Abstract
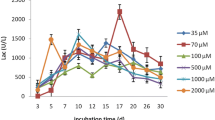
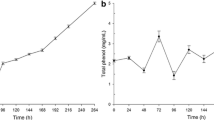
The present study investigated the screening of mono and co-culture fungal cultivations for laccase production using extracted lignin as the substrate obtained from cauliflower wastes by two different pretreatment methods. Amongst mono and mixed culture fungal cultivations, monoculture of Aspergillus oryzae exhibited the highest enzymatic activity of 29.7 ± 0.6 U mL−1 under submerged conditions and using alkali extracted lignin as substrate. Under the optimal conditions (pH 4.5, 30 °C, 12 days, 1% (w/v) lignin and 0.5 mM Cu2+ concentration) the maximum laccase activity was estimated to be 41.3 ± 2.8 U mL−1 and production yield of 153.3 ± 2.4 mg L−1. Maximum decolorization of pigment extracted from Aspergillus heteromorphus CBS 117.55 cultivated culture media was achieved by administration of 40 U g−1 of crude enzyme concentration. Thermal and pH stability of crude laccase was observed over wide ranges. The dye decolorization efficiency of crude A. oryzae laccase was studied and Congo Red exhibited maximum decolorization percentage (64 ± 1.3%) at 15 µM, 50 °C and pH 4.5. The kinetic study of different dye (Congo Red) concentrations obtained Vmax and Km values of 0.123 × 10−3 M and 0.724 mol L−1 min−1, respectively.







Similar content being viewed by others
References
Chapman J, Ismail A, Dinu C (2018) Industrial applications of enzymes: recent advances, techniques, and outlooks. Catalysts 8(6):238
Li SH, Liu S, Colmenares JC, Xu YJ (2016) A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem 18(3):594–607
Nanayakkara S, Patti AF, Saito K (2014) Chemical depolymerization of lignin involving the redistribution mechanism with phenols and repolymerization of depolymerized products. Green Chem 16(4):1897–1903
Peralta RM, da Silva BP, Côrrea RCG, Kato CG, Seixas FAV, Bracht A (2017) Enzymes from basidiomycetes—peculiar and efficient tools for biotechnology. Biotechnology of microbial enzymes. Academic Press, pp 119–149
Moreno LF, Feng P, Weiss VA, Vicente VA, Stielow JB, de Hoog S (2017) Phylogenomic analyses reveal the diversity of laccase-coding genes in Fonsecaea genomes. PLoS ONE 12(2):e0171291
Roopan SM (2017) An overview of natural renewable bio-polymer lignin towards nano and biotechnological applications. Int J Biol Macromol 103:508–514
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res 2014:1–21
Gonzalez JC, Medina SC, Rodriguez A, Osma JF, Alméciga-Díaz CJ, Sánchez OF (2013) Production of Trametes pubescens laccase under submerged and semi-solid culture conditions on agro-industrial wastes. PLoS ONE 8(9):e73721
Khedkar MA, Nimbalkar PR, Chavan PV, Chendake YJ, Bankar SB (2017) Cauliflower waste utilization for sustainable biobutanol production: revelation of drying kinetics and bioprocess development. Bioprocess Biosyst Eng 40(10):1493–1506
Zhou C, Dong A, Wang Q, Yu Y, Fan X, Cao Y, Li T (2017) Effect of common metal ions and anions on laccase catalysis of guaiacol and lignocellulosic fiber. BioResources 12(3):5102–5117
Ijoma GN, Tekere M (2017) Potential microbial applications of co-cultures involving ligninolytic fungi in the bioremediation of recalcitrant xenobiotic compounds. Int J Environ Sci Technol 14(8):1787–1806
Ferreira FL, Dall’Antonia CB, Shiga EA, Alvim LJ, Pessoni RAB (2018) Sugarcane bagasse as a source of carbon for enzyme production by filamentous fungi. Hoehnea 45(1):134–142
Hu HL, den Brink J, Van Gruben BS, Wösten HAB, Gu JD, de Vries RP (2011) Improved enzyme production by co- cultivation of Aspergillus oryzae and with other fungi. Int Biodeterior Biodegrad 65:248–252
Koziróg A, Otlewska A, Gapińska M, Michlewska S (2019) Influence of gemini surfactants on biochemical profile and ultrastructure of Aspergillus brasiliensis. Appl Sci 9(2):245
Pankar SA, Bornare DT (2018) Studies on cauliflower leave powder and its waste utilization in traditional product. Int J Agric Eng 11:95–98
Wadhwa M, Bakshi MPS, Makkar HPS (2015) Wastes to worth: value added products from fruit and vegetable wastes. CAB Int 1–25
Kumar R, Kaur J, Jain S, Kumar A (2016) Optimization of laccase production from Aspergillus flavus by design of experiment technique: partial purification and characterization. J Genet Eng Biotechnol 14(1):125–131
Pollegioni L, Tonin F, Rosini E (2015) Lignin degrading enzymes. FEBS J 282(7):1190–1213
Adekunle AE, Guo C, Liu CZ (2017) Lignin-enhanced laccase production from Trametes versicolor. Waste Biomass Valor 8(4):1061–1066
El Monssef RAA, Hassan EA, Ramadan EM (2016) Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann Agric Sci 61(1):145–154
Jaber SM, Shah UKM, Asa’ari AZM, Ariff AB (2017) Optimization of laccase production by locally isolated Trichoderma muroiana IS1037 using rubber wood dust as substrate. BioResources 12(2):3834–3849
Saito K, Horikawa Y, Sugiyama J, Watanabe T, Kobayashi Y, Takabe K (2016) Effect of thermochemical pretreatment on lignin alteration and cell wall microstructural degradation in Eucalyptus globulus: comparison of acid, alkali, and water pretreatments. J Wood Sci 62(3):276–284
Majumdar S, Naha A, Bhattacharyya DK, Bhowal J (2019) Effective delignification and decrystallization of cauliflower wastes by using dilute phosphoric acid for efficient enzymatic digestibility to produce fermentable sugars. Biomass Bioenerg 125:169–179
Wu M, Zhao D, Pang J, Zhang X, Li M, Xu F, Sun R (2015) Separation and characterization of lignin obtained by catalytic hydrothermal pretreatment of cotton stalk. Ind Crops Prod 66:123–130
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. LAP 1617:1–16
Kalra K, Chauhan R, Shavez M, Sachdeva S (2013) Isolation of laccase producing Trichoderma spp. and effect of pH and temperature on its activity. Int J Chem Environ Technol 5(5):2229–2235
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Li M, Foster C, Kelkar S, Pu Y (2012) Structural characterization of alkaline hydrogen peroxide pretreated grasses exhibiting diverse lignin phenotypes. Biotechnol Biofuels 5(1):38
Kaur R, Uppal SK (2015) Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid Polym Sci 293(9):2585–2592
Grimaldi MP, Marques MP, Laluce C, Cilli EM (2015) Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol Biofuels 8(1):205
Chen X, Li H, Sun S, Cao X (2016) Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci Rep 6:39354
Wang Y, Chantreau M, Sibout R, Hawkins S (2013) Plant cell wall lignification and monolignol metabolism. Front Plant Sci 4:220
Bunzel M, Seiler A, Steinhart H (2005) Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. J Agric Food Chem 53(24):9553–9559
Osma JF, Moilanen U, Toca-Herrera JL, Rodríguez-Couto S (2011) Uses of laccases in the food industry. FEMS Microbiol Lett 318(1):27–34
Singh A, Bajar S, Bishnoi NR, Singh N (2010) Laccase production by Aspergillus heteromorphus using distillery spent wash and lignocellulosic biomass. J Hazard Mater 176(1–3):1079–1082
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67(3):369–385
Ranieri D, Colao MC, Ruzzi M, Romagnoli G, Bianchi MM (2009) Optimization of recombinant fungal laccase production with strains of the yeast Kluyveromyces lactis from the pyruvate decarboxylase promoter. FEMS Yeast Res 9(6):892–902
Du W, Sun C, Wang J, Xie W, Wang B, Liu X, Fan Y (2017) Conditions and regulation of mixed culture to promote Shiraia bambusicola and Phoma sp. BZJ6 for laccase production. Sci Rep 7(1):17801
Pan K, Zhao N, Yin Q, Zhang T, Xu X, Fang W, Xiao Y (2014) Induction of a laccase Lcc9 from Coprinopsis cinerea by fungal coculture and its application on indigo dye decolorization. Biores Technol 162:45–52
Kannaiyan R, Mahinpey N, Kostenko V, Martinuzzi RJ (2015) Nutrient media optimization for simultaneous enhancement of the laccase and peroxidases production by coculture of Dichomitus squalens and Ceriporiopsis subvermispora. Biotechnol Appl Biochem 62(2):173–185
Hailei W, Guangli Y, Ping L, Yanchang G, Jun L, Guosheng L, Jianming Y (2009) Overproduction of Trametes versicolor laccase by making glucose starvation using yeast. Enzyme Microb Technol 45(2):146–149
Baldi A, Jain A, Gupta N, Srivastava AK, Bisaria VS (2008) Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxins: a first report. Biotechnol Lett 30(9):1671
Díaz R, Alonso S, Sánchez C, Tomasini A, Bibbins-Martinez M, Díaz-Godínez G (2011) Characterization of the growth and laccase activity of strains of Pleurotus ostreatus in submerged fermentation. BioResources 6(1):282–290
Sidhu AK, Darade SB, Bhavsar PP, Gaikwad VB, Patil SN (2017) Isolation, screening and optimization for laccase production by Scytalidium lignicolapesante under submerged fermentation. Int J Curr Microbiol Appl Sci 6(4):2477–2491
Sathishkumar P, Murugesan K, Palvannan T (2010) Production of laccase from Pleurotus florida using agro-wastes and efficient decolorization of reactive blue 198. J Basic Microbiol 50(4):360–367
Nadeem A, Baig S, Sheikh N (2014) Mycotechnological production of laccase by Pleurotus ostreatus P1 and its inhibition study. J Anim Plant Sci 24(2):492–502
Brijwani K, Rigdon A, Vadlani PV (2010) Fungal laccases: production, function, and applications in food processing. Enzyme Res 2010:1–10
Tychanowicz GK, Souza DFD, Souza CG, Kadowaki MK, Peralta RM (2006) Copper improves the production of laccase by the white-rot fungus Pleurotus pulmonarius in solid state fermentation. Braz Arch Biol Technol 49(5):699–704
Zilly A, da Silva C-M, Bracht A, De Souza CGM, Carvajal AE, Koehnlein EA, Peralta RM (2011) Influence of NaCl and Na2SO4 on the kinetics and dye decolorization ability of crude laccase from Ganoderma lucidum. Int Biodeterior Biodegrad 65(2):340–344
Yang J, Li W, Ng TB, Deng X, Lin J, Ye X (2017) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8:832
More SS, Renuka PS, Malini S (2011) Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Res (Article ID 248735)
Shrestha P, Joshi B, Joshi J, Malla R, Sreerama L (2016) Isolation and physicochemical characterization of laccase from Ganoderma lucidum-CDBT1 isolated from its native habitat in Nepal. BioMed Res Int (Article ID 3238909)
Nawaz H, Shad MA, Muzaffar S (2018) Phytochemical composition and antioxidant potential of brassica. In: Brassica germplasm: characterization, breeding and utilization. Intechopen 7
Konkol N, McNamara C, Sembrat J, Rabinowitz M (2009) Enzymatic decolorization of bacterial pigments from culturally significant marble. J Cult Herit 10(3):362–366
Forootanfar H, Moezzi A, Aghaie-Khozani M, Mahmoudjanlou Y (2012) Synthetic dye decolorization by three sources of fungal laccase. Iran J Environ Health 9(1):27
Bankole PO, Adekunle AA, Obidi OF, Chandanshive VV (2018) Biodegradation and detoxification of Scarlet RR dye by a newly isolated filamentous fungus, Peyronellaea prosopidis. Sustain Environ Res 28(5):214–222
Moilanen U, Osma JF, Winquist E, Leisola M (2010) Decolorization of simulated textile dye baths by crude laccases from Trametes hirsuta and Cerrena unicolor. Eng Life Sci 10(3):242–247
Sarkar S, Banerjee A, Halder U, Biswas R, Bandopadhyay R (2017) Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv Sci Eng 2(4):121–131
Rezaei S, Tahmasbi H, Mogharabi M, Ameri A, Forootanfar H, Khoshayand MR, Faramarzi MA (2015) Laccase-catalyzed decolorization and detoxification of Acid Blue 92: statistical optimization, microtoxicity, kinetics, and energetic. J Environ Health Sci Eng 13(1):31
Acknowledgements
Authors are thankful to Indian Institute of Engineering Science and Technology, Shibpur (Formerly Bengal Engineering and Science University, Shibpur), West Bengal, India for providing facilities for the completion of this research work. Research Scholar (S. Majumdar) is grateful to UGC for providing financial support as Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
Both the authors contributed equally to this research work.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majumdar, S., Bhowal, J. Studies on production and evaluation of biopigment and synthetic dye decolorization capacity of laccase produced by A. oryzae cultivated on agro-waste. Bioprocess Biosyst Eng 45, 45–60 (2022). https://doi.org/10.1007/s00449-021-02638-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02638-z