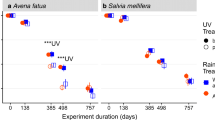
Abstract
Litter decomposition is a key ecosystem process that determines rates of carbon and nutrient cycling. Photodegradation and soil-litter mixing have emerged as important drivers of dryland litter decomposition, but how these processes interact with decomposing microorganisms has received less attention. In this study, we examined the effects of ultraviolet-B radiation (UV-B; 280–315 nm) and soil-litter mixing on the decomposition of litter and its associated microbial community in an arid shrubland. We performed a full factorial litter decomposition experiment using leaf litter from a dominant shrub (Prosopis velutina) and a dominant grass (Eragrostis lehmanniana) that were exposed to solar radiation with near-ambient or attenuated UV-B, and were either soil-free or soil-covered; we then quantified litter decomposition and microbial community composition over a 12 month period. In general, shrub litter decomposed more rapidly than grass litter regardless of soil coverage, likely due to its lower C:N. Attenuation of UV-B had modest effects on decomposition but UV-B exposure did increase fungal biomass, perhaps reflecting facilitative aspects of photodegradation. Both bacteria and fungi emerged as important regulators of decomposition, and microbial decomposition was indirectly mediated by litter C:N, soil coverage, and UV-B effects on the microbial community. Bacterial colonization was inhibited in soil-free treatments, but was facilitated when litter was soil-covered. These findings suggest that UV-B may play an important role in facilitating fungal decomposition of litter, while soil-litter mixing is fundamental for promoting bacterial decomposition of litter.

Highlights
-
Soil-litter mixing enhanced bacterial colonization of litter.
-
Fungi are present on dryland litter regardless of soil and UV-B conditions.
-
Soil-litter mixing initially accelerated litter decomposition but subsequently reduced it.










Similar content being viewed by others
References
Adair EC, Parton WJ, King JY, Brandt LA, Lin Y. 2017. Accounting for photodegradation dramatically improves prediction of carbon losses in dryland systems. Ecosphere 8:e01892.
Allen MF. 2007. Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone Journal 6:291–297.
Archer S, Andersen E, Predick K, Schwinning S, Steidl R, Woods S. 2017. Woody plant encroachment: causes and consequences. In: Briske D, Ed. Rangeland systems: processes, management and challenges, . Springer. pp 25–84.
Austin AT, Ballaré CL. 2010. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci USA 107:4618–4622.
Austin AT, Méndez MS, Ballaré CL. 2016. Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. PNAS 113:4392–4397.
Baker NR, Allison SD. 2015. Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate. Ecology 96:1994–2003.
Bärlocher F, Gessner MO, Graça MAS, Eds. 2020. Cham: Springer International Publishing.
Barnard RL, Osborne CA, Firestone MK. 2013. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. The ISME Journal 7:2229–2241.
Barnes PW, Throop HL, Hewins DB, Abbene ML, Archer SR. 2012. Soil Coverage Reduces Photodegradation and Promotes the Development of Soil-Microbial Films on Dryland Leaf Litter. Ecosystems 15:311–321.
Barnes PW, Robson TM, Zepp RG, Bornman JF, Jansen MAK, Ossola R, Wang Q-W, Robinson SA, Foereid B, Klekociuk AR, Martinez-Abaigar J, Hou W-C, Mackenzie R, Paul ND. 2023. Interactive effects of changes in UV radiation and climate on terrestrial ecosystems, biogeochemical cycles, and feedbacks to the climate system. Photochemical & Photobiological Sciences: In Press.
Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:1–48.
Baumert VL, Vasilyeva NA, Vladimirov AA, Meier IC, Kögel-Knabner I, Mueller CW. 2018. Root Exudates Induce Soil Macroaggregation Facilitated by Fungi in Subsoil. Front Environ Sci 6:140.
Belnap J, Phillips SL, Flint S, Money J, Caldwell M. 2008. Global change and biological soil crusts: effects of ultraviolet augmentation under altered precipitation regimes and nitrogen additions. Global Change Biology 14:670–686.
Berenstecher P, Vivanco L, Pérez LI, Ballaré CL, Austin AT. 2020. Sunlight doubles aboveground carbon loss in a seasonally dry woodland in Patagonia. Current Biology 30(3243–3251):E3243. https://doi.org/10.1016/j.cub.2020.06.005.
Bonkowski M. 2004. Protozoa and plant growth: the microbial loop in soil revisited. New Phytologist 162:617–631.
Bornman JF, Barnes PW, Robson TM, Robinson SA, Jansen MAK, Ballaré CL, Flint SD. 2019. Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochem Photobiol Sci 18:681–716.
Bosco T, Bertiller MB, Carrera AL. 2016. Combined effects of litter features, UV radiation, and soil water on litter decomposition in denuded areas of the arid Patagonian Monte. Plant Soil 406:71–82.
Bradford MA, Wieder WR, Bonan GB, Fierer N, Raymond PA, Crowther TW. 2016. Managing uncertainty in soil carbon feedbacks to climate change. Nature Climate Change 6:751–758.
Brandt LA, King JY, Hobbie SE, Milchunas DG, Sinsabaugh RL. 2010. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 13:765–781.
Browning D, Archer S, Asner G, McClaran M, Wessman C. 2008. Woody plants in grasslands: post-encroachment stand dynamics. Ecological Applications 18:928–944.
Charney N, Record S, Charney MN. 2016. Vegetarian: Jost Diversity Measures for Community Data. https://CRAN.R-project.org/package=vegetarian.
Collins SL, Sinsabaugh RL, Crenshaw C, Green L, Porras-Alfaro A, Stursova M, Zeglin LH. 2008. Pulse dynamics and microbial processes in aridland ecosystems. Journal of Ecology 96:413–420.
Crowther TW, Maynard DS, Leff JW, Oldfield EE, McCulley RL, Fierer N, Bradford MA. 2014. Predicting the responsiveness of soil biodiversity to deforestation: a cross-biome study. Global Change Biology 20:2983–2994.
Day TA, Bliss MS. 2019. A spectral weighting function for abiotic photodegradation based on photochemical emission of CO2 from leaf litter in sunlight. Biogeochemistry 146:173–190.
Day TA, Bliss MS. 2020. Solar photochemical emission of CO2 from leaf litter: sources and significance to C loss. Ecosystems 23:1344–1361.
Day TA, Bliss MS, Tomes AR, Ruhland CT, Guénon R. 2018. Desert leaf litter decay: Coupling of microbial respiration, water-soluble fractions and photodegradation. Global Change Biology 24:5454–5470.
Ding J, Eldridge DJ. 2022. Drivers of soil biodiversity vary with organism type along an extensive aridity gradient. Applied Soil Ecology 170:104271.
Evans S, Todd-Brown KEO, Jacobson K, Jacobson P. 2020. Non-rainfall moisture: A key driver of microbial respiration from standing litter in arid, semiarid, and mesic grasslands. Ecosystems 23:1154–1169. https://doi.org/10.1007/s10021-019-00461-y
Feng W, Zhang Y, Yan R, Lai Z, Qin S, Sun Y, She W, Liu Z. 2020. Dominant soil bacteria and their ecological attributes across the deserts in northern China. European Journal of Soil Science 71:524–535.
Findlay RH, Dobbs FC. 1993. Quantitative description of microbial communities using lipid analysis. Handbook of Methods Aquatic Microbial Ecology, . CRC Press.
Flint SD, Caldwell MM. 2003. A biological spectral weighting function for ozone depletion research with higher plants. Physiologia Plantarum 117:137–144.
Gao Q, Garcia-Pichel F. 2011. Microbial ultraviolet sunscreens. Nature Reviews Microbiology 9:791–802.
Gliksman D, Rey A, Seligmann R, Dumbur R, Sperling O, Navon Y, Haenel S, Angelis PD, Arnone JA, Grünzweig JM. 2017. Biotic degradation at night, abiotic degradation at day: positive feedbacks on litter decomposition in drylands. Global Change Biology 23:1564–1574.
Grünzweig JM, De Boeck HJ, Rey A, Santos MJ, Adam O, Bahn M, Belnap J, Deckmyn G, Dekker SC, Flores O, Gliksman D, Helman D, Hultine KR, Liu L, Meron E, Michael Y, Sheffer E, Throop HL, Tzuk O, Dan Yakir D. 2022. Dryland mechanisms could widely control ecosystem functioning in a drier and warmer world. Nature Ecolology & Evolution 6:1064–1076. https://doi.org/10.1038/s41559-022-01779-y.
Hewins DB, Archer SR, Okin GS, McCulley RL, Throop HL. 2013. Soil–litter mixing accelerates decomposition in a Chihuahuan desert grassland. Ecosystems 16:183–195.
Hewins DB, Sinsabaugh RL, Archer SR, Throop HL. 2017. Soil–litter mixing and microbial activity mediate decomposition and soil aggregate formation in a sandy shrub-invaded Chihuahuan Desert grassland. Plant Ecol 218:459–474.
Hicks LC, Ang R, Leizeaga A, Rousk J. 2019. Bacteria constrain the fungal growth response to drying-rewetting. Soil Biology and Biochemistry 134:108–112.
Homyak PM, Blankinship JC, Marchus K, Lucero DM, Sickman JO, Schimel JP. 2016. Aridity and plant uptake interact to make dryland soils hotspots for nitric oxide (NO) emissions. Proceedings of the National Academy of Sciences 113:E2608–E2616.
Hooper D, Coughlan J, Mullen MR. 2008. Structural equation modelling: guidelines for determining model fit. Electronic Journal of Business Research Methods 6:53–60.
Joly F-X, Kurupas KL, Throop HL. 2017. Pulse frequency and soil-litter mixing alter the control of cumulative precipitation over litter decomposition.
King JY, Brandt LA, Adair EC. 2012. Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry 111:57–81.
Klein DA, Paschke MW. 2004. Filamentous fungi: the indeterminate lifestyle and microbial ecology. Microbial Ecology 47:224–235.
Kotas P, Choma M, Šantrůčková H, Lepš J, Tříska J, Kaštovská E. 2017. Linking Above- and Belowground Responses to 16 Years of Fertilization, Mowing, and Removal of the Dominant Species in a Temperate Grassland. Ecosystems 20:354–367.
Lee H, Rahn T, Throop H. 2012. An accounting of C-based trace gas release during abiotic plant litter degradation. Global Change Biology 18:1185–1195.
Lee H, Fitzgerald J, Hewins DB, McCulley RL, Archer SR, Rahn T, Throop HL. 2014. Soil moisture and soil-litter mixing effects on surface litter decomposition: A controlled environment assessment. Soil Biology and Biochemistry 72:123–132.
Lenth R, Singmann H, Love J, Buerkner P, Herve M. 2020. emmeans: Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeans.
Levi EM, Archer SR, Throop HL, Rasmussen C. 2020. Soil-litter mixing promotes decomposition and soil aggregate formation on contrasting geomorphic surfaces in a shrub-invaded Sonoran Desert grassland. Plant Soil 450:397–415.
Lin Y, Scarlett RD, King JY. 2015. Effects of UV photodegradation on subsequent microbial decomposition of Bromus diandrus litter. Plant Soil 395:263–271.
Logan JR, Barnes P, Evans SE. 2022. Photodegradation of plant litter cuticles enhances microbial decomposition by increasing uptake of non-rainfall moisture. Functional Ecology 36:1727–1738.
McClaran MP, Browning DM, Huang C. 2010. Temporal dynamics and spatial variability in desert grassland vegetation. Pages 145–166 in D. E. B. R.H. Webb, R.M. Turner editor. Repeat Photography: Methods and Applications in the Natural Sciences. Island Press.
McClaran MP. 2003. A century of vegetation change on the Santa Rita Experimental Range. In: McClaran, Mitchel P; Ffolliott, Peter F; Edminster, Carleton B, tech coords Santa Rita Experimental Range: 100 years (1903 to 2003) of accomplishments and contributions; conference proceedings; 2003 October 30-November 1; Tucson, AZ Proc RMRS-P-30 Ogden, UT: US Department of Agriculture, Forest Service, Rocky Mountain Research Station p 16–33 30:16–33.
Meentemeyer V. 1978. Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472.
Moody SA, Newsham KK, Ayres PG, Paul ND. 1999. Variation in the responses of litter and phylloplane fungi to UV-B radiation (290–315 nm). Mycological Research 103:1469–1477.
Moorhead DL, Reynolds JF. 1991. A general model of litter decomposition in the northern Chihuahuan Desert. Ecological Modelling 56:197–219.
Němcová L, Bystrianský L, Hujslová M, Malinská HA, Hršelová H, Gryndler M. 2022. Detection of biofilm and planktonic microbial communities in litter/soil mixtures. Applied Soil Ecology 179:104589.
Okin GS, Heras MMDL, Saco PM, Throop HL, Vivoni ER, Parsons AJ, Wainwright J, Peters DP. 2015. Connectivity in dryland landscapes: shifting concepts of spatial interactions. Frontiers in Ecology and the Environment 13(1):20–27.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2019. vegan: Community Ecology Package. https://CRAN.R-project.org/package=vegan. Last accessed 25/02/2020
Ortiz C, Fernández M, Kitzler B, Díaz-Pinés E, Saiz G, Rubio A, Benito M. 2022. Variations in soil aggregation, microbial community structure and soil organic matter cycling associated to long-term afforestation and woody encroachment in a Mediterranean alpine ecotone. Geoderma 405:115450.
Osburn ED, Hoch PJ, Lucas JM, McBride SG, Strickland MS. 2022A. Evaluating the roles of microbial functional breadth and home-field advantage in leaf litter decomposition. Functional Ecology 36:1258–1267.
Osburn ED, McBride SG, Kupper JV, Nelson JA, McNear DH, McCulley RL, Barrett JE. 2022B. Accurate detection of soil microbial community responses to environmental change requires the use of multiple methods. Soil Biology and Biochemistry 169:108685.
Pancotto VA, Sala OE, Cabello M, López NI, Matthew Robson T, Ballaré CL, Caldwell MM, Scopel AL. 2003. Solar UV-B decreases decomposition in herbaceous plant litter in Tierra del Fuego, Argentina: potential role of an altered decomposer community: UV-B radiation, decomposition and decomposers. Global Change Biology 9:1465–1474.
Paradis E, Blomberg S, Bolker B, Brown J, Claude J, Cuong HS, Desper R, Didier G. 2018. Package ‘ape’. Analyses of phylogenetics and evolution, version:2–4.
Pieristè M, Chauvat M, Kotilainen TK, Jones AG, Aubert M, Robson TM, Forey E. 2019. Solar UV-A radiation and blue light enhance tree leaf litter decomposition in a temperate forest. Oecologia 191:191–203.
Pieristè M, Forey E, Lounès-Hadj Sahraoui A, Meglouli H, Laruelle F, Delporte P, Robson TM, Chauvat M. 2020. Spectral composition of sunlight affects the microbial functional structure of beech leaf litter during the initial phase of decomposition. Plant Soil 451:515–530.
Predick KI, Archer SR, Aguillon SM, Keller DA, Throop HL, Barnes PW. 2018. UV-B radiation and shrub canopy effects on surface litter decomposition in a shrub-invaded dry grassland. Journal of Arid Environments 157:13–21.
Rodríguez-Echeverría S, Delcgado-Baquerizo M, Morillo JA, Gaxiola A, Manzano M, Marquet PA, González L, Cavieres LA, Pugnaire FI, Armas C. 2021. Azorella cushion plants and aridity are important drivers of soil microbial communities in Andean ecosystems. Ecosystems. https://doi.org/10.1007/s10021-021-00603-1.
Rosseel Y. 2012. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software 48:1–36.
Sagi N, Zaguri M, Hawlena D. 2021. Macro-detritivores assist resolving the dryland decomposition conundrum by engineering an underworld heaven for decomposers. Ecosystems 24:56–67.
Schussman H, Geiger E, Mau-Crimmins T, Ward J. 2006. Spread and current potential distribution of an alien grass, Eragrostis lehmanniana Nees, in the southwestern USA: comparing historical data and ecological niche models. Diversity and Distributions 12:582–592.
Song X, Peng C, Jiang H, Zhu Q, Wang W. 2013. Direct and indirect effects of UV-B exposure on litter decomposition: a meta-analysis. PLoS ONE 8:e68858.
Sterflinger K, Tesei D, Zakharova K. 2012. Fungi in hot and cold deserts with particular reference to microcolonial fungi. Fungal Ecology 5:453–462.
Su Y, Le J, Ma X, Zhou X, Zhang Y, Gong Y, Han W, Li K, Liu X. 2021. Soil burial has a greater effect on litter decomposition rate than nitrogen enrichment in alpine grasslands. Journal of Plant Ecology 14:1047–1059.
Teixeira MM, Moreno LF, Stielow BJ, Muszewska A, Hainaut M, Gonzaga L, Abouelleil A, Patané JSL, Priest M, Souza R, Young S, Ferreira KS, Zeng Q, da Cunha MML, Gladki A, Barker B, Vicente VA, de Souza EM, Almeida S, Henrissat B, Vasconcelos ATR, Deng S, Voglmayr H, Moussa TAA, Gorbushina A, Felipe MSS, Cuomo CA, de Hoog GS. 2017. Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota). Studies in Mycology 86:1–28.
Throop HL, Archer SR. 2007. Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecological Applications 17:1809–1823.
Throop HL, Archer SR. 2009. Resolving the dryland decomposition conundrum: some new perspectives on potential drivers. Progress in botany, . Springerpp 171–194.
Throop HL, Belnap J. 2019. Connectivity dynamics in dryland litter cycles: moving decomposition beyond spatial stasis. BioScience 69:602–614.
Walvoord MA, Phillips FM, Stonestrom DA, Evans RD, Hartsough PC, Newman BD, Striegl RG. 2003. A reservoir of nitrate beneath desert soils. Science 302:1021–1024.
Wang Y, Li FY, Song X, Wang X, Suri G, Baoyin T. 2020. Changes in litter decomposition rate of dominant plants in a semi-arid steppe across different land-use types: Soil moisture, not home-field advantage, plays a dominant role. Agriculture, Ecosystems & Environment 303:107119.
Waterman PG, Mole S. 1994. Analysis of phenolic plant metabolites. Blackwell Scientific.
Wheeler CW, Archer SR, Asner GP, McMurtry CR. 2007. Climatic/edaphic controls on soil carbon/nitrogen response to shrub encroachment in desert grassland. Ecological Applications 17:1911–1928.
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ. 1979. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40:51–62.
Whitford WG, Meentemeyer V, Seastedt TR, Cromack K, Crossley DA, Santos P, Todd RL, Waide JB. 1981. Exceptions to the AET model: deserts and clear-cut forest. Ecology 62:275–277.
Wilkinson S. 2002. PLFA profiles of microbial communities in decomposing conifer litters subject to moisture stress. Soil Biology and Biochemistry 34:189–200.
Xu X, Thornton PE, Post WM. 2013. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Global Ecology and Biogeography 22:737–749.
Acknowledgments
This collaborative research was supported in part by NSF Ecosystems grants DEB 0816162, DEB 0815808, DEB 0815897, and DEB 081446, by Arizona Agricultural Experimentation Project ARZT-1360540-H12-199. This work is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture Hatch Program under KY006045. SM was supported on a United States Department of Agriculture Postdoctoral Fellowship NIFA 1023307. Laboratory and field assistance was provided by M. Tobler (Loyola University), M. del Refugio Bravo-Garza, M. Ferman and S. Riley (University of Arizona), and Jihan Ahmed and A. Elizabeth Carlisle (University of Kentucky). S. Husman and his staff at the Tucson Area Agriculture Centers provided critical logistical support. Jay Ripley of Steelheart Tattoo for graphic design of Figure 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions: SGM performed all data analysis and wrote the manuscript. EL performed the experiment, collected data, and contributed to writing the manuscript. JN and KP performed laboratory assays, collected data, and contributed to writing the manuscript. SA, PB, HT, and RM developed research questions and approach, oversaw research, and contributed to writing the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McBride, S.G., Levi, E.M., Nelson, J.A. et al. Soil-Litter Mixing Mediates Drivers of Dryland Decomposition along a Continuum of Biotic and Abiotic Factors. Ecosystems 26, 1349–1366 (2023). https://doi.org/10.1007/s10021-023-00837-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00837-1