Abstract
Objective
Herein, we evaluated pinealectomy-induced melatonin absence to determine its effects on craniofacial and dental development in the offspring.
Design
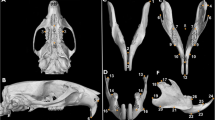
Female Wistar rats in three groups, i.e., intact pregnant rats, pinealectomized pregnant rats (PINX), and pinealectomized pregnant rats subjected to oral melatonin replacement therapy, were crossed 30 days after surgery. The heads of 7-day-old pups were harvested for cephalometric and histological analyses, and maxillae and incisors were collected for mRNA expression analysis.
Results
The PINX pups exhibited a reduction in neurocranial and facial parameters such as a decrease in alveolar bone area, incisor size and proliferation, and an increase in odontoblasts and the dentin layer. Based on incisor mRNA expression analysis, we found that Dmp1 expression was upregulated, whereas Col1a1 expression was downregulated. Maxillary mRNA expression revealed that Rankl expression was upregulated, whereas that of Opn and Osx was downregulated.
Conclusion
Our results demonstrated that the absence of maternal melatonin during early life could affect dental and maxillary development in offspring, as well as delay odontogenesis and osteogenesis in maxillary tissues.
Clinical relevance
Our findings suggest that disruptions or a lack of melatonin during pregnancy may cause changes in craniofacial and dental development, at least in animal experiments; however, in humans, these feedings are still poorly understood, and thus careful evaluations of melatonin levels in humans need to be investigated in craniofacial alterations.







Similar content being viewed by others
Data availability
Not applicable.
References
Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA (2014) Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update 20:293–307. https://doi.org/10.1093/humupd/dmt054
Valenzuela FJ, Vera J, Venegas C, Pino F, Lagunas C (2015) Circadian System and Melatonin Hormone: Risk Factors for Complications during Pregnancy. Obstet Gynecol Int 2015:825802. https://doi.org/10.1155/2015/825802
Hardeland R (2008) Melatonin, hormone of darkness and more: occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci 65:2001–2018. https://doi.org/10.1007/s00018-008-8001-x
Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J Pineal Res 56:371–381. https://doi.org/10.1111/jpi.12137
Buonfiglio D, Parthimos R, Dantas R et al (2018) Melatonin Absence Leads to Long-Term Leptin Resistance and Overweight in Rats. Front Endocrinol (Lausanne) 9:122. https://doi.org/10.3389/fendo.2018.00122
Ferreira DS, Amaral FG, Mesquita CC et al (2012) Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. PLoS One 7:e38795. https://doi.org/10.1371/journal.pone.0038795
Motta-Teixeira LC, Machado-Nils AV, Battagello DS et al (2018) The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm Behav 105:146–156. https://doi.org/10.1016/j.yhbeh.2018.08.006
Tao J, Zhai Y, Park H et al (2016) Circadian Rhythm Regulates Development of Enamel in Mouse Mandibular First Molar. PLoS One 11:e0159946. https://doi.org/10.1371/journal.pone.0159946
Zheng L, Ehardt L, McAlpin B et al (2014) The tick tock of odontogenesis. Exp Cell Res 325:83–89. https://doi.org/10.1016/j.yexcr.2014.02.007
Kumasaka S, Shimozuma M, Kawamoto T et al (2010) Possible involvement of melatonin in tooth development: expression of melatonin 1a receptor in human and mouse tooth germs. Histochem Cell Biol 133:577–584. https://doi.org/10.1007/s00418-010-0698-6
Sharan K, Lewis K, Furukawa T, Yadav VK (2017) Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J Pineal Res 63. https://doi.org/10.1111/jpi.12423
Liu J, Huang F, He HW (2013) Melatonin effects on hard tissues: bone and tooth. Int J Mol Sci 14:10063–10074. https://doi.org/10.3390/ijms140510063
Liu J, Zhou H, Fan W et al (2013) Melatonin influences proliferation and differentiation of rat dental papilla cells in vitro and dentine formation in vivo by altering mitochondrial activity. J Pineal Res 54:170–178. https://doi.org/10.1111/jpi.12002
Liu Q, Fan W, He Y et al (2017) Effects of melatonin on the proliferation and differentiation of human dental pulp cells. Arch Oral Biol 83:33–39. https://doi.org/10.1016/j.archoralbio.2017.06.034
Maria S, Samsonraj RM, Munmun F et al (2018) Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res 64. https://doi.org/10.1111/jpi.12465
Tian Y, Gong Z, Zhao R, Zhu Y (2021) Melatonin inhibits RANKLinduced osteoclastogenesis through the miR882/Reverbalpha axis in Raw264.7 cells. Int J Mol Med 47:633–642. https://doi.org/10.3892/ijmm.2020.4820
Udagawa N, Koide M, Nakamura M et al (2021) Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39:19–26. https://doi.org/10.1007/s00774-020-01162-6
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann N Y Acad Sci 1092:385–396. https://doi.org/10.1196/annals.1365.035
Komori T (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99:1233–1239. https://doi.org/10.1002/jcb.20958
Yu T, Klein OD (2020) Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development 147. https://doi.org/10.1242/dev.184754
Chen S, Gluhak-Heinrich J, Wang YH et al (2009) Runx2, osx, and dspp in tooth development. J Dent Res 88:904–909. https://doi.org/10.1177/0022034509342873
Hoffman RA, Reiter RJ (1965) Rapid pinealectomy in hamsters and other small rodents. Anat Rec 153:19–21. https://doi.org/10.1002/ar.1091530103
Calsa B, Masiero BC, Esquisatto MAM, Catisti R, Santamaria M Jr (2020) Gestational protein restriction alters the RANKL/OPG system in the dental germ of offsprings. J Oral Biol Craniofac Res 10:743–746. https://doi.org/10.1016/j.jobcr.2020.10.007
Calsa B, Bortolanca TJ, Masiero BC et al (2022) Maxillary and dental development in the offspring of protein-restricted female rats. Eur J Oral Sci. https://doi.org/10.1111/eos.12895e12895.10.1111/eos.12895
Cesani MF, Orden AB, Oyhenart EE, Zucchi M, Mune MC, Pucciarelli HM (2006) Growth of functional cranial components in rats submitted to intergenerational undernutrition. J Anat 209:137–147. https://doi.org/10.1111/j.1469-7580.2006.00603.x
Quintero FA, Castro LE, Luna ME et al (2012) Growth of functional cranial components in rats with intrauterine growth retardation after treatment with growth hormone. Eur J Orthod 34:710–718. https://doi.org/10.1093/ejo/cjr101
Luna ME, Quintero FA, Cesani MF et al (2014) Craniofacial catch-up growth in intrauterine growth retarded rats following postnatal nutritional rehabilitation. Clin Exp Obstet Gynecol 41:530–533
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160. https://doi.org/10.1177/096228029900800204
Kim HY (2013) Statistical notes for clinical researchers: Evaluation of measurement error 2: Dahlberg’s error, Bland-Altman method, and Kappa coefficient. Restor Dent Endod 38:182–185. https://doi.org/10.5395/rde.2013.38.3.182
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Cohen J (1992) A power primer. Psychol Bull 112:155–159. https://doi.org/10.1037//0033-2909.112.1.155
Bellavia SL, Carpentieri AR, Vaque AM, Macchione AF, Vermouth NT (2006) Pup circadian rhythm entrainment–effect of maternal ganglionectomy or pinealectomy. Physiol Behav 89:342–349. https://doi.org/10.1016/j.physbeh.2006.06.018
Yellon SM, Tamarkin L, Goldman BD (1985) Maturation of the pineal melatonin rhythm in long- and short-day reared Djungarian hamsters. Experientia 41:651–652. https://doi.org/10.1007/BF02007704
Ivanov DO, Evsyukova II, Mironova ES, Polyakova VO, Kvetnoy IM, Nasyrov RA (2021) Maternal melatonin deficiency leads to endocrine pathologies in children in early ontogenesis. Int J Mol Sci 22:2058. https://doi.org/10.3390/ijms22042058
Varcoe TJ, Wight N, Voultsios A, Salkeld MD, Kennaway DJ (2011) Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS One 6:e18504. https://doi.org/10.1371/journal.pone.0018504
Boyce BF (2013) Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res 92:860–867. https://doi.org/10.1177/0022034513500306
Walsh MC, Choi Y (2014) Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol 5:511. https://doi.org/10.3389/fimmu.2014.00511
Lu X, Yu S, Chen G et al (2021) Insight into the roles of melatonin in bone tissue and bonerelated diseases (Review). Int J Mol Med 47. https://doi.org/10.3892/ijmm.2021.4915
Munmun F, Witt-Enderby PA (2021) Melatonin effects on bone: Implications for use as a therapy for managing bone loss. J Pineal Res 71:e12749. https://doi.org/10.1111/jpi.12749
Dalla-Costa K, Yurtsever FV, Penteado J, Martinez EF, Sperandio M, Peruzzo DC (2020) Melatonin has a stimulatory effect on osteoblasts by upregulating col-i and opn expression/secretion. Acta Odontol Latinoam 33:125
Tresguerres IF, Tamimi F, Eimar H et al (2014) Melatonin dietary supplement as an anti-aging therapy for age-related bone loss. Rejuvenation Res 17:341–346. https://doi.org/10.1089/rej.2013.1542
Han Y, Kim YM, Kim HS, Lee KY (2017) Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Sci Rep 7:5716. https://doi.org/10.1038/s41598-017-06304-x
Icer MA, Gezmen-Karadag M (2018) The multiple functions and mechanisms of osteopontin. Clin Biochem 59:17–24. https://doi.org/10.1016/j.clinbiochem.2018.07.003
Ogata Y (2008) Bone sialoprotein and its transcriptional regulatory mechanism. J Periodontal Res 43:127–135. https://doi.org/10.1111/j.1600-0765.2007.01014.x
Hunter GK (2013) Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int 93:348–354. https://doi.org/10.1007/s00223-013-9698-6
Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG (1992) Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res 281:275–294. https://pubmed.ncbi.nlm.nih.gov/1499220/
Matsumura H, Ogata Y (2014) Melatonin regulates human bone sialoprotein gene transcription. J Oral Sci 56:67–76. https://doi.org/10.2334/josnusd.56.67
Couve E, Osorio R, Schmachtenberg O (2013) The amazing odontoblast: activity, autophagy, and aging. J Dent Res 92:765–772. https://doi.org/10.1177/0022034513495874
Aplin HM, Hirst KL, Crosby AH, Dixon MJ (1995) Mapping of the human dentin matrix acidic phosphoprotein gene (DMP1) to the dentinogenesis imperfecta type II critical region at chromosome 4q21. Genomics 30:347–349. https://doi.org/10.1006/geno.1995.9867
Zhan FL, Liu XY, Wang XB (2018) The Role of MicroRNA-143-5p in the Differentiation of Dental Pulp Stem Cells into Odontoblasts by Targeting Runx2 via the OPG/RANKL Signaling Pathway. J Cell Biochem 119:536–546. https://doi.org/10.1002/jcb.26212
Feng JQ, Luan X, Wallace J et al (1998) Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem 273:9457–9464. https://doi.org/10.1074/jbc.273.16.9457
Acknowledgements
The authors thank Sra. Mirelle Oliveira for radiographic image assistance; Me. Lucas Eduardo Orzari and Sra. Renata Barbieri provided technical assistance.
Funding
This work was supported by grants from the Hermínio Ometto Foundation-FHO and the São Paulo Research Foundation (FAPESP - 16/23237-4). T.J. Bortolança was the recipient of an undergraduate fellowship from Programa Iniciação Científica do Conselho Nacional de Pesquisa (PIBIC/CNPq - 143262/2021-1).
Author information
Authors and Affiliations
Contributions
Conceptualization: B Calsa, LS de Camargo, FG do Amaral, M Santamaria-Jr; Data curation: B Calsa; Formal analysis: B Calsa, LS de Camargo, CA de Oliveira, R Catisti, FG do Amaral, M Santamaria-Jr; Funding acquisition: M Santamaria-Jr; Investigation: B Calsa, LS de Camargo, TJ Bortolança, CA de Oliveira; Methodology: B Calsa, LS de Camargo, R Catisti, FG do Amaral, M Santamaria-Jr; Project administration: FG do Amaral, M Santamaria-Jr; Supervision: R Catisti, FG do Amaral, M Santamaria-Jr; Writing - original draft: B Calsa, LS de Camargo, TJ Bortolança; Writing - review & editing: B Calsa, CA de Oliveira, R Catisti, FG do Amaral, M Santamaria-Jr.
Corresponding author
Ethics declarations
Ethical approval
All the protocols followed by this research were previously approved by the Ethical Committee in Animal Experimentation from the Federal University of São Paulo, Brazil (#8074220415).
Competing interests
None.
Conflicts of Interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calsa, B., de Camargo, L.S., Bortolança, T.J. et al. Absence of melatonin during development impairs craniofacial and dental onset in rats. Clin Oral Invest 27, 5353–5365 (2023). https://doi.org/10.1007/s00784-023-05155-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05155-3