Abstract
Purpose
The introduction of hypofractionated stereotactic radiosurgery (hSRS) extended the treatment modalities beyond the well-established single-fraction stereotactic radiosurgery and fractionated radiotherapy. Here, we report the efficacy and side effects of hSRS using Cyberknife® (CK-hSRS) for the treatment of patients with critical brain metastases (BM) and a very poor prognosis. We discuss our experience in light of current literature.
Methods
All patients who underwent CK-hSRS over 3 years were retrospectively included. We applied a surface dose of 27 Gy in 3 fractions. Rates of local control (LC), systemic progression-free survival (PFS), and overall survival (OS) were estimated using Kaplan–Meier method. Treatment-related complications were rated using the Common Terminology Criteria for Adverse Events (CTCAE).
Results
We analyzed 34 patients with 75 BM. 53% of the patients had a large tumor, tumor location was eloquent in 32%, and deep seated in 15%. 36% of tumors were recurrent after previous irradiation. The median Karnofsky Performance Status was 65%.
The actuarial rates of LC at 3, 6, and 12 months were 98%, 98%, and 78.6%, respectively.
Three, 6, and 12 months PFS was 38%, 32%, and 15%, and OS was 65%, 47%, and 28%, respectively. Median OS was significantly associated with higher KPS, which was the only significant factor for survival. Complications CTCAE grade 1–3 were observed in 12%.
Conclusion
Our radiation schedule showed a reasonable treatment effectiveness and tolerance. Representing an optimal salvage treatment for critical BM in patients with a very poor prognosis and clinical performance state, CK-hSRS may close the gap between surgery, stereotactic radiosurgery, conventional radiotherapy, and palliative care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Single fraction stereotactic radiosurgery (SRS) is a well-established part of standard care in BM. Its application is limited by tumor size (cross-sectional diameter > 3 cm), the number of targets, and a close proximity to critical structures (i.e., brainstem, cerebellar nuclei; sensorimotor, language or visual cortex; basal ganglia, hypothalamus or thalamus; internal capsule; optic pathway). A pre-irradiation [e.g., SRS, brachytherapy, fractionated radiation therapy (fRT), or WBRT] can limit the possible radiation dose. Cyberknife® hSRS may overcome these limitations by combining the advantages of SRS (i.e., shorter treatment period, steep dose decay) with the radiobiological advantages of fRT in terms of re-oxygenation of hypoxic tumor tissue, redistribution of the cell cycle to a more sensitive phase, and sparing of radio-sensible adjacent brain structures by lower doses (Brenner et al. 1991). Thus, hypofractionated stereotactic radiosurgery (hSRS) extends the indications of stereotactic radiation techniques and of fractionated radiotherapy beyond the previous constraints.
To date, clinical data regarding feasibility, local control, and toxicity of Cyberknife® hSRS (CK-hSRS) for the treatment of BM are scarce.
Materials and methods
Subjects and patients
In this single center retrospective analysis, we included all patients who underwent CK-hSRS between September 2014 and August 2017. We queried our database for demographic, disease, and treatment-related parameters. Recursive partitioning analysis (RPA) classification was determined for all patients according to Gaspar et al. (1997).
Indications for CK-hSRS
Decision for hSRS was made by an interdisciplinary neurooncological tumor board. The treatment indications were: (1) BM located in close proximity to or in eloquent brain areas, (2) deep-seated BM in recurrence after previous irradiation, and (3) large BM (> 3 cm) if surgery is not feasible. Additional small BMs were co-treated in the same setting to simplify the treatment management. Neurological or radiological evidence of carcinomatous meningitis was an exclusion criterion. Patients with a poor Karnofsky performance status (< 40%) or without further oncological options were not treated with this protocol. Informed consent was obtained from all patients.
Follow-up and end points
Clinical assessment and follow-up MRI were carried out in at least 3 month intervals or earlier in cases of significant neurologic deterioration. The tumor size was defined as the largest cross-sectional diameter on T1-weighted contrast-enhanced axial sequences. Each lesion was measured for local tumor response and graded using the RANO criteria for brain metastases: local tumor control (LC) was defined as either complete remission, partial remission or stable disease. An increase in tumor size ≥ 30% was suspected as local failure a progressive disease (Lin et al. 2015). To rule out a pseudo-progress a short-term follow-up MRI, [18F]-fluoro-ethyl-l-tyrosine PET (FET-PET) or stereotactical biopsy was performed in confounding cases. We evaluated systemic progression-free survival (PFS), overall survival (OS), and treatment related early and late adverse events according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Cause of the death was classified due to primary disease and comorbidities, neurological disease, or unknown. Neurological death was defined as death from any impact of intracranial metastases, e.g., tumor recurrence, carcinomatous meningitis, or cerebral dissemination.
Radiosurgery technique
A high-resolution contrast-enhanced cranial computed tomography (CCT) and MRI scans were obtained a few days before SRS. We used an MRI protocol consisting of 4 MRI modalities: T1-weighted contrast-enhanced axial sequences with 2 mm sl (3D T1) and coronal sequences with 1.2 mm sl (3D T1w FFE), T2-weighted axial sequences with 2 mm sl (T2 TSE), and axial FLAIR sequences with 2 mm sl (FLAIR long TR). The Cyberknife® treatment planning was carried out with the software Multiplan v4.5 (MultiPlan, Inc., New York, USA). The tumor targets and the critical structures were contoured on stereotactic MRI scans (1.5 or 3 T; Philips, Hamburg, Germany), which were obtained a few days before hSRS. PTV was outlined according to a suspected CTV as GTV with a 1–2 mm margin. The cMRI scans were fused with the stereotactic CCT (1 mm slice thickness [sl], Toshiba). For Cyberknife® treatment, the patient`s head was immobilized with a custom-made aquaplast mask.
Following a prospective protocol, we applied a surface dose of 27 Gy in three fractions prescribed on the 65% isodose. The prescribed surface dose (Gy), isodose level, mean dose, minimum dose, maximum dose, tumor coverage, homogeneity index, conformity index, new conformity index, VOI 16 (volume of healthy brain tissue that is irradiated with a total dose of at least 16 Gy), collimator, and the number of radiation beams were recorded. All treatments were rendered by an experienced team of SRS physicians and medical physicists.
Statistical analysis
Statistical analyses were performed with SPSS, version 23 (IBM Corp., Armonk, New York, USA). Local control (LC), systemic progression-free survival (PFS), and overall survival (OS) were analyzed using the Kaplan–Meier method. Prognostic factors were identified by the log-rank test. A p value < 0.05 was considered statistically significant.
Results
Patient characteristics and follow-up
34 patients with 75 brain metastases were included (Table 1). Large tumor size was the main indication in 53% of patients (n = 18). Eloquent location of BM indicated hSRS in 32% (n = 11) and recurrent, deep-seated BM after previous irradiation in 15% (n = 5) of cases. We co-treated all small or not eloquent located additional BM to simplify the treatment management, sparing medical resources and patients time, or due to previous irradiation. Since more than 50% of patients harbored 2–5 BMs requiring treatment, the majority of the treated tumors were additional. All hSRS treatments were completed within 5–7 days. A total of 24 patients (70.6%) with 57 treated BM (76%) were evaluated by imaging follow-up (FU). The median radiological FU was 8.0 months (range 2–28). In 10 patients (29.4%) with 18 treated tumors (24%), FU imaging was not available due to clinical worsening and consecutive death. The median clinical FU for all 34 patients was 6 months (range 1–28).
Tumor characteristics
Details of the treated tumors are summarized in Table 2. The median maximum tumor diameter was 2.0 cm (range 0.4–4.3 cm) and the median tumor volume in terms of PTV amounted to 4.6 cm3 (range 0.03–24.8 cm).
In 48 BM (64%), CK-hSRS was carried out as de-novo treatment. 27 BM (36%) were recurrences after irradiation, including 13 new distant lesions (17%) after WBRT, 11 locally recurrent lesions (15%) after WBRT, and 3 locally recurrent lesions (4%) after other irradiation modalities (brachytherapy and single shot radiosurgery).
Local tumor control (LC)
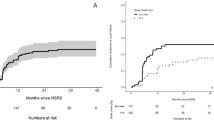
The actuarial radiologic local tumor control rate was 98% at 3 months as well as at 6 months, and 78.6% at 12 month (Fig. 1a).
Kaplan–Meier estimates. a Actuarial local tumor control rates: at 3 months, at 6 months = 98%, and at 12 months = 78.6%. b Systemic progression-free survival rates: at 3 months = 38%, 6 months = 32%, and 12 months = 15%. c Overall survival rates: at 3 months = 65%, 6 months = 47%, and 12 months = 28%. d Overall Survival rates in RPA classes at 6 months after CK-hSRS: 82% in class 1 + 2 (median OS = 14 months, 95% CI 8.8–19.1 months) and 12% in class 3 (median OS = 2 months, 95% CI 0.7–3.3 months), p < 0.01
Overall LC failure was observed in 6 of 57 (10.5%) tumors and in 5 of 24 patients (20.8%) with complete follow-up. Two of these patients were subsequently treated with surgical resection and adjuvant RT of the cavity. Another patient was treated with WBRT and a boost on recurrent metastases after the histological proof by stereotactical biopsy and the other two patients were treated in a purely palliative setting due to extracranially progressive disease. The median time interval between hSRS and the diagnosis of local control failure was 8 months (range 3–11).
According to RANO criteria for brain metastasis (Lin et al. 2015), partial remission was found in 33.3% of patients (n = 8/24) and stable disease in 12.5% (n = 6/24). Progressive disease was found in 54.2% (n = 13/24), with the majority (33.3%, n = 8/24) due to new brain metastases (i.e., distant brain control failure) and fewer due to local tumor control failure (20.8%, n = 5/24). In terms of the individual tumors, 5.3% (n = 3/57) were in complete remission, 68.4% (n = 39/57) were in a partial remission, 15.8% (n = 9/57) were stable, and 10.5% (n = 6/57) showed a local progression at last radiologic follow-up. Complete local remission was only observed in tumors less than 0.6 cm and local progressive disease occurred only in tumors exceeding at least 2 cm in the maximum diameter or a PTV of 4.6 cm3 (Table 3).
Systemic progression-free survival, overall survival, and death events
PFS rates were 38%, 32% and 15% at 3, 6, and 12 months, respectively (Fig. 1b). The median PFS amounted to 3 months. OS rates were 65%, 47%, and 28% at 3, 6, and 12 months, respectively (Fig. 1c). The median OS amounted to 6 months.
All patients were assessed in terms of RPA classification and were divided in class I (n = 7), class II (n = 10), and class III (n = 17) according to Gaspar et al. (1997). Since the patient number in class I was very low and the status of the extra-cranial disease was not always up to date, we merged class I with class II comparing them together with class III. There was a significant inter-class difference of overall survival rates based on Kaplan–Meier Curves (p < 0.01), the median overall survival was 14 months in RPA class I + II patients and 2 months in class III (Fig. 1d).
KPS was the only factor, which was significantly associated with survival. The BM count, PTV, prior WBRT, and age were not significant (Table 4).
At last follow-up, 79% of patients had died (n = 27/34). The cause of death was extra-cranial progression of the primary or through other comorbidities in 65% (n = 22/34), distant meningeosis in 6% (n = 2/34), and unknown in 9% (n = 3/34).
Adverse events and toxicities
Overall, four patients (12%) experienced early (< 6 weeks after hSRS) or late (> 6 weeks after hSRS) adverse events grade 1–3 according to CTCAE.
Among early complications (n = 2/34, 6%), one patient suffered a cerebral edema with a need for steroid treatment (CTCAE grade 2). Another patient had a deterioration of hemiparesis and additional structural focal epilepsy, which led to hospitalization and medical treatment (CTCAE grade 3).
Among late complications (n = 2/34, 6%), there was one asymptomatic patient with an extensive radiogenic edema, but no need for treatment (CTCAE grade 1) and another patient experienced severe headache and nausea as a cause of a radiation necrosis with an extensive edema and a need of hospitalization (CTCAE grade 3). All above-mentioned patients improved during medical therapy.
Ultimately, there was no case of permanent morbidity as a result of treatment.
Discussion
Hypofractionated stereotactic radiosurgery by Cyberknife® (CK-hSRS) is often confused with hypofractionated stereotactic radiotherapy (HFSRT, by linear accelerator). Since 1993, clinical application of fractionated stereotactic radiotherapy for brain metastases performed by LINAC was extensively described in the literature (Salles et al. 1993; Manning et al. 2000; Feuvret et al. 2014; Lehrer et al. 2019; Rajakesari et al. 2014; Rades et al. 2017; Baliga et al. 2017; Aoyama et al. 2003; Ernst-Stecken et al. 2006; Aoki et al. 2006; Narayana et al. 2007; Kwon et al. 2009; Saitoh et al. 2010; Kim et al. 2011; Minniti et al. 2014). However, there are only few heterogeneous reports about the CK-hSRS for BM elucidating its application in different patient populations and with different study specifics (Table 5) (Inoue et al. 2014; Murai et al. 2014; Royer et al. 2017; Nakamura et al. 2017; Lesueur et al. 2018; Guan et al. 2020; Mengue et al. 2020). It is difficult to compare the results due to different study designs.
In accordance to previous reports, tumor size was the main indication for treatment in our collective on the basis of poor performance state or patient’s will, while eloquent location and deep-seated BM recurrences were indications, due to limits of conventional treatment modalities.
The median FU of our patients and the actuarial LC rates of the treated tumors are in the range of the current literature on the CK-hSRS (Table 5). Longer reported FU may result from a better performance state of treated patients.
In comparison to other series, our OS rates were rather poor. This may be explained by the lower median Karnofsky Performance State and oncologic status of the majority of patients in our study, so classified the RPA the half of our patients as class III, i.e., gravely ill. In accordance with the current literature, 65% of our patients died due to extra-cranial progression.
In line with the most oncologic studies, the better median OS of our patients was significantly associated with higher KPS. For this reason, KPS is an important part of all prognostic scores (e.g., Johung et al. 2016).
Other than reported, there was no evidence of death events due to Local Control Failure in our patient collective, where the neurologic death cases (6%, n = 2/34) resulted from a distant meningeosis. That may be due to a relatively low total number of patients and a relatively high number of patients with a low performance state.
Our results are in line with the reported LC rates (68.6–87%, related to the number of treated targets) and median survival time range (7–13.9 months) for single shot stereotactic radiosurgery by different modalities (LINAC, Gamma Knife or Cyberknife®) (Flickinger et al. 1994; Li et al. 2000; Hoffman et al. 2001; Aoyama et al. 2006; Kocher et al. 2011; Murovic et al. 2017; Brown et al. 2016).
Regarding hypofractionated stereotactic radiosurgery using LINAC, our results are in the range of the reported LC rates (56–88%) and at the lower end of the range for median survival time (6–14.8 months), as well as for the median follow-up (6–17.4 months), due to a preponderance of RPA class III patients in our cohort (Salles et al. 1993; Manning et al. 2000; Feuvret et al. 2014; Rajakesari et al. 2014; Rades et al. 2017; Baliga et al. 2017; Aoyama et al. 2003; Ernst-Stecken et al. 2006; Aoki et al. 2006; Narayana et al. 2007; Kwon et al. 2009; Saitoh aet al/ 2010; Kim et al. 2011; Minniti et al. 2014).
Compared to postoperative fractionated local 3D-conformal radiotherapy (3DRT) for resected BM with a reported range of LC rates of 81.2–91.4% at 12 months, our LC rates are at the lower end (78.6%). Due to a healthier sample, they showed longer median follow-ups (9.7–19.1 months) and survival rates at 12 months (68–77.7%) (Hashimoto et al. 2011; Shin et al. 2015; Igaki et al. 2017; Ayas et al. 2018).
The additional co-treating of small tumors by CK-hSRS proved no disadvantages in our study, as especially smaller tumors showed the tendency to better response and additional treatment modality was spared. This concept simplifies the management, may spare medical resources, and provide additional time for, e.g., a systemic therapy or just improve the quality of life. Backing the above-mentioned tendency to better response of smaller tumors, Lesueur et al. (2018) estimated a significantly decreased risk of local control failure (from 36 to 17% at 12 months, p = 0.007) for the CK-hSRS therapy of radio-resistant BM (melanoma and renal carcinoma) showing < 1 cm in diameter and Mengue et al. (2020) showed the target size of < 2.5 cm to be an independent factor improving LC in CK-hSRS therapy of BM (p = 0.01).
Among the tumors with local control failure after CK-hSRS (n = 6/57) was no adverse tendency for pre-irradiated ones (33%, n = 2/6), for location of the targets or for the total number of the targets per patient, but there was a strong tendency for larger BMs, so were all recurrent targets at least 2 cm in the diameter.
Like reported by Nakamura et al. (2017), we detected the most LC failures at the second half year after the treatment. On the contrary, Murai et al. (2014) and Lesueur et al. (2018) observed distinct more LC failures at the first half year.
Interestingly, 67% (n = 4/6) of targets with LC failure in our study originated from breast cancer primary, whereas there is an increasing evidence of not existing radio-resistance against radiosurgery (Lesueur et al. 2018). Further statistical estimation of LC failure was not feasible due to a very low number of events.
Our rates of Adverse Events and Toxicities according to CTCAE with 12% Grade 1–3 were moderate compared to the reported range of 5–25% Grade 1–4 (Table 5). They may correlate with extra-large target size and eloquent location.
In addition to the common limitations, as the retrospective character with the risk of hidden selection bias, a small sample size, a heterogeneity of cancer histology, and the treatment indications, there is a reduced FU rate after CK-hSRS in our study with at least 24 of 34 patients (70.6%), due to the poor general condition of our patients and a progressive systemic cancer.
Conclusion
Outcome reports of CK-hSRS and the reported patient collectives are scarce and heterogeneous to date. This study demonstrated hypofractionated stereotactic radiosurgery using Cyberknife® as an important salvage treatment for cerebral metastases even in pre-irradiated cases. Different Cyberknife® hypofractination schedules proved to be reasonably tolerated and effective. CK-hSRS is a relatively young treatment modality, but it is capable to fill the gap between surgery, single-fraction stereotactic radiosurgery, fractionated radiotherapy, and pure palliative care.
Availability of data and materials
Datasets generated for this study are available upon request to the corresponding author.
References
Aoki M, Abe Y, Hatayama Y et al (2006) Clinical outcome of hypofractionated conventional conformation radiotherapy for patients with single and no more than three metastatic brain tumors, with noninvasive fixation of the skull without whole brain irradiation. Int J Radiat Oncol 64:414–418. https://doi.org/10.1016/j.ijrobp.2005.03.017
Aoyama H, Shirato H, Onimaru R et al (2003) Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol 56:793–800. https://doi.org/10.1016/S0360-3016(03)00014-2
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. JAMA 295:2483. https://doi.org/10.1001/jama.295.21.2483
Ayas AW, Grau S, Jablonska K et al (2018) Postoperative local fractionated radiotherapy for resected single brain metastases. Strahlenther Onkol 194:1163–1170. https://doi.org/10.1007/s00066-018-1368-1
Baliga S, Garg MK, Fox J et al (2017) Fractionated stereotactic radiation therapy for brain metastases: a systematic review with tumour control probability modelling. Br J Radiol 90:20160666. https://doi.org/10.1259/bjr.20160666
Brenner DJ, Martel MK, Hall EJ (1991) Fractionated regimens for stereotactic radiotherapy of recurrent tumors in the brain. Int J Radiat Oncol 21:819–824. https://doi.org/10.1016/0360-3016(91)90703-7
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA 316:401. https://doi.org/10.1001/jama.2016.9839
Ernst-Stecken A, Ganslandt O, Lambrecht U et al (2006) Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 81:18–24. https://doi.org/10.1016/j.radonc.2006.08.024
Feuvret L, Vinchon S, Martin V et al (2014) Stereotactic radiotherapy for large solitary brain metastases. Cancer/Radiothérapie 18:97–106. https://doi.org/10.1016/j.canrad.2013.12.003
Flickinger JC, Kondziolka D, Dade Lunsford L et al (1994) A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol 28:797–802. https://doi.org/10.1016/0360-3016(94)90098-1
Gaspar L, Scott C, Rotman M et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol 37:745–751. https://doi.org/10.1016/S0360-3016(96)00619-0
Guan Y, Wang C, Zhu H et al (2020) Hypofractionated radiosurgery plus bevacizumab for locally recurrent brain metastasis with previously high-dose irradiation. World Neurosurg 133:e252–e258. https://doi.org/10.1016/j.wneu.2019.08.233
Hashimoto K, Narita Y, Miyakita Y et al (2011) Comparison of clinical outcomes of surgery followed by local brain radiotherapy and surgery followed by whole brain radiotherapy in patients with single brain metastasis: single-center retrospective analysis. Int J Radiat Oncol 81:e475–e480. https://doi.org/10.1016/j.ijrobp.2011.02.016
Hoffman R, Sneed PK, McDermott MW et al (2001) Radiosurgery for brain metastases from primary lung carcinoma. Cancer J 7:121–131
Igaki H, Harada K, Umezawa R et al (2017) Outcomes of surgery followed by local brain radiotherapy compared with surgery followed by whole brain radiotherapy for single brain metastasis. Tumori J 103:367–373. https://doi.org/10.5301/tj.5000657
Inoue HK, Sato H, Suzuki Y et al (2014) Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: a single-institutional prospective study. Radiat Oncol 9:231. https://doi.org/10.1186/s13014-014-0231-5
Johung KL, Yeh N, Desai NB et al (2016) Extended survival and prognostic factors for patients with ALK—rearranged non–small-cell lung cancer and brain metastasis. J Clin Oncol 34:123–129. https://doi.org/10.1200/JCO.2015.62.0138
Kim Y-J, Cho KH, Kim J-Y et al (2011) Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol 81:483–489. https://doi.org/10.1016/j.ijrobp.2010.05.033
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 Study. J Clin Oncol 29:134–141. https://doi.org/10.1200/JCO.2010.30.1655
Kwon AK, DiBiase SJ, Wang B et al (2009) Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer 115:890–898. https://doi.org/10.1002/cncr.24082
Lehrer EJ, Peterson JL, Zaorsky NG et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol 103:618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Lesueur P, Lequesne J, Barraux V et al (2018) Radiosurgery or hypofractionated stereotactic radiotherapy for brain metastases from radioresistant primaries (melanoma and renal cancer). Radiat Oncol 13:138. https://doi.org/10.1186/s13014-018-1083-1
Li B, Yu J, Suntharalingam M et al (2000) Comparison of three treatment options for single brain metastasis from lung cancer. Int J Cancer 90:37–45. https://doi.org/10.1002/(SICI)1097-0215(20000220)90:1%3c37::AID-IJC5%3e3.0.CO;2-7
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Manning MA, Cardinale RM, Benedict SH et al (2000) Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. Int J Radiat Oncol 47:603–608. https://doi.org/10.1016/S0360-3016(00)00475-2
Mengue L, Bertaut A, Ngo Mbus L et al (2020) Brain metastases treated with hypofractionated stereotactic radiotherapy: 8 years experience after Cyberknife installation. Radiat Oncol 15:82. https://doi.org/10.1186/s13014-020-01517-3
Minniti G, D’Angelillo RM, Scaringi C et al (2014) Fractionated stereotactic radiosurgery for patients with brain metastases. J Neurooncol 117:295–301. https://doi.org/10.1007/s11060-014-1388-3
Murai T, Ogino H, Manabe Y et al (2014) Fractionated stereotactic radiotherapy using cyberknife for the treatment of large brain metastases: a dose escalation study. Clin Oncol 26:151–158. https://doi.org/10.1016/j.clon.2013.11.027
Murovic J, Ding V, Han SS et al (2017) Impact of cyberknife radiosurgery on overall survival and various parameters of patients with 1–3 versus ≥ 4 brain metastases. Cureus 9:1–19. https://doi.org/10.7759/cureus.1798
Nakamura M, Nishimura H, Mayahara H et al (2017) Investigation of the efficacy and safety of CyberKnife hypofractionated stereotactic radiotherapy for brainstem metastases using a new evaluation criterion: ‘symptomatic control.’ J Radiat Res 58:834–839. https://doi.org/10.1093/jrr/rrx042
Narayana A, Chang J, Yenice K et al (2007) Hypofractionated stereotactic radiotherapy using intensity-modulated radiotherapy in patients with one or two brain metastases. Stereotact Funct Neurosurg 85:82–87. https://doi.org/10.1159/000097923
Rades D, Janssen S, Dziggel L et al (2017) A matched-pair study comparing whole-brain irradiation alone to radiosurgery or fractionated stereotactic radiotherapy alone in patients irradiated for up to three brain metastases. BMC Cancer 17:30. https://doi.org/10.1186/s12885-016-2989-3
Rajakesari S, Arvold ND, Jimenez RB et al (2014) Local control after fractionated stereotactic radiation therapy for brain metastases. J Neurooncol 120:339–346. https://doi.org/10.1007/s11060-014-1556-5
Royer P, Salleron J, Vogin G et al (2017) Radiothérapie stéréotaxique hypofractionnée des métastases cérébrales : bénéfice de l’irradiation encéphalique totale ? Cancer/Radiothérapie 21:731–740. https://doi.org/10.1016/j.canrad.2017.02.005
Saitoh I, Saito Y, Kazumoto T et al (2010) Therapeutic effect of linac-based stereotactic radiotherapy with a micro-multileaf collimator for the treatment of patients with brain metastases from lung cancer. Jpn J Clin Oncol 40:119–124. https://doi.org/10.1093/jjco/hyp128
Salles AAF, Hariz M, Bajada CL et al (1993) Comparison between radiosurgery and stereotactic fractionated radiation for the treatment of brain metastases. Advances in stereotactic and functional neurosurgery 10. Springer Vienna, Vienna, pp 115–118
Shin SM, Vatner RE, Tam M et al (2015) Resection followed by involved-field fractionated radiotherapy in the management of single brain metastasis. Front Oncol 5:12–13. https://doi.org/10.3389/fonc.2015.00206
Acknowledgments
No source of financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
ST carried out the study including acquisition, analysis, and interpretation of the data, and prepared and wrote the manuscript. DR, MR, and MK conceived and supervised the study. DR, MR, KJ, HT, and ST participated in the clinical, radiation and image data collection. SG, RG, and NS revised the manuscript critically for important intellectual content. All authors reviewed the manuscript before submission and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/competing interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Telentschak, S., Ruess, D., Grau, S. et al. Cyberknife® hypofractionated stereotactic radiosurgery (CK-hSRS) as salvage treatment for brain metastases. J Cancer Res Clin Oncol 147, 2765–2773 (2021). https://doi.org/10.1007/s00432-021-03564-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03564-z