajpcell.2001.280.3.C540
Lowe DA, Thomas DD, Thompson LV (2002) Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol 283:C187–C192. https://doi.org/10.1152/ajpcell.00008.2002
Luden N, Minchev K, Hayes E et al (2008) Human vastus lateralis and soleus muscles display divergent cellular contractile properties. Am J Physiol Regul Integr Comp Physiol 295:1593–1598. https://doi.org/10.1152/ajpregu.90564.2008
Luff AR (1981) Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol 313:161–171. https://doi.org/10.1113/jphysiol.1981.sp013656
Lynch GS, Stephenson DG, Williams DA (1991) Endurance exercise effects on the contractile properties of single, skinned skeletal muscle fibres of young rats. Pflugers Arch 418:161–167. https://doi.org/10.1007/BF00370466
Lynch GS, Stephenson DG, Williams DA (1995) Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms. J Muscle Res Cell Motil 16:65–78. https://doi.org/10.1007/BF00125311
Macintosh BR, Glumpak JJ, MacNaughton MB, Rassier DE (2011) Pattern of summation with fatigue and inhibition of calcium release in rat muscle. Muscle Nerve 44:410–417. https://doi.org/10.1002/mus.22073
Marsh E, Sale D, McComas AJ, Quinlan J (1981) Influence of joint position on ankle dorsiflexion in humans. J Appl Physiol 51:160–167. https://doi.org/10.1152/jappl.1981.51.1.160
Martyn DA, Gordon AM (1988) Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength. J Muscle Res Cell Motil 9:428–445. https://doi.org/10.1007/BF01774069
Martyn DA, Gordon AM (2001) Influence of length on force and activation-dependent changes in troponin C structure in skinned cardiac and fast skeletal muscle. Biophys J 80:2798–2808. https://doi.org/10.1016/S0006-3495(01)76247-9
Maughan DW, Molloy JE, Brotto MA, Godt RE (1995) Approximating the isometric force-calcium relation of intact frog muscle using skinned fibers. Biophys J 69:1484–1490. https://doi.org/10.1016/S0006-3495(95)80019-6
Mayfield DL, Lichtwark GA, Cronin NJ et al (2015) Doublet potentiation in the triceps surae is limited by series compliance and dynamic fascicle behavior. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00403.2015
Mayfield DL, Cresswell AG, Lichtwark GA (2016a) Effects of series elastic compliance on muscle force summation and the rate of force rise. J Exp Biol 219:3261–3270. https://doi.org/10.1242/jeb.142604
Mayfield DL, Launikonis BS, Cresswell AG, Lichtwark GA (2016b) Additional in-series compliance reduces muscle force summation and alters the time course of force relaxation during fixed-end contractions. J Exp Biol 219:3587–3596. https://doi.org/10.1242/jeb.143123
Mayfield DL, Lichtwark GA (2022) Muscle-Model. https://doi.org/10.5281/ZENODO.6423696
Mazara N, Zwambag DP, Noonan AM et al (2021) Rate of force development is Ca2+-dependent and influenced by Ca2+-sensitivity in human single muscle fibres from older adults. Exp Gerontol 150:111348. https://doi.org/10.1016/j.exger.2021.111348
McCrum C, Leow P, Epro G et al (2018) Alterations in leg extensor muscle-tendon unit biomechanical properties with ageing and mechanical loading. Front Physiol 9:1–7. https://doi.org/10.3389/fphys.2018.00150
McNeil CJ, Doherty TJ, Stashuk DW, Rice CL (2005) Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31:461–467. https://doi.org/10.1002/mus.20276
McNeil CJ, Vandervoort AA, Rice CL (2007) Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol 102:1962–1968. https://doi.org/10.1152/japplphysiol.01166.2006
Miller MS, Bedrin NG, Callahan DM et al (2013) Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol 115:1004–1014. https://doi.org/10.1152/japplphysiol.00563.2013
Monti E, Reggiani C, Franchi MV et al (2021) Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J Physiol 599:3037–3061. https://doi.org/10.1113/JP281365
Moo EK, Leonard TR, Herzog W (2020) The sarcomere force-length relationship in an intact muscle-tendon unit. J Exp Biol 223:215020. https://doi.org/10.1242/jeb.215020
Moran AL, Warren GL, Lowe DA (2005) Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol 40:966–975. https://doi.org/10.1016/j.exger.2005.09.005
Morse CI, Thom JM, Birch KM, Narici MV (2005) Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand 183:291–298. https://doi.org/10.1111/j.1365-201X.2004.01404.x
Muramatsu T, Muraoka T, Takeshita D et al (2001) Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J Appl Physiol 90:1671–1678
Nakagawa Y, Hayashi K, Yamamoto N, Nagashima K (1996) Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol Occup Physiol 73:7–10. https://doi.org/10.1007/BF00262803
Narayanan N, Jones DL, Xu A, Yu JC (1996) Effects of aging on sarcoplasmic reticulum function and contraction duration in skeletal muscles of the rat. Am J Physiol Cell Physiol 271:C1032–C1040. https://doi.org/10.1152/ajpcell.1996.271.4.C1032
Narici MV, Bordini M, Cerretelli P (1991) Effect of aging on human adductor pollicis muscle function. J Appl Physiol 71:1277–1281. https://doi.org/10.1152/jappl.1991.71.4.1277
Narici MV, Maganaris CN, Reeves ND, Capodaglio P (2003) Effect of aging on human muscle architecture. J Appl Physiol 95:2229–2234. https://doi.org/10.1152/japplphysiol.00433.2003
Nelson CR, Fitts RH (2014) Effects of low cell pH and elevated inorganic phosphate on the pCa-force relationship in single muscle fibers at near-physiological temperatures. Am J Physiol Cell Physiol 306:C670–C678. https://doi.org/10.1152/ajpcell.00347.2013
Nilwik R, Snijders T, Leenders M et al (2013) The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48:492–498. https://doi.org/10.1016/j.exger.2013.02.012
Ochala J, Frontera WR, Dorer DJ et al (2007) Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62:375–381. https://doi.org/10.1093/gerona/62.4.375
Olsson K, Cheng AJ, Al-Ameri M et al (2020) Impaired sarcoplasmic reticulum Ca2+ release is the major cause of fatigue-induced force loss in intact single fibres from human intercostal muscle. J Physiol 598:773–787. https://doi.org/10.1113/JP279090
Onambele GL, Narici MV, Maganaris CN (2006) Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 100:2048–2056. https://doi.org/10.1152/japplphysiol.01442.2005
Ong CF, Geijtenbeek T, Hicks JL, Delp SL (2019) Predicting gait adaptations due to ankle plantarflexor muscle weakness and contracture using physics-based musculoskeletal simulations. PLoS Comput Biol 15:e1006993. https://doi.org/10.1371/journal.pcbi.1006993
Otten E (1987) A myocybernetic model of the jaw system of the rat. J Neurosci Methods 21:287–302. https://doi.org/10.1016/0165-0270(87)90123-3
Pardes AM, Beach ZM, Raja H et al (2017) Aging leads to inferior Achilles tendon mechanics and altered ankle function in rodents. J Biomech 60:30–38. https://doi.org/10.1016/j.jbiomech.2017.06.008
Pettigrew FP, Gardiner PF (1987) Changes in rat plantaris motor unit profiles with advanced age. Mech Ageing Dev 40:243–259. https://doi.org/10.1016/0047-6374(87)90022-4
Plant DR, Lynch GS (2001) Rigor force responses of permeabilized fibres from fast and slow skeletal muscles of aged rats. Clin Exp Pharmacol Physiol 28:779–781. https://doi.org/10.1046/j.1440-1681.2001.03521.x
Power GA, Minozzo FC, Spendiff S et al (2016) Reduction in single muscle fiber rate of force development with aging is not attenuated in world class older masters athletes. Am J Physiol Cell Physiol 310:C318–C327. https://doi.org/10.1152/ajpcell.00289.2015
Rack PM, Westbury DR (1969) The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol 204:443–460. https://doi.org/10.1113/jphysiol.1969.sp008923
Rack PM, Westbury DR, (1984) Elastic properties of the cat soleus tendon and their functional importance. J Physiol 347:479–495. https://doi.org/10.1113/jphysiol.1984.sp015077
Ranatunga KW (1982) Temperature-dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol 329:465–483. https://doi.org/10.1113/jphysiol.1982.sp014314
Ranatunga KW (1998) Temperature dependence of mechanical power output in mammalian (rat) skeletal muscle. Exp Physiol 83:371–376. https://doi.org/10.1113/expphysiol.1998.sp004120
Ranatunga KW, Thomas PE (1990) Correlation between shortening velocity, force-velocity relation and histochemical fibre-type composition in rat muscles. J Muscle Res Cell Motil 11:240–250. https://doi.org/10.1007/BF01843577
Reid KF, Doros G, Clark DJ et al (2012) Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol 112:2289–2301. https://doi.org/10.1007/s00421-011-2200-0
Rice KM, Linderman JK, Kinnard RS, Blough ER (2005) The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology 6:335–343. https://doi.org/10.1007/s10522-005-4808-0
Roberts TJ (2002) The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol - A Mol Integr Physiol 133:1087–1099. https://doi.org/10.1016/S1095-6433(02)00244-1
Roos MR, Rice CL, Connelly DM, Vandervoort AA (1999) Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22:1094–1103. https://doi.org/10.1002/(SICI)1097-4598(199908)22:8%3c1094::AID-MUS14%3e3.0.CO;2-G
Rubenson J, Pires NJ, Loi HO et al (2012) On the ascent: the soleus operating length is conserved to the ascending limb of the force–length curve across gait mechanics in humans. J Exp Biol 215:3539–3551. https://doi.org/10.1242/jeb.070466
Ruff RL (1989) Calcium sensitivity of fast- and slow-twitch human muscle fibers. Muscle Nerve 12:32–37. https://doi.org/10.1002/mus.880120107
Ruff RL, Whittlesey D (1991) Ca-, Sr-tension relationships and contraction velocities of human muscle fibers. Muscle Nerve 14:1219–1226. https://doi.org/10.1002/mus.880141214
Russ DW, Grandy JS, Toma K, Ward CW (2011) Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol 201:391–403. https://doi.org/10.1111/j.1748-1716.2010.02191.x
Russ DW, Wills AM, Boyd IM, Krause J (2014) Weakness, SR function and stress in gastrocnemius muscles of aged male rats. Exp Gerontol 50:40–44. https://doi.org/10.1016/j.exger.2013.11.018
Sale D, Quinlan J, Marsh E et al (1982) Influence of joint position on ankle plantarflexion in humans. J Appl Physiol Respir Environ Exerc Physiol 52:1636–1642
Sandercock TG (2006) Extra force from asynchronous stimulation of cat soleus muscle results from minimizing the stretch of the common elastic elements. J Neurophysiol 96:1401–1405. https://doi.org/10.1152/jn.01304.2005
Sawicki GS, Roberts TJ (2009) Isometric force production requires asymmetric muscle-tendon length trajectory, in: 33rd Annual Meeting of American Society of Biomechanics. State College, PA: ASB.
Seow CY (2013) Hill’s equation of muscle performance and its hidden insight on molecular mechanisms. J Gen Physiol 142:561–573. https://doi.org/10.1085/jgp.201311107
Short KR, Vittone JL, Bigelow ML et al (2005) Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol 99:95–102. https://doi.org/10.1152/japplphysiol.00129.2005
Simoneau E, Martin A, Van Hoecke J (2005) Muscular performances at the ankle joint in young and elderly men. J Gerontol A Biol Sci Med Sci 60:439–447. https://doi.org/10.1093/gerona/60.4.439
Soendenbroe C, Dahl CL, Meulengracht C et al (2022) Preserved stem cell content and innervation profile of elderly human skeletal muscle with lifelong recreational exercise. J Physiol 600:1969–1989. https://doi.org/10.1113/JP282677
Song S, Geyer H (2018) Predictive neuromechanical simulations indicate why walking performance declines with ageing. J Physiol 596:1199–1210. https://doi.org/10.1113/JP275166
Sonjak V, Jacob K, Morais JA et al (2019) Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol 597:5009–5023. https://doi.org/10.1113/JP278261
Stenroth L, Peltonen J, Cronin NJ et al (2012) Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113:1537–1544. https://doi.org/10.1152/japplphysiol.00782.2012
Stephenson DG, Williams DA (1981) Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol 317:281–302. https://doi.org/10.1113/jphysiol.1981.sp013825
Stephenson DG, Williams DA (1982) Effects of sarcomere length on the force—pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol 333:637–653. https://doi.org/10.1113/jphysiol.1982.sp014473
Straight CR, Ades PA, Toth MJ, Miller MS (2018) Age-related reduction in single muscle fiber calcium sensitivity is associated with decreased muscle power in men and women. Exp Gerontol 102:84–92. https://doi.org/10.1016/j.exger.2017.12.007
Sullivan VK, Powers SK, Criswell DS et al (1995) Myosin heavy chain composition in young and old rat skeletal muscle: effects of endurance exercise. J Appl Physiol 78:2115–2120. https://doi.org/10.1152/jappl.1995.78.6.2115
Sun YB, Lou F, Edman KAP (1996) The relationship between the intracellular Ca2+ transient and the isometric twitch force in frog muscle fibres. Exp Physiol 81:711–724. https://doi.org/10.1113/expphysiol.1996.sp003971
Sundberg CW, Hunter SK, Trappe SW et al (2018) Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans. J Physiol 596:3993–4015. https://doi.org/10.1113/JP276018
Svensson RB, Heinemeier KM, Couppé C et al (2016) Effect of aging and exercise on the tendon. J Appl Physiol 121:1353–1362. https://doi.org/10.1152/japplphysiol.00328.2016
Teigen LE, Sundberg CW, Kelly LJ et al (2020) Ca2+ dependency of limb muscle fiber contractile mechanics in young and older adults. Am J Physiol Cell Physiol 318:C1238–C1251. https://doi.org/10.1152/ajpcell.00575.2019
Tevald MA, Foulis SA, Lanza IR, Kent-Braun JA (2009) Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Regul Integr Comp Physiol 298:R729–R739. https://doi.org/10.1152/ajpregu.00713.2009
Thelen DG (2003) Adjustment of muscle mechanics model parameters to simulate dynamic contractions in older adults. J Biomech Eng 125:70–77. https://doi.org/10.1115/1.1531112
Thom JM, Morse CI, Birch KM, Narici MV (2007) Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol 100:613–619. https://doi.org/10.1007/s00421-007-0481-0
Thomas MM, Vigna C, Betik AC et al (2010) Initiating treadmill training in late middle age offers modest adaptations in Ca2+ handling but enhances oxidative damage in senescent rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298:R1269–R1278. https://doi.org/10.1152/ajpregu.00663.2009
Thompson LV, Brown M (1999) Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol 86:881–886. https://doi.org/10.1152/jappl.1999.86.3.881
Thompson LV, Johnson SA, Shoeman JA (1998) Single soleus muscle fiber function after hindlimb unweighting in adult and aged rats. J Appl Physiol 84:1937–1942. https://doi.org/10.1152/jappl.1998.84.6.1937
Trappe S, Gallagher P, Harber M et al (2003) Single muscle fibre contractile properties in young and old men and women. J Physiol 552:47–58. https://doi.org/10.1113/jphysiol.2003.044966
Trestik CL, Lieber RL (1993) Relationship between achilles tendon mechanical properties and gastrocnemius muscle function. J Biomech Eng 115:225–230. https://doi.org/10.1115/1.2895479
Umanskaya A, Santulli G, Xie W et al (2014) Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci USA 111:15250–15255. https://doi.org/10.1073/pnas.1412754111
van Schaik CS, Hicks AL, McCartney N (1994) An evaluation of the length-tension relationship in elderly human ankle dorsiflexors. J Gerontol 49:B121–B127. https://doi.org/10.1093/geronj/49.3.b121
Vandervoort AA, McComas AJ (1986) Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol 61:361–367. https://doi.org/10.1152/jappl.1986.61.1.361
Wakeling JM, Johnston IA (1999) Predicting muscle force generation during fast-starts for the common carp Cyprinus carpio. J Comp Physiol B 169:391–401. https://doi.org/10.1007/s003600050235
Wakeling JM, Lee SSM, Arnold AS et al (2012) A muscle’s force depends on the recruitment patterns of its fibers. Ann Biomed Eng 40:1708–1720. https://doi.org/10.1007/s10439-012-0531-6
Walker JS, Li X, Buttrick PM (2010) Analysing force–pCa curves. J Muscle Res Cell Motil 31:59–69. https://doi.org/10.1007/s10974-010-9208-7
Walters TJ, Sweeney HL, Farrar RP (1990) Aging does not affect contractile properties of type IIb FDL muscle in Fischer 344 rats. Am J Physiol Cell Physiol 258:C1031–C1035. https://doi.org/10.1152/ajpcell.1990.258.6.C1031
Wang ZM, Messi ML, Delbono O (2000) L-type Ca2+ channel charge movement and intracellular Ca2+ in skeletal muscle fibers from aging mice. Biophys J 78:1947–1954. https://doi.org/10.1016/S0006-3495(00)76742-7
Wang Z-M, Messi ML, Delbono O (2002) Sustained overexpression of IGF-1 prevents age-dependent decrease in charge movement and intracellular Ca2+ in mouse skeletal muscle. Biophys J 82:1338–1344. https://doi.org/10.1016/S0006-3495(02)75489-1
Westerblad H, Allen DG (1991) Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98:615–635. https://doi.org/10.1085/jgp.98.3.615
Westerblad H, Allen DG (1993) The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol 468:729–740. https://doi.org/10.1113/jphysiol.1993.sp019797
Westerblad H, Duty S, Allen DG (1993) Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75:382–388. https://doi.org/10.1152/jappl.1993.75.1.382
Widrick JJ, Trappe SW, Costill DL, Fitts RH (1996) Force-velocity and force-power properties of single muscle fibers from elite master runners and sedentary men. Am J Physiol 271:C676–C683. https://doi.org/10.1152/ajpcell.1996.271.2.C676
Widrick JJ, Norenberg KM, Romatowski JG et al (1998) Force-velocity-power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol 85:1949–1956. https://doi.org/10.1152/jappl.1998.85.5.1949
Widrick JJ, Stelzer JE, Shoepe TC, Garner DP (2002) Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283:R408–R416. https://doi.org/10.1152/ajpregu.00120.2002
Williams T, Bowtell G, Curtin NA (1998) Predicting force generation by lamprey muscle during applied sinusoidal movement using a simple dynamic model. J Exp Biol 201:869–875. https://doi.org/10.1242/jeb.201.6.869
Winters TM, Takahashi M, Lieber RL, Ward SR (2011) Whole muscle length-tension relationships are accurately modeled as scaled sarcomeres in rabbit hindlimb muscles. J Biomech 44:109–115. https://doi.org/10.1016/j.jbiomech.2010.08.033
Wisdom KM, Delp SL, Kuhl E (2015) Use it or lose it: multiscale skeletal muscle adaptation to mechanical stimuli. Biomech Model Mechanobiol 14:195–215. https://doi.org/10.1007/s10237-014-0607-3
Wood LK, Brooks SV (2016) Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J Orthop Res 34:346–353. https://doi.org/10.1002/jor.22824
Wood LK, Arruda EM, Brooks SV (2011) Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol 111:999–1006. https://doi.org/10.1152/japplphysiol.00460.2011
Xu H, Lamb GD, Murphy RM (2017) Changes in contractile and metabolic parameters of skeletal muscle as rats age from 3 to 12 months. J Muscle Res Cell Motil 38:405–420. https://doi.org/10.1007/s10974-017-9484-6
Yu F, Hedström M, Cristea A et al (2007) Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol 190:229–241. https://doi.org/10.1111/j.1748-1716.2007.01699.x
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17:359–411
Zhong S, Lowe DA, Thompson LV (2006) Effects of hindlimb unweighting and aging on rat semimembranosus muscle and myosin. J Appl Physiol 101:873–880. https://doi.org/10.1152/japplphysiol.00526.2005
Zuurbier CJ, Everard AJ, van der Wees P, Huijing PA (1994) Length-force characteristics of the aponeurosis in the passive and active muscle condition and in the isolated condition. J Biomech 27:445–453. https://doi.org/10.1016/0021-9290(94)90020-5
Acknowledgements
This study benefited from the contributions of members belonging to the University of Jyväskylä Neuromuscular Research Centre, in particular, Drs Janne Avela and Marko Korhonen. We are also grateful to Dr Andrew Cresswell for insightful discussions, the participants who volunteered for this study, and two anonymous reviewers for their constructive comments.
Funding
No funds, grants, or other support was received to directly support this work. G.A.L. is supported by an Australian Research Council Future Fellowship (FT190100129).
Author information
Authors and Affiliations
Contributions
Conceptualization: D.L.M., N.J.C., and G.A.L.; Investigation: D.L.M. and N.J.C.; Software: D.L.M. and G.A.L.; Formal analysis: D.L.M.; Visualisation: D.L.M; Writing—original draft: D.L.M.; Writing—review & editing: D.L.M., N.J.C., and G.A.L.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing or financial interests.
Ethical approval
Experimental work was approved by and performed in accordance with the guidelines outlined by the human research ethical review committee of the University of Jyväskylä. Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayfield, D.L., Cronin, N.J. & Lichtwark, G.A. Understanding altered contractile properties in advanced age: insights from a systematic muscle modelling approach. Biomech Model Mechanobiol 22, 309–337 (2023). https://doi.org/10.1007/s10237-022-01651-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-022-01651-9