Abstract
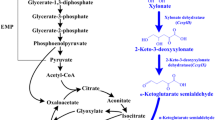
Microbial biorefinery is a promising route toward sustainable production of glycolic acid (GA), a valuable raw material for various industries. However, inherent microbial GA production has limited substrate consumption using either d-xylose or d-glucose as carbon catabolite repression (CCR) averts their co-utilization. To bypass CCR, a GA-producing strain using d-xylose via Dahms pathway was engineered to allow cellobiose uptake. Unlike glucose, cellobiose was assimilated and intracellularly degraded without repressing d-xylose uptake. The final GA-producing E. coli strain (CLGA8) has an overexpressed cellobiose phosphorylase (cep94A) from Saccharophagus degradans 2–40 and an activated glyoxylate shunt pathway. Expression of cep94A improved GA production reaching the maximum theoretical yield (0.51 g GA g−1 xylose), whereas activation of glyoxylate shunt pathway enabled GA production from cellobiose, which further increased the GA titer (2.25 g GA L−1). To date, this is the highest reported GA yield from d-xylose through Dahms pathway in an engineered E. coli with cellobiose as co-substrate.






Similar content being viewed by others
References
Girio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Lukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101(13):4775–4800. https://doi.org/10.1016/j.biortech.2010.01.088
Zhang Y, Guo S, Wang Y, Liang X, Xu P, Gao C, Ma C (2019) Production of d-xylonate from corn cob hydrolysate by a metabolically engineered Escherichia coli strain. ACS Sustain Chem Eng 2:2160–2168. https://doi.org/10.1021/acssuschemeng.8b04839
Babilas P, Knie U, Abels C (2012) Cosmetic and dermatologic use of alpha hydroxyl acids. J Dtsch Dermatol Ges 10:488–491. https://doi.org/10.1111/j.1610-0387.2012.07939.x
Salusjärvi L, Havukainen S, Koivistoinen O, Toivari M (2019) Biotechnological production of glycolic acid and ethylene glycol: current state and perspectives. Appl Microbiol Biotechnol 103:2525–2535. https://doi.org/10.1007/s00253-019-09640-2
Fredenberg S, Wahlgren M, Reslow M, Axelsson A (2011) The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm 415:34–52. https://doi.org/10.1016/j.ijpharm.2011.05.049
Tang S-C, Yang J-H (2018) Dual effects of alpha-hydroxy acids in the skin. Molecules 23(4):863. https://doi.org/10.3390/molecules23040863
Cunha JT, Soares PO, Baptista SL, Costa CE, Domingues L (2020) Engineered Saccharomyces cerevisiae for lignocellulosic valorization: a review and perspectives on bioethanol production. Bioengineered 11(1):883–903. https://doi.org/10.1080/21655979.2020.1801178
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311(5760):484–489
Stephanopoulos G (2007) Challenges in engineering microbes for biofuels production. Science 315(5813):801–804. https://doi.org/10.1126/science.1139612
Liu H, Ramos KRM, Valdehuesa KNG, Nisola GM, Lee W-K, Chung W-J (2013) Biosynthesis of ethylene glycol in Escherichia coli. Appl Microbiol Biotechnol 97(8):3409–3417. https://doi.org/10.1007/s00253-012-4618-7
Martin CH, Dhamankar H, Tseng HC, Sheppard MJ, Reisch CR, Prather KLJ (2013) A platform pathway for production of 3-hydroxyacids provides a biosynthetic route to 3-hydroxy-γ-butyrolactone. Nat Commun 4(1933):1–9. https://doi.org/10.1038/ncomms2418
Koivistoinen OM, Kuivanen J, Barth D, Turkia H, Pitkanen JP, Penttila M, Richard P (2013) Glycolic acid production in the engineered yeasts Saccharomyces cerevisiae and Kluyveromyces lactis. Microb Cell Fact 12(82):1–16. https://doi.org/10.1186/1475-2859-12-82
Zahoor A, Otten A, Wendisch VF (2014) Metabolic engineering of Corynebacterium glutamicum for glycolate production. J Biotechnol 192(Pt B):366–375. https://doi.org/10.1016/j.jbiotec.2013.12.020
Deng Y, Mao Y, Zhang X (2015) Metabolic engineering of E. coli for efficient production of glycolic acid from glucose. Biochem Eng J 103:256–262. https://doi.org/10.1016/j.bej.2015.08.008
Nanchen A, Schicker A, Revelles O, Sauer U (2008) Cyclic AMP-dependent catabolite repression is the dominant control mechanism of metabolic fluxes under glucose limitation in Escherichia coli. J Bacteriol 90(7):2323–2330. https://doi.org/10.1128/JB.01353-07
Cam Y, Alkim C, Trichez D, Trebosc V, Vax A, Bartolo F, Besse P, Francois JM, Walther T (2015) Engineering of a synthetic metabolic pathway for the assimilation of (d)-xylose into value-added chemicals. ACS Synth Biol 5(7):607–618. https://doi.org/10.1021/acssynbio.5b00103
Alkim C, Trichez D, Cam Y, Spina L, Francois JM, Walther T (2016) The synthetic xylulose-1 phosphate pathway increases production of glycolic acid from xylose-rich sugar mixtures. Biotechnol Biofuels. https://doi.org/10.1186/s13068-016-0610-2
Pereira B, Li ZJ, De Mey M, Lim CG, Zhang H, Hoeltgen C, Stephanopoulos G (2016) Efficient utilization of pentoses for bioproduction of the renewable two-carbon compounds ethylene glycol and glycolate. Metab Eng 34:80–87. https://doi.org/10.1016/j.ymben.2015.12.004
Salusjarvi L, Toivari M, Vehkomaki ML, Koivistoinen O, Mojzita D, Niemela K, Penttila M, Ruohonen L (2017) Production of ethylene glycol or glycolic acid from D-xylose in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 101(22):8151–8163. https://doi.org/10.1007/s00253-017-8547-3
Bañares AB, Valdehuesa KNG, Ramos KRM, Nisola GM, Lee W-K, Lee C-R, Chung W-J (2019) Discovering a novel d-xylonate-responsive promoter: the PyjhI-driven genetic switch towards better 1,2,4-butanetriol production. Appl Microbiol Biotechnol 103:8063–8074. https://doi.org/10.1007/s00253-019-10073-0
Cabulong RB, Valdehuesa KNG, Ramos KRM, Nisola GM, Lee W-K, Lee CR, Chung WJ (2017) Enhanced yield of ethylene glycol production from d-xylose by pathway optimization in Escherichia coli. Enzyme Microb Technol 97:11–20. https://doi.org/10.1016/j.enzmictec.2016.10.020
Cabulong RB, Lee WK, Bañares AB, Ramos KRM, Nisola GM, Valdehuesa KNG, Chung W-J (2018) Engineering Escherichia coli for glycolic acid production from d-xylose through the Dahms pathway and glyoxylate bypass. Appl Microbiol Biotechnol 102(5):2179–2189. https://doi.org/10.1007/s00253-018-8744-8
Valdehuesa KNG, Liu H, Ramos KRM, Park S-J, Nisola GM, Lee W-K, Chung WJ (2014) Direct bioconversion of d-xylose to 1,2,4-butanetriol in an engineered Escherichia coli. Process Biochem 49(1):25–32. https://doi.org/10.1016/j.procbio.2013.10.002
Valdehuesa KNG, Lee WK, Ramos KRM, Cabulong RB, Choi JS, Liu H, Nisola GM, Chung WJ (2015) Identification of aldehyde reductase catalyzing the terminal step for conversion of xylose to butanetriol in engineered Escherichia coli. Bioprocess Biosyst Eng 38:1761–1772
Vinuselvi P, Chandran SP, Mukhopadhyay A, Lee S-K, Keasling JD (2017) Intracellular cellobiose metabolism and its applications in lignocellulose-based biorefineries. Bioresour Technol 239:496–506. https://doi.org/10.1016/j.biortech.2017.05.001
Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS (2012) Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol 30(5):274–282. https://doi.org/10.1016/j.tibtech.2012.01.005
Sekar R, Shin HD, Chen R (2012) Engineering Escherichia coli cells for cellobiose assimilation through a phosphorolytic mechanism. Appl Environ Microbiol 78(5):1611–1614. https://doi.org/10.1128/AEM.06693-11
Shin HD, Wu J, Chen R (2014) Comparative engineering of Escherichia coli for cellobiose utilization: hydrolysis versus phosphorolysis. Metab Eng 24:9–17. https://doi.org/10.1016/j.ymben.2014.04.002
Vinuselvi P, Lee SK (2011) Engineering Escherichia coli for efficient cellobiose utilization. Appl Microbiol Biotechnol 92(1):125–132. https://doi.org/10.1007/s00253-011-3434-9
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6(5):343–345. https://doi.org/10.1038/nmeth.1318
Xu P, Vansiri A, Bhan N, Koffas MAG (2012) ePathBrick: a synthetic biology platform for engineering metabolic pathways in E. coli. ACS Synth Biol 1(7):256–266. https://doi.org/10.1021/sb300016b
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor, Cold Spring Harbor Laboratory
Hall BG, Betts PW (1987) Cryptic genes for cellobiose utilization in natural isolates in Escherichia coli. Genetics 115(3):431–439
Cabulong RB, Valdehuesa KNG, Bañares AB, Ramos KRM, Nisola GM, Lee W-K, Lee C-R, Chung WJ (2019) Improved cell growth and biosynthesis of glycolic acid by overexpression of membrane-bound pyridine nucleotide transhydrogenase. J Ind Microbiol Biotechnol 46:159–169. https://doi.org/10.1007/s10295-018-2117-2
Lawford HG, Rousseau JD (1994) Relative rates of sugar utilization by an ethanologenic recombinant Escherichia coli using mixtures of glucose, mannose, and xylose. Appl Biochem Biotechnol 45–46:367–381. https://doi.org/10.1007/BF02941812
Bezerra RMF, Dias AA (2005) Enzymatic kinetic of cellulose hydrolysis inhibition by ethanol and cellobiose. Appl Biochem Biotechnol 126:49–59. https://doi.org/10.1007/s12010-005-0005-5
Kricka W, James TC, Fitzpatrick J, Bond U (2015) Engineering Saccharomyces pastorianus for the co-utilization of xylose and cellulose from biomass. Microb Cell Fact 14(61):1–11. https://doi.org/10.1186/s12934-015-0242-4
Ha SJ, Galazka JM, Kim SR, Choi JH, Yang XM, Seo JH, Glass NL, Cate JHD, Jin YS (2011) Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc Natl Acad Sci USA 108:504–509
Gong Z, Wang Q, Shen H, Hu C, Jin G, Zhao ZK (2012) Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour Technol 117:20–24
Nichols NN, Dien BS, Bothast RJ (2001) Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Appl Microbiol Biotechnol 56:120–125
Sawisit A, Jantama SS, Kanchanatawee S, Jantama K (2015) Efficient utilization of cassava pulp for succinate production by metabolically engineered E scherichia coli KJ122. Bioprocess Biosyst Eng 38:175–187
Gruno M, Väljamäe P, Pettersson G, Johansson G (2004) Inhibition of the Trichoderma reesei cellulases by cellobiose is strongly dependent on the nature of the substrate. Biotechnol Bioeng 86:503–511. https://doi.org/10.1002/bit.10838
Zhao Y, Wu B, Yan B, Gao P (2004) Mechanism of cellobiose inhibition in cellulose hydrolysis by cellobiohydrolase. Sci China Ser C 47:8–24. https://doi.org/10.1360/02yc0163
Deng Y, Ma N, Zhu K, Mao Y, Wei Z, Zhao Y (2018) Balancing the carbon flux distributions between the TCA cycle and glyoxylate shunt to produce glycolate at high yield and titer in Escherichia coli. Metab Eng 46:28–34
Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol 72(5):3653–3661
Kornberg HL (1966) The role and control of the glyoxylate cycle in Escherichia coli. Biochem J 99(1):1–11. https://doi.org/10.1042/bj0990001
Kurata H (2019) Self-replenishment cycles generate a threshold response. Sci Rep 9:17139. https://doi.org/10.1038/s41598-019-53589-1
Valdehuesa KNG, Ramos KRM, Nisola GM, Bañares AB, Cabulong RB, Lee WK, Liu H, Chung WJ (2018) Everyone loves an underdog: metabolic engineering of the xylose oxidative pathway in recombinant microorganisms. Appl Microbiol Biotechnol 102(18):7703–7716. https://doi.org/10.1007/s00253-018-9186-z
Liu H, Valdehuesa KNG, Nisola GM, Ramos KRM, Chung W-J (2012) High yield production of d-xylonic acid from d-xylose using engineered Escherichia coli. Bioresour Technol 115:244–248. https://doi.org/10.1016/j.biortech.2011.08.065
Bañares AB, Valdehuesa KNG, Ramos KRM, Nisola GM, Lee WK, Lee CR, Chung WJ (2020) A pH-responsive genetic sensor for the dynamic regulation of d-xylonic acid accumulation in Escherichia coli. Appl Microbiol Biotechnol 104:2097–2108. https://doi.org/10.1007/s00253-019-10297-0
Vinuselvi P, Lee S-K (2012) Engineered Escherichia coli capable of co-utilization of cellobiose and xylose. Enzyme Microb Technol 50(1):1–4. https://doi.org/10.1016/j.enzmictec.2011.10.001
Moen B, Ao J, Langsrud S, Langsrud O, Hobman JL, Constantinidou C, Kohler A, Rudi K (2009) Global responses of Escherichia coli to adverse conditions determined by microarrays and FT-IR spectroscopy. Can J Microbiol 55:714–728
Zheng J, Yates SP, Jia Z (2012) Structural and mechanistic insights into the bifunctional enzyme isocitrate dehydrogenase kinase/phosphatase AceK. Phil Trans R Soc B 367:2656–2668
Acknowledgements
The authors would like to acknowledge the National Research Foundation of Korea (NRF) under the Basic Science Research Program through the Ministry of Education and the Korea Institute of Technology Evaluation and Planning (KETEP) funded by the Ministry of Trade, Industry & Energy for funding the research.
Funding
This work was supported by the National Research Foundation of Korea (NRF) under the Basic Science Research Program through the Ministry of Education (2018R1D1A1B07043993 and 2020R1A6A1A03038817) and by the Korea Institute of Technology Evaluation and Planning (KETEP) funded by the Ministry of Trade, Industry & Energy (MOTIE No. 20194010201750).
Author information
Authors and Affiliations
Contributions
RBC and ABB conceived and designed the study, performed the experiments, analyzed the data, and wrote the manuscript. GMN analyzed the data, supervised, wrote, and revised the manuscript. WKL and WJC acquired the funding and resources, supervised, and revised the manuscript. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cabulong, R.B., Bañares, A.B., Nisola, G.M. et al. Enhanced glycolic acid yield through xylose and cellobiose utilization by metabolically engineered Escherichia coli. Bioprocess Biosyst Eng 44, 1081–1091 (2021). https://doi.org/10.1007/s00449-020-02502-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02502-6