Abstract
Objectives
The aim of this study was investigate the cranium dimensions of adult female rats, who suffered estrogen deficiency during the prepubertal stage, to assess the impact of estrogen deficiency on craniofacial morphology.
Material and methods
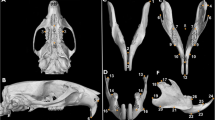
Twenty-two female Wistar rats were divided into ovariectomy (OVX) (n = 11) and sham-operated control (n = 11) groups. Bilateral ovariectomy were performed in both groups at 21 days old (prepubertal stage), and rats were euthanized at an age of 63 days (post-pubertal stage). Micro-CT scans were performed with rat skulls, and the cranium morphometric landmark measurements were taken in the dorsal, lateral, and ventral view positions. Differences in measurements between the OVX and sham control groups were assessed using t test with an established alpha error of 5%.
Results
The measures of the rats’ skull showed that the inter-zygomatic arch width and anterior cranial base length were significantly larger in OVX group (p = 0.020 and p = 0.050, respectively), whereas the length of parietal bone was significantly higher in the sham group (p = 0.026). For the remaining measurements no significant differences between groups were detected (p > 0.05).
Conclusion
This study provides evidence that ovariectomized rats had alterations in cranial bone dimensions, demonstrating that estrogens during puberty are important for skull morphology.
Clinical relevance
To understand the role of estrogen on the postnatal cranium development will impact the clinical diagnose and therapy during childhood and adolescence.


Similar content being viewed by others
References
Smith DG, Schenk MP (2001) A dissection guide & atlas to the rat, 1st edn. Morton Publishing Company, Englewood, Colorado
Yazdi FT, Adriaens D, Darvish J (2011) Geographic pattern of cranial differentiation in the Asian Midday Jird Meriones meridianus (Rodentia: Muridae: Gerbilinae) and its taxonomic implications. J Zool Syst Evol Res 50:157–164
VanderBerg JR, Buschang PH, Hinton RJ (2004) Absolute and relative growth of the rat craniofacial skeleton. Archives of oral biology 49:477–484
Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP (2005) Cytokine dysregulation, inflammation, and well-being. Neuro Immuno Modulation 12:255–269
Shibazaki R, Dechow PC, Maki K, Opperman LA (2007) Biomechanical strain and morphologic changes with age in rat calvarial bone and sutures. Plast Reconstr Surg 119:2167–2181
Vecchione L, Byron C, Cooper GM, Barbano T, Hamrick MW, Sciote JJ, Mooney MP (2007) Craniofacial morphology in myostatin-deficient mice. J Dent Res 86:1068–1072
Santaolalla-Montoya F, Martinez-Ibargüen A, Sánchez-Fernández JM, Sánchez-Del-Rey A (2012) Principles of cranial base ossification in humans and rats. Acta OtoLaryngologica 132:349–354
Moss ML, Rankow RM (1968) The role of the functional matrix in mandibular growth. Angle Orthod 38:95–103
Van Erum R, Carels C, Verbeke G, Zegher F (1997) Craniofacial growth in short children born small for gestational age: two years follow-up after high-dose growth hormone treatment. J Craniofac Genet Dev Biol 17:184–189
DeSesso JM, Scialli AR (2018) Bone development in laboratory mammals used in developmental toxicity studies. Birth Defects Research 110:1157–1187
Cauley JÁ (2015) Estrogen and bone health in men and women. Steroids 99:1115
Cutler GB (1997) The role of estrogen in bone growth and a maturation during childhood and adolescence. J Steroid Biochem Mol Biol 61:141–144
Perry RJ, Farquharson C, Ahmed SF (2008) The role of sex steroids in controlling pubertal growth. Clin Endocrinol 68:4–15
Emons J, Chagin AS, Sävendahl L, Karperien M, Wit JM (2011) Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res Paediatr 75:383–391
Cohen SP, LaChappelle AR, Walker BS, Lassiter CS (2014) Modulation of estrogen causes disruption of craniofacial chondrogenesis in Danio rerio. Aquat Toxicol 152:113–120
Fujita T, Ohtani J, Shigekawa M, Kawata T, Kaku M, Kohno S, Tsutsui K, Tenjo K, Motokawa M, Tohma Y, Tanne K (2004) Effects of sex hormone disturbances on craniofacial growth in newborn mice. J Dent Res 83:250–254
Fujita T, Kawata T, Tokimasa C, Tanne K (2001) Influence of oestrogen and androgen on modelling of the mandibular condylar bone in ovariectomized and orchiectomized growing mice. Arch Oral Biol 46:57–65
Fujita T, Ohtani J, Shigekawa M, Kawata T, Kaku M, Kohno S, Motokawa M, Tohma Y, Tanne K (2006) Influence of sex hormone disturbances on the internal structure of the mandible in newborn mice. Eur J Orthod 28:190–194
Ejiri S, Tanaka M, Watanabe N, Anwar RB, Yamashita E, Yamada K, Ikegame M (2008) Estrogen deficiency and its effect on the jaw bones. J Bone Miner Metab 26:409–415
Seifi M, Ashiri M, Hedayati M (2008) Effect of sexual hormone elimination on the changes of craniofacial dimensions in rats. J Dent Sch 25:365–372
Hsu PY, Tsai MT, Wang SP, Chen YJ, Wu J, Hsu JT (2016) Cortical bone morphological and trabecular bone microarchitectural changes in the mandible and femoral neck of ovariectomized rats. PLoS ONE. 11:e0154367. https://doi.org/10.1371/journal.pone.0154367
Hernandez RA, Ohtani J, Fujita T et al (2011) Sex hormones receptors play a crucial role in the control of femoral and mandibular growth in newborn mice. Eur J Orthod. 33:564–569
Omori MA, Marañón-Vásquez GA, Romualdo PC, Martins-Neto EC, Stuani MBS, Matsumoto MAM, Nelson-Filho P, Proff P, León JE, Kirschneck C, Küchler EC (2020) Effect of ovariectomy on maxilla and mandible dimensions of female rats. Orthod Craniofac Res 23:342–350
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 1:94–99
Chen GX, Zheng S, Qin S, Zhong ZM, Wu XH, Huang ZP, Li W, Ding RT, Yu H, Chen JT (2014) Effect of low-magnitude whole-body vibration combined with alendronate in ovariectomized rats: a random controlled osteoporosis prevention study. PloS One 9:e96181
Sengupta P (2013) The laboratory rat: relating its age with human’s. Int J Prev Med 4:624–630
Wei X, Thomas N, Hatch NE, Hu M, Liu F (2017) Postnatal craniofacial skeletal development of female C57BL/6NCrl mice. Front Physiol 14:697–697
Jin SW, Sim KB, Kim SD (2016) Development and growth of the normal cranial vault: An embryologic review. J Kor Neur Soc 59:192–196 7
Siebert JR (1982) The ethmoid bone: implications for normal and abnormal facial development. J Craniofac Genet Dev Biol 1:381–389
Nakahara K, Haga-Tsujimura M, Iizuka T, Saulacic N (2016) Periosteum-induced bone formation by distraction osteogenesis: histologic and microcomputed tomography analysis. Int J Oral Maxill Imp 31:785–792. https://doi.org/10.11607/jomi.4316
Teng CS, Ting M, Farmer JT, Brockop M, Maxson RE, Crump JG (2018) Alterated bone growth dynamics prefigure craniosynostosis in a zebrafish model of Saethre-Chotzen syndrome. eLife 7:1–23
Streicher C, Heyny A, Andrukhova O, Haigl B, Slavic S, Schüler C et al (2017) Estrogen regulates bone turnover by targeting RANKL expression in bone lining cells. Sci Rep 7:1–14
Bord S, Ireland DC, Beavan SR, Compston JE (2003) The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 32:136–141
Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H (2002) Effects of ovariectomy on trabecular structures of rat alveolar bone. J Periodontal Res http://doi.wiley.com/10.1034/j.1600-0765.2002.01601.x 37:161–165
Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S (2003) Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95:495–502
Väänänen HK, Härkönen PL (1996) Estrogen and bone metabolism. Maturitas 23:S65–S69
Frank GR (2003) Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol 41:217–221
Chidi-Ogbolu N, Baar K (2019) Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 9. https://doi.org/10.3389/fphys.2018.01834
Opperman LA (2000) Cranial sutures as intramembranous bone growth sites. Dev Dyn 219:472–485
Acknowledgments
We thank gratefully the RCBE (Regensburg Center of Biomedical Engineering) for the support by the μCT facility and Dr. Birgit Striegl for performing the μCT analysis, and we acknowledge the support from the Deutsche Forschungsgemeinschaft (DFG) in frame of the program “Forschungsgeräte” (INST 102/11-1 FUGG).
Funding
Support for this work was provided by FAPESP (the State of São Paulo Research Foundation, number 2015/06866-5), and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and Alexander-von-Humboldt-Foundation (Küchler/Kirschneck accepted in July 4, 2019) for funding and support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Lara, R.M., dos Santos, M.C., Omori, M.A. et al. The role of postnatal estrogen deficiency on cranium dimensions. Clin Oral Invest 25, 3249–3255 (2021). https://doi.org/10.1007/s00784-020-03655-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03655-0