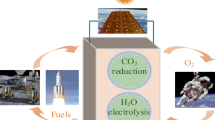
Abstract
In recent years, the space exploration is developing rapidly; therefore, human beings need the long-term sustainable supply of oxygen and fuel in the activities of space exploration. In situ resource utilization (ISRU) is a sustainable and low-cost solution, and it can reduce the dependence on carrying resources from the earth. ISRU technology might realize extraterrestrial crewed exploration, future space immigration, and other extraterrestrial activities. Taking Mars exploration as an example, the main component of Martian atmosphere is CO2, which can be used as a source of propulsion fuel. In this paper, a device based on microfluidic technology for ISRU was proposed. The reaction principle of the device is described, including the electrolysis reaction at the anode, the mixing of two-phase flow at the cathode, and the CO2 reduction reaction at the cathode. The device uses electrochemical catalysis to convert carbon dioxide and water into oxygen and fuel, which can achieve energy and matter transformation with a higher efficiency. Based on the device, the fluid flow and chemical reaction were studied; the research was carried out by simulation and experiment. The effects of microchip structural parameters and reaction conditions were analyzed. It is found that the electrochemical catalytic synthesis of CH3OH is affected by the gas–liquid phase flow rate, the microchannel structural parameters, the applied potential, and other conditions.





















Similar content being viewed by others
References
Yang L, Zhang C, Yu X et al (2021) Extraterrestrial artificial photosynthetic materials for in-situ resource utilization. Natl Sci Rev 8:nwab104. https://doi.org/10.1093/20nsr/nwab104
Starr SO, Muscatello AC (2020) Mars in situ resource utilization: a review. Planet Space Sci 182:104824. https://doi.org/10.1016/j.pss.2019.104824
Sridhar K, Iacomini C, Finn J (2004) Combined H2O/CO2 solid oxide electrolysis for mars in situ resource utilization. J Propul Power 20:892–901. https://doi.org/10.2514/1.3480
Wang L, Pérez-Fortes M, Madi H et al (2018) Optimal design of solid-oxide electrolyzer based power-to-methane systems: a comprehensive comparison between steam electrolysis and co-electrolysis. Appl Energy 211:1060–1079. https://doi.org/10.1016/j.apenergy.2017.11.050
Gruenwald J (2014) Human outposts on Mars: engineering and scientific lessons learned from history. CEAS Space J 6:73–77. https://doi.org/10.1007/s12567-014-0059-8
Snoeckx R, Bogaerts A (2017) Plasma technology- a novel solution for CO2 conversion. Chem Soc Rev 46:5805–5863. https://doi.org/10.1039/c6cs00066e
Brooks KP, Hu J, Zhu H et al (2007) Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem Eng Sci 62:1161–1170.
Blauth S, Leithäuser C, Pinnau R (2021) Optimal control of the Sabatier process in microchannel reactors. J Eng Math 128:1–28. https://doi.org/10.1007/s10665-021-10134-2
Sridhar K, Finn J, Kliss MH (2000) In-situ resource utilization technologies for Mars life support systems. Adv Space Res 25:249–255. https://doi.org/10.1016/S0273-1177
Matsushima H, Kiuchi D, Fukunaka et al (2009) Single bubble growth during water electrolysis under microgravity. Electrochem Commun 11:1721–1723. https://doi.org/10.1016/j.elecom.2009.07.009
Guo H, Zhao JF, Ye F et al (2008) Two-phase flow in fuel cells in short-term microgravity condition. Microgravity Sci Technol 20:265–269. https://doi.org/10.1007/s12217-008-9038-z
Sakurai M, Sone Y, Nishida T et al (2013) Fundamental study of water electrolysis for life support system in space. Electrochim Acta 100:350–357. https://doi.org/10.1016/j.electacta.2012.11.112
Van Rooij GJ, Van Den Bekerom DCM, Den Harder N et al (2015) Taming microwave plasma to beat thermodynamics in CO2 dissociation. Faraday Discuss 183:233–248. https://doi.org/10.1039/c5fd00045a
Gruenwald J (2018) Comment on The case for in situ resource utilization for oxygen production on Mars by non-equilibrium plasmas. Plasma Sources Sci Technol 27:028001. https://doi.org/10.1088/1361-6595/aaa873
Guerra V, Silva T, Ogloblina P et al (2017) The case for in situ resource utilisation for oxygen production on Mars by non-equilibrium plasmas. Plasma Sources Sci Technol 26:11LT01. https://doi.org/10.1088/1361-6595/aa8dcc
Ogloblina P, Morillo-Candas AS, Silva AF et al (2021) Mars in situ oxygen and propellant production by non-equilibrium plasmas. Plasma Sources Sci Technol 30. https://doi.org/10.1088/201361-6595/abec28
Krödel M (2020) Rational design of ion exchange membrane material properties limits the crossover of CO2 reduction products in artificial photosynthesis devices. ACS Appl Mater Interfaces 12:12030–12042. https://doi.org/10.1021/acsami.9b21415
Kumar A, Raizada P, Thakur VK et al (2021) An overview on polymeric carbon nitride assisted photocatalytic CO2 reduction: strategically manoeuvring solar to fuel conversion efficiency. Chem Eng Sci 230:116219. https://doi.org/10.1016/j.ces.2020.116219
Tufa RA, Chanda D, Ma M et al (2020) Towards highly efficient electrochemical CO2 reduction: cell designs, membranes and electrocatalysts. Appl Energy 277:115557. https://doi.org/10.1016/j.apenergy.2020.115557
Yadav DK et al (2021) Cobalt oxide decorated zirconium oxide immobilized multiwalled carbon nanotubes as scaffolds for supercapacitors and the CO2 reduction reaction. J Energy Storage 44:103312. https://doi.org/10.1016/j.est.2021.103312
Wang F, Meng Y, Chen X et al (2022) Effect of nickel-based electrocatalyst size on electrochemical carbon dioxide reduction: a density functional theory study. J Colloid Interface Sci 615:587–596. https://doi.org/10.1016/j.jcis.2022.02.032
Ouyang T (2022) A comprehensive evaluation for microfluidic fuel cells from anti-vibration viewpoint using phase field theory. Renewable Energy 189:676–693. https://doi.org/10.1016/j.renene.2022.03.067
Lee CY, Fu LM (2018) Recent advances and applications of micromixers. Sens Actuators B Chem 259:677–702. https://doi.org/10.1016/j.snb.2017.12.034
Zeng W, Jacobi I, Beck DJ et al (2015) Characterization of syringe-pump-driven induced pressure fluctuations in elastic microchannels. Lab on a Chip 15:1110–1115. https://doi.org/10.1039/c4lc01347f
Jong WR, Kuo TH, Ho SW et al (2007) Flows in rectangular microchannels driven by capillary force and gravity. Int. Commun Heat Mass Transf 34:186–196. https://doi.org/10.1016/j.icheatmasstransfer.2006.09.011
Malik MI, Malaibari ZO, Atieh M et al (2016) Electrochemical reduction of CO2 to methanol over MWCNTs impregnated with Cu2O. Chem Eng Sci 152:468–477. https://doi.org/10.1016/j.ces.2016.06.035
Toyao T (2019) Heterogeneous Pt and MoO x co-loaded TiO2 catalysts for low-temperature CO2 hydrogenation to form CH3OH. ACS Catal 9:8187–8196. https://doi.org/10.1021/acscatal.9b01225
Liu L (2022) Distribution of liquid–liquid two-phase flow in branching T-junction microchannels. Chem Eng J 431:133939. https://doi.org/10.1016/j.cej.2021.133939
Falconi CJ, Lehrenfeld C, Marschall H, et al (2016) Numerical and experimental analysis of local flow phenomena in laminar Taylor flow in a square mini-channel. Phys Fluids 28:012109. https://doi.org/10.1063/1.4939498
Abiev R, Svetlov S, Haase S (2017) Hydrodynamics and mass transfer of gas-liquid and liquid-liquid Taylor flow in microchannels. Chem Eng Technol 40:1985–1998. https://doi.org/10.1002/ceat.201700041
Abiev RS (2022) Taylor vortex center, film thickness, velocity and frequency of circulations in slugs and plugs for non-Newtonian and Newtonian fluids in two-phase Taylor flow in microchannels. Chem Eng Sci 3:117380. https://doi.org/10.1016/j.ces.2021.117380
Jin Z (2020) Investigation of hydrodynamic and heat transfer characteristics of gas-liquid Taylor flow in square microchannel. Int J Chem React Eng 18: https://doi.org/10.1515/ijcre-2019-0139
Svetlov SD, Abiev RS (2018) Formation mechanisms and lengths of the bubbles and liquid slugs in a coaxial-spherical micro mixer in Taylor flow regime. Chem Eng J 354:269–284. https://doi.org/10.1016/j.cej.2018.07.213
Salman W, Gavriilidis A, Angeli P (2006) On the formation of Taylor bubbles in small tubes. Chem Eng Sci 61:6653–6666. https://doi.org/10.1016/j.ces.2006.05.036
Abiev R, Butler C, Cid E et al (2019) Mass transfer characteristics and concentration field evolution for gas-liquid Taylor flow in milli channels. Chem Eng Sci 207:1331–1340. https://doi.org/10.1016/j.ces.2019.07.046
Abiev R (2023) Analysis of segmented flow in microchannel reactors. Springer, Singapore. https://doi.org/10.1007/978-981-4585-86-6_30-1
Rufat R (2020) Gas-liquid and gas-liquid-solid mass transfer model for Taylor flow in micro (milli) channels: a theoretical approach and experimental proof. Chem Eng J Adv 4:100065. https://doi.org/10.1016/j.ceja.2020.100065
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rizhi, D., Qingjun, Y., Rui, Z. et al. Microchannel reactor for extraterrestrial in situ resource utilization. J Solid State Electrochem 28, 319–333 (2024). https://doi.org/10.1007/s10008-023-05684-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05684-7