Abstract
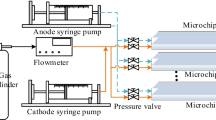
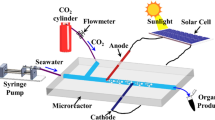
In-situ resource utilization technology(ISRU) is the key technology for human beings to go out of the earth and realize deep space exploration. As an important resource in outer space, CO2 can be reduced to produce O2 and fuel. In this paper, the reduction of CO2 by ISRU is studied. Artificial photoelectrocatalytic synthesis technology is applied through a microfluidic device to realize the generation of O2 and the production of organic substances in extraterrestrial space. A multi-physical field coupled mathematical model was established for the reduction of CO2 in electrochemical catalytic reaction. The flow and diffusion of gas-liquid two-phase flow in microchannel was studied, and the mechanism of mass transfer and chemical reaction in microscale was analyzed. The gas-liquid mass transfer performance during electrochemical reaction was studied, and the effects of microchannel structure parameters on mass transfer were analyzed.























Similar content being viewed by others
Data availability
Data available on request from the authors.
Abbreviations
- \(\rho\) [kg/m3]:
-
density;
- t [s]:
-
time;
- \(\vec{u}\) [m/s]:
-
the velocity vector;
- \(\varphi\) [-]:
-
the volume fraction;
- \(p\) [Pa]:
-
pressure;
- \(\mu\) [Pa.s]:
-
the dynamic viscosity;
- g [m/s2]:
-
gravitational acceleration;
- \(D_{{}}^{i,eff}\) [m2/s]:
-
effective diffusion coefficient;
- C [mol/L]:
-
molar concentration;
- \(\vec{u}_{f}\) [m/s]:
-
flow rate of the liquid film;
- A f [m2]:
-
area of the liquid film;
- A O2 [m2]:
-
cross-sectional area of the bubble;
- A d [m2]:
-
cross-sectional area of the microchannel;
- \(\eta\) [-]:
-
porosity of the anode porous medium;
- S [kg]:
-
mass source phase;
- M [g/mol]:
-
molar mass;
- F [-]:
-
Faraday's constant;
- R [A/m2]:
-
exchange current density;
- \(K\) [-]:
-
permeability;
- A [m2]:
-
area of the proton exchange membrane;
- n d [-]:
-
electric traction coefficient;
- I [A]:
-
current;
- t m [m]:
-
thickness of the proton exchange membrane;
- \(\phi\) [V]:
-
overpotential;
- Rg [-]:
-
ideal gas constant,
- T [K]:
-
reaction temperature,
- \(\alpha\) [kmol/(m2.s.kPa)]:
-
transfer coefficient;
- \(\phi_{s}\) [V]:
-
potential of the microreactor chip skeleton structure;
- \(\phi_{m}\) [V]:
-
potential of the electrolyte;
- \(U_{{}}^{0}\) [V]:
-
equilibrium potential;
- \(\varsigma\) [m2]:
-
active surface area;
- \(j\) [A/m2]:
-
exchange current density;
- \(\gamma\) [-]:
-
concentration index;
- \(v\) [m/s]:
-
the flow rate;
- \(d\) [m]:
-
the equivalent diameter;
- \(\sigma\) [N/m]:
-
interfacial tension;
- An :
-
the anode microchannel;
- El :
-
transfer by electromigration;
- Sp :
-
transfer by diffusion;
- Cat :
-
cathode microchannel;
- L :
-
gas Phase;
- G :
-
liquid phase
References
Alekseev M (2021) Exploration, utilisation and exploitation of space resources: Legal challenges and perspectives. Advances in the Astronautical Sciences 174:403–410
Rummel J, Hipkin VJ (2014) In situ resource development on the Moon and Mars: Considerations for their appropriation and use. Proceedings of the International Astronautical Congress 13:9203–9204
Yang Q, Dong R, Zhu R, et al (2023) Flow Characteristics of Electrochemical Catalytic Reduction of CO2 in Microchannel. Energies 16: 3929. https://doi.org/10.3390/en16093929
McClean J B, Hoffman J A, Hecht M H, et al (2022) Pre-landing plans for Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE) science operations. Acta Astronautica 192: 301-313. https://doi.org/10.1016/j.actaastro.2021.12.003
Hinterman E, Jeffrey A H (2020) Simulating oxygen production on Mars for the Mars oxygen in-situ resource utilization experiment. Acta Astronautica 170: 678-685. https://doi.org/10.1016/j.actaastro.2020.02.043
Fais G, Manca A, Concas A, et al. (2022) A novel process to grow edible microalgae on Mars by exploiting in situ-available resources: Experimental investigation. Acta Astronautica 201: 454-463. https://doi.org/10.1016/j.actaastro.2022.09.058
Saude B, LaSart N, Blair J, et al (2022) Microgrid-Based Wind and Solar Power Generation on Moon and Mars. IEEE Transactions on Smart Grid, 2: 1329-1332. https://doi.org/10.1109/TSG.2022.3210774
Fan K, Yan L, Wei Y, et al (2022) The solar wind plasma upstream of Mars observed by Tianwen-1: Comparison with Mars Express and MAVEN. Science China Earth Sciences, 65: 759-768. https://doi.org/10.1007/s11430-021-9917-0
Li P, Xu J, Jing H, et al (2014) Wedged N-doped CuO with more negative conductive band and lower overpotential for high efficiency photoelectric converting CO2 to methanol. Applied Catalysis B: Environmental 156: 134-140. https://doi.org/10.1016/j.apcatb.2014.03.011
Yang Z, Xu J, Wu C, et al (2014) New insight into photoelectric converting CO2 to CH3OH on the one-dimensional ribbon CoPc enhanced Fe2O3 NTs. Applied Catalysis B: Environmental, 156: 249-256. https://doi.org/10.1016/j.apcatb.2014.03.012
Ola O, Maroto-Valer M M (2014) Role of catalyst carriers in CO2 photoreduction over nanocrystalline nickel loaded TiO2-based photo catalysts. Journal of Catalysis 309: 300-308. https://doi.org/10.1016/j.jcat.2013.10.016
Yuan K, Yang L, Du X, et al (2014) Performance analysis of photo catalytic CO2 reduction in optical fiber monolith reactor with multiple inverse lights. Energy Conversion and Management 81: 98-105. https://doi.org/10.1016/j.enconman.2014.02.027
Chen H, Chu F, Yang L, et al (2018) Enhanced photo catalytic reduction of carbon dioxide in optical fiber monolith reactor with transparent glass balls. Applied Energy 230: 1403-1413. https://doi.org/10.1016/j.apenergy.2018.09.081
Nguyen T V, Wu J C S (2008) Photoreduction of CO2 in an optical-fiber photoreactor: effects of metals addition and catalyst carrier. Applied Catalysis A:General, 335: 112-120. https://doi.org/10.1016/j.apcata.2007.11.022
Lachaux J, Hwang G, Arouche N, et al (2021) A compact integrated microfluidic oxygenator with high gas exchange efficiency and compatibility for long-lasting endothelialization. Lab on a Chip, 21: 4791-4804. https://doi.org/10.1039/d1lc00356a
Kotb Y, Fateen S E K, Albo J, et al (2017) Modeling of a Microfluidic Electrochemical Cell for the Electro-Reduction of CO2 to CH3OH. Journal of The Electrochemical Society 164: E391. https://doi.org/10.1149/2.0741713jes
Behdani B, Alhameedi H A (2021) Computational Fluid Dynamics Study of Taylor Flow in Microreactors: Investigating the Effect of Surface Tension and Contact Angle on the Heatand Mass Transfer. arXiv preprint arXiv, 2102: 04006.
Peng Z, Wang G, Moghtaderi B, et al (2022) A review of microreactors based on slurry Taylor (segmented) flow. Chemical Engineering Science 247: 117040. https://doi.org/10.1016/j.ces.2021.117040
Kharati-Koopaee M, Rezaee M (2017) Investigation of turbulent flow through microchannels consisting of different micropost arrangements. Engineering Computations 34: 1367-1392. https://doi.org/10.1108/EC-02-2016-0069
Abiev R S (2022) Taylor vortex center, film thickness, velocity and frequency of circulations in slugs and plugs for non-Newtonian and Newtonian fluids in two-phase Taylor flow in microchannels. Chemical Engineering Science 250: 117380. https://doi.org/10.1016/j.ces.2021.117380
Cattaneo C R, Rodríguez Y, Rene E R, et al (2022) Biogas bioconversion into poly (3-hydroxybutyrate) by a mixed microbial culture in a novel Taylor flow bioreactor. Waste Management, 150: 364-372. https://doi.org/10.1016/j.wasman.2022.07.017
Mahmoudirad S, Shirani E, Aloui F (2021) Structural Analysis of Couette-Taylor Flow With Periodic Oscillation of the Inner Cylinder in Different Flow Regimes. Fluids Engineering Division Summer Meeting. 85307: V003T05A032. https://doi.org/10.1115/FEDSM2021-65626
Yin Y, Zhang X, Zhu C, et al (2021) Hydrodynamics and gas-liquid mass transfer in a cross-flow T-junction microchannel: Comparison of two operation modes. Separation and Purification Technology 255: 117697. https://doi.org/10.1016/j.seppur.2020.117697
Xiong R, Chung J N (2007) An experimental study of the size effect on adiabatic gas-liquid two-phase flow patterns and void fraction in microchannels. Physics of Fluids 19: 1139-1158. https://doi.org/10.1063/1.2567903
Li W, Liu K, Simms R, et al (2012) Microfluidic study of fast gas-liquid reactions. Journal of the American Chemical Society 134: 3127-3132. https://doi.org/10.1021/ja2101278
Yang Z, Xu J, Wu C, et al (2014) New insight into photoelectric converting CO2 to CH3OH on the one-dimensional ribbon CoPc enhanced Fe2O3 NTs. Applied Catalysis B: Environmental 156: 249-256. https://doi.org/10.1016/j.apcatb.2014.03.012
El-Shafie O A, El-Maghraby R M, Albo J, et al (2020) Modeling and numerical investigation of the performance of gas diffusion electrodes for the electrochemical reduction of carbon dioxide to methanol. Industrial & Engineering Chemistry Research 59: 20929-20942. https://doi.org/10.1021/acs.iecr.0c02358
Jamalabadi M Y A (2020) Numerical simulation of carbon dioxide sorption circulating fluidized bed used in solid oxide fuel cell. Journal of Thermal Analysis and Calorimetry 139: 2565-2575. https://doi.org/10.1007/s10973-019-08565-2
Abiev R S(2008) Simulation of the slug flow of a gas-liquid system in capillaries. Theoretical Foundations of Chemical Engineering 42: 105-117. https://doi.org/10.1134/S0040579508020012
Falconi C J, Lehrenfeld C, Marschall H, et al (2016) Numerical and experimental analysis of local flow phenomena in laminar Taylor flow in a square mini-channel. Physics of Fluids 28: 012109. https://doi.org/10.1063/1.4939498
Abiev R, Svetlov S, Haase S (2017) Hydrodynamics and mass transfer of gas-liquid and liquid-liquid Taylor flow in microchannels. Chemical Engineering & Technology 40: 1985-1998. https://doi.org/10.1002/ceat.201700041
Svetlov, S. D., and R. Sh Abiev (2018) Formation mechanisms and lengths of the bubbles and liquid slugs in a coaxial-spherical micro mixer in Taylor flow regime. Chemical Engineering Journal, 354: 269-284. https://doi.org/10.1016/j.cej.2018.07.213
Salman W, Gavriilidis A, Angeli P (2006) On the formation of Taylor bubbles in small tubes. Chemical Engineering Science 61:6653–6666. https://doi.org/10.1016/j.ces.2006.05.036
Fletcher David F, Brian SH (2017) CFD simulation of Taylor flow: Should the liquid film be captured or not? Chem. Eng. Sci 167:334–335. https://doi.org/10.1016/j.ces.2016.08.034
Butler C, Lalanne B, Sandmann K, etal, (2018) Mass transfer in Taylor flow: Transfer rate modelling from measurements at the slug and film scale. International Journal of Multiphase Flow 105:185–201. https://doi.org/10.1016/j.ijmultiphaseflow.2018.04.005
Van Baten JM, Krishna R (2004) CFD simulations of mass transfer from Taylor bubbles rising in circular capillaries. Chemical Engineering Science 59: 2535-2545. https://doi.org/10.1016/j.ces.2004.03.010
Yin Y, Fu T, Zhu C, et al (2019) Dynamics and mass transfer characteristics of CO2 absorption into MEA/[Bmim][BF4] aqueous solutions in a microchannel. Separation and Purification Technology 210: 541-552. https://doi.org/10.1016/j.seppur.2018.08.045
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qingjun, Y., Rizhi, D., Rui, Z. et al. Mass transfer of electrochemical CO2 reduction in microchannel. J Solid State Electrochem (2023). https://doi.org/10.1007/s10008-023-05643-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-023-05643-2