Abstract
Background
Serum cystatin C (CysC) is a promising biomarker of kidney function, which has higher accuracy and sensitivity when compared with creatinine. To better utilize serum CysC in clinical practice, this study aimed to establish continuous paediatric reference intervals (RIs) for serum CysC.
Methods
The study subjects consisted of healthy term neonates and children aged 30 days to 18 years. Venous blood samples were collected and serum CysC levels were measured using the immunoturbidimetric measurement principle. Fractional polynomial regression model and quantile regression was applied in the statistical analysis to generate continuous RIs.
Results
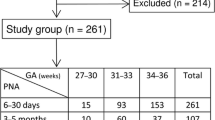
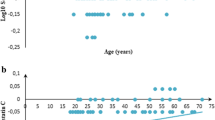
A total of 378 samples with equal numbers of males and females were analysed for serum CysC. No outliers were found in this analysis. The continuous RIs are presented as equations and graphical scatterplots.
Conclusions
This study established continuous paediatric reference intervals (RIs) for serum CysC in healthy term neonates and children. The continuous RIs generated from this study show age-based dynamic changes as well as blood group and gender-specific differences for serum CysC.

Graphical abstract



Similar content being viewed by others
Data availability
Not applicable.
References
Ferguson TW, Komenda P, Tangri N (2015) Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens 24:295–300
Mussap M, Plebani M (2004) Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci 41:467–550
Grubb A, Lofberg H (1982) Human gamma-trace, a basic microprotein: amino acid sequence and presence in the adenohypophysis. Proc Natl Acad Sci U S A 79:3024–3027
Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH (2005) Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 16:1404–1412
Kaeser SA, Herzig MC, Coomaraswamy J, Kilger E, Selenica ML, Winkler DT, Staufenbiel M, Levy E, Grubb A, Jucker M (2007) Cystatin C modulates cerebral beta-amyloidosis. Nat Genet 39:1437–1439
Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F (2001) Codeposition of cystatin C with amyloid-beta protein in the brain of Alzheimer disease patients. J Neuropathol Exp Neurol 60:94–104
Kos J, Werle B, Lah T, Brunner N (2000) Cysteine proteinases and their inhibitors in extracellular fluids: markers for diagnosis and prognosis in cancer. Int J Biol Markers 15:84–89
Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40:221–226
Park MY, Choi SJ, Kim JK, Hwang SD, Lee YW (2013) Urinary cystatin C levels as a diagnostic and prognostic biomarker in patients with acute kidney injury. Nephrology 18:256–262
Glassock RJ, Warnock DG, Delanaye P (2017) The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 13:104–114
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483
Swedko PJ, Clark HD, Paramsothy K, Akbari A (2003) Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 163:356–360
Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE (2004) Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65:1416–1421
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Horowitz GL, Altaie S, Boyd J, Ceriotti F, Garg U, Horn P, Pesce A, Harrison E, Zakowski J (2010) EP28-A3C defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. Clinical Laboratory Standards Institute
Liu C, Wen J, Xiang J, Ouyang X, Yang Y, Lu W, Wang J, Huang J, Min X (2019) Age-and sex-specific reference intervals for the serum cystatin C/creatinine ratio in healthy children (0–18 years old). J Int Med Res 47:3151–3159
Edinga-Melenge BE, Yakam AT, Nansseu JR, Bilong C, Belinga S, Minkala E, Noudjeu PA, Ondhoua M, Kokola SW, Ama Moor VJ, Ashuntantang G (2019) Reference intervals for serum cystatin C and serum creatinine in an adult sub-Saharan African population. BMC Clin Pathol 19:4
Wei L, Ye X, Pei X, Wu J, Zhao W (2014) Reference intervals for serum cystatin C and factors influencing cystatin C levels other than renal function in the elderly. PLoS One 9:e86066
Zierk J, Arzideh F, Rechenauer T, Haeckel R, Rascher W, Metzler M, Rauh M (2015) Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem 61:964–973
Loh TP, Antoniou G, Baghurst P, Metz MP (2014) Development of paediatric biochemistry centile charts as a complement to laboratory reference intervals. Pathology 46:336–343
Higgins V, Adeli K (2018) Advances in pediatric reference intervals: from discrete to continuous. J Lab Precis Med 3:3
Adeli K, Higgins V, Trajcevski K, White-Al Habeeb N (2017) The Canadian laboratory initiative on pediatric reference intervals: a CALIPER white paper. Crit Rev Clin Lab Sci 54:358–413
Harmoinen A, Ylinen E, Ala-Houhala M, Janas M, Kaila M, Kouri T (2000) Reference intervals for cystatin C in pre- and full-term infants and children. Pediatr Nephrol 15:105–108
Asgari S, Higgins V, McCudden C, Adeli K (2019) Continuous reference intervals for 38 biochemical markers in healthy children and adolescents: comparisons to traditionally partitioned reference intervals. Clin Biochem 73:82–89
Zierk J, Arzideh F, Haeckel R, Cario H, Fruhwald MC, Gross HJ, Gscheidmeier T, Hoffmann R, Krebs A, Lichtinghagen R, Neumann M, Ruf HG, Steigerwald U, Streichert T, Rascher W, Metzler M, Rauh M (2017) Pediatric reference intervals for alkaline phosphatase. Clin Chem Lab Med 55:102–110
Hoq M, Matthews S, Karlaftis V, Burgess J, Cowley J, Donath S, Carlin J, Yen T, Ignjatovic V, Monagle P, HAPPI Kids study team (2019) Reference values for 30 common biochemistry analytes across 5 different analyzers in neonates and children 30 days to 18 years of age. Clin Chem 65:1317–1326
Hoq M, Karlaftis V, Mathews S, Burgess J, Donath SM, Carlin J, Monagle P, Ignjatovic V (2019) A prospective, cross-sectional study to establish age-specific reference intervals for neonates and children in the setting of clinical biochemistry, immunology and haematology: the HAPPI Kids study protocol. BMJ Open 9:e025897
Barbato G, Barini EM, Genta G, Levi R (2011) Features and performance of some outlier detection methods. J Appl Stat 38:2133–2149
StataCorp L (2017) Stata statistical software: release 15 college station, TX
Daly CH, Liu X, Grey VL, Hamid JS (2013) A systematic review of statistical methods used in constructing pediatric reference intervals. Clin Biochem 46:1220–1227
Esezobor CI, Soriyan OO, Iroha E (2011) Serum cystatin C levels in Nigerian children: reference intervals and relationship to demographic and anthropometric variables. West Afr J Med 30:188–192
Randers E, Krue S, Erlandsen EJ, Danielsen H, Hansen LG (1999) Reference interval for serum cystatin C in children. Clin Chem 45:1856–1858
Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J (1998) Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 12:125–129
Dorum S, Silfeler I, Dorum BA, Silfeler DB, Canbak Y, Say A (2012) Reference values of serum cystatin-C for full-term and preterm neonates in Istanbul. Indian J Pediatr 79:1037–1042
Nakashima T, Inoue H, Fujiyoshi J, Matsumoto N (2016) Longitudinal analysis of serum cystatin C for estimating the glomerular filtration rate in preterm infants. Pediatr Nephrol 31:983–989
Ziegelasch N, Vogel M, Muller E, Tremel N, Jurkutat A, Loffler M, Terliesner N, Thiery J, Willenberg A, Kiess W, Dittrich K (2019) Cystatin C serum levels in healthy children are related to age, gender, and pubertal stage. Pediatr Nephrol 34:449–457
Finney H, Newman DJ, Thakkar H, Fell JM, Price CP (2000) Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child 82:71–75
Uemura O, Ushijima K, Nagai T, Yamada T, Hayakawa H, Nabeta Y, Shinkai Y, Koike K, Kuwabara M (2010) Reference serum cystatin C levels in Japanese children. Clin Exp Nephrol 14:453–456
Noordzij M, Tripepi G, Dekker FW, Zoccali C, Tanck MW, Jager KJ (2010) Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant 25:1388–1393
Royston P (1991) Constructing time-specific reference ranges. Stat Med 10:675–690
Kristensen K, Strevens H, Lindström V, Grubb A, Wide-Swensson D (2008) Increased plasma levels of ß2-microglobulin, cystatin C and ß-trace protein in term pregnancy are not due to utero-placental production. Scand J Clin Lab Invest 68:649–653
Cataldi L, Mussap M, Bertelli L, Ruzzante N, Fanos V, Plebani M (1999) Cystatin C in healthy women at term pregnancy and in their infant newborns: relationship between maternal and neonatal serum levels and reference values. Am J Perinatol 16:287–295
Bahar A, Yilmaz Y, Unver S, Gocmen I, Karademir F (2003) Reference values of umbilical cord and third-day cystatin C levels for determining glomerular filtration rates in newborns. J Int Med Res 31:231–235
Filler G, Lepage N (2013) Cystatin C adaptation in the first month of life. Pediatr Nephrol 28:991–994
Li DD, Zou MN, Hu X, Zhang M, Jia CY, Tao CM, Wang LL, Ying BW (2012) Reference intervals and factors contributing to serum Cystatin C levels in a C hinese population. J Clin Lab Anal 26:49–54
Al Wakeel JS, Memon NA, Chaudhary A, Mitwalli AH, Tarif N, Isnani A, Hammad D (2008) Normal reference levels of serum cystatin C in Saudi adults. Saudi J Kidney Dis Transpl 19:361–370
Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K (2012) Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58:854–868
Ozcurumez MK, Haeckel R (2018) Biological variables influencing the estimation of reference limits. Scand J Clin Lab Inv 78:337–345
Jang JH, Seo JY, Bang SH, Park IA, Kim HJ, Kim SH (2010) Establishment of reference intervals for von Willebrand factor antigen and eight coagulation factors in a Korean population following the clinical and laboratory standards institute guidelines. Blood Coagul Fibrinolysis 21:251–255
Acknowledgements
The authors thank the staff of the Pathology Collection Department at The Royal Children’s Hospital for obtaining the consent of participants and the collection of samples. The authors thank the staff of the Anaesthetic, Surgical, and Post-Natal Departments at the Royal Children’s Hospital and collaborating laboratory.
Funding
This study was funded by the Royal Children's Hospital Foundation, with supplementary funding from Ortho Clinical Diagnostics and in-kind reagents supplied from the Medical and Scientific Affairs at Roche Diagnostics International during the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study protocol was approved by The Royal Children’s Hospital, Melbourne, Australia, Ethics in Human Research Committee (HREC) and subsequently approved by the HRECs of all participating hospitals (HREC 34183 A).
Consent to participate
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, T., Karlaftis, V., Hearps, S. et al. Reference intervals for serum cystatin C in neonates and children 30 days to 18 years old. Pediatr Nephrol 35, 1959–1966 (2020). https://doi.org/10.1007/s00467-020-04612-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04612-5