Abstract
Traditionally, clinicians consider lactate as a waste product of anaerobic glycolysis. Interestingly, research has shown that lactate may serve as an alternative fuel for the brain to protect it against harm. The increasing scientific awareness of the potential beneficial side of lactate, however, is entering the clinic rather slowly. Following this, and realizing that the application of potential novel therapeutic strategies in pediatric populations often lags behind the development in adults, this review summarizes the key data on therapeutic use of intravenous infusion of sodium lactate in humans. PubMed and clinicaltrial.gov were searched up until November 2021 focusing on interventional studies in humans. Thirty-four articles were included in this review, with protocols of lactate infusion in adults with diabetes mellitus, traumatic brain injury, Alzheimer’s disease, and cardiac disease. One study on lactate infusion in children was also included. Results of our literature search show that sodium lactate can be safely administrated, without major side effects. Additionally, the present literature clearly shows the potential benefits of therapeutic lactate infusion under certain pathological circumstances, including rather common clinical conditions like traumatic brain injury.
Conclusion: This review shows that lactate is a save, alternative energy source for the adult brain warranting studies on the potential therapeutic effects of sodium lactate infusion in children.
What is Known: • Lactate is generally considered a waste product of anaerobic glycolysis. However, lactate also is an alternative fuel for different organs, including the brain. • Lactate infusion is not incorporated in standard care for any patient population. | |
What is New: • Thirty-four studies investigated the therapeutic use of intravenous sodium lactate in different patient populations, all with different study protocols. • Literature shows that lactate infusion may have beneficial effects in case of hypoglycemia, traumatic brain injury, and cardiac failure without the risk of major side effects. |
Similar content being viewed by others
Introduction
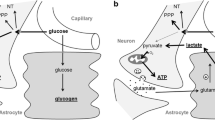
In some rare inborn errors of metabolism (e.g., glucose transporter 1 deficiency syndrome (Glut1DS) and glycogen storage disease 1 (GSD1)) and under certain clinical circumstances (e.g., recurrent hypoglycemia), lactate may serve as a fuel to the brain [1]. This knowledge could be of use in developing treatment options for various neurometabolic disorders. However, a persisting negative connotation of lactate can be found almost everywhere else in medicine, especially in clinical practice since an increasing level of lactate in blood is a well-known marker for severe illness and hypoxia. The negative connotation of lactate can also be explained in a historical context. Lactate was first discovered in 1780 by Scheele [2] and in 1808, Berzelius found an elevated concentration of lactate in “the muscles of hunted stags.” A century and many studies on lactate later [3], Fletcher and Hopkins reported in their landmark paper in 1907 that the level of lactate in muscles is low in resting conditions, increases under conditions of hypoxia or when muscles are activated, and decreases again when placed in an oxygen-rich environment [4]. The theory that lactate production and accumulation are a result of the insufficient availability of oxygen became generally accepted.
Nonetheless, more recent studies again showed the other, beneficial side of lactate. In the eighties of last century, Brooks et al. formulated the “cell to cell lactate shuttle hypothesis,” which is based on the principle that one cell may excrete lactate that is formed after glycolysis, while another cell may take up and further process lactate for the benefit of energy metabolism [5, 6]. Other studies have also shown the beneficial side of lactate as an alternative energy source for different organs, including the brain [7, 8]. Lactate can cross the blood–brain barrier, and when oxidized in the brain, it can provide up to 8–10% of the brain’s energy requirement. This contribution could even be larger when the concentration of lactate in blood is artificially raised by lactate infusion [9,10,11]. Pellerin and Magistretti developed the astrocyte neuron lactate shuttle (ANLS) theory, which proposes that astrocytes take up glucose, turn it into lactate through aerobic glycolysis, and transport it to neurons, where it can be used as a source of energy [12, 13]. Thereafter, many studies concluded that their results are in accordance with the ANLS theory [14,15,16,17].
Unfortunately, there is still little attention for lactate and its beneficial side in clinical practice. The aim of this review is to thoroughly review available literature on the use of lactate in a clinical setting and to provide an overview of protocols infusing lactate in different patient groups. By doing so, we intend to bring attention to the potential therapeutic effects of lactate and to catalyze the discussion on the possible benefits of therapeutic lactate infusion among pediatricians and other pediatric specialists. We hope to increase the awareness of the therapeutic use of lactate and to facilitate the development of future clinical trial protocols in pediatric populations, which so often tremendously lag behind the developments in adults.
Materials and methods
A scoping review was performed, focusing on study designs and used protocols of lactate infusion in humans. The PRISMA guidelines were followed wherever possible (see supplemental text for the checklist and exact MeSH terms) [18]. We searched PubMed up to November 2021, and searched on clinicaltrial.gov for additional relevant studies not yet published. Limits were used for “English” and “Dutch.” After correction for duplicates, we identified 282 articles. Following screening of title and abstracts of all articles, 42 relevant articles remained. Once we checked references of all articles after 2010, we found another 76 articles, 9 of which were included. Exclusion criteria were articles without the aim to increase normal lactate concentration in blood and articles that did not infuse lactate in humans in a clinical setting. After applying these exclusion criteria, 34 articles remained; only one of which involved children. We abstracted data on patient characteristics (diagnosis, number of patients included), protocol details (infusion concentration, rate and duration, as well as total infused lactate, and peak blood concentration of lactate), and reported beneficial and adverse effects.
Results
The included studies are listed in Table 1. In all studies, solutions of sodium lactate were intravenously administered, except in the studies of Gallagher et al. and Jalloh et al. who used a technique based on cerebral microdialysis. Unless otherwise indicated, all studies included adults in their research.
Lactate as alternative energy source for the brain (n = 20/34 articles)
Sodium lactate has been infused to patients with diabetes mellitus [19,20,21,22,23,24,25], traumatic brain injury (TBI) [11, 26,27,28,29,30,31,32,33,34], and Alzheimer’s disease [35,36,37].
Studies among people with or without diabetes mellitus suggest that the brain is able to use lactate during hypoglycaemia [19,20,21,22,23,24,25]. Several studies showed that lactate administration suppresses the counterregulatory hormone and symptom responses to hypoglycemia [19, 20, 23, 25], whereas a fall in brain lactate concentration during hypoglycemia among people with type 1 diabetes and impaired awareness of hypoglycemia also suggests lactate consumption [38]. These studies conclude that lactate infusion reduces the awareness of hypoglycemia and prevents clinical signs and symptoms of neuroglycopenia.
TBI can be divided into primary and secondary injury. Primary injury is defined as brain damage directly resulting from external forces of trauma. Secondary injury develops minutes to days after the acute insult as a result of a complex cascade of metabolic and chemical changes induced by the primary tissue damage [39]. In TBI, a high lactate concentration in plasma predicts poor outcome. Interestingly, Gallagher et al. and Jalloh et al. showed that the brains of patients with and without TBI are able to utilize lactate as energy source [11, 33] when lactate was administered through local microdialysis. Additionally, Glenn et al. reported that the cerebral uptake of glucose in patients with TBI was significantly decreased compared to the control group. Nonetheless, the cerebral uptake of lactate did not differ between both groups and in both groups lactate was oxidized to CO2 [29]. Other studies showed that administering lactate was associated with a better long-term outcome than standard care in patients with TBI [26, 30,31,32, 34]. Moreover, studies showed that infusion of lactate in patients with TBI causes a less severe increase in intracranial pressure, a known and feared complication in TBI [27, 28, 33].
Lactate may lead to vasodilatation in cerebral vasculature [32]. Impaired cerebral microcirculation and glucose metabolism are established markers of Alzheimer’s disease. To our knowledge, three studies have investigated whether lactate could serve as an alternative energy source and positively influence cerebral blood flow in patients with Alzheimer’s disease [35,36,37]. Unfortunately, it did not seem to improve cognitive functioning of the patients.
Lactate infusion in cardiology (n = 4/34 articles)
It is well known that free fatty acids rather than glucose are the preferred energy substrate for the myocardium, but a stressed myocardium increases the net uptake and utilization of lactate [7]. Four studies have shown that hypertonic lactate infusion induces an increase in cardiac output compared with traditional infusion fluids after cardiac surgery [40,41,42,43]. Additionally, this was accomplished with less totally infused volume of sodium lactate than that of the comparator fluid, thus reducing the risk fluid overloads in patients with potentially compromised cardiac function [40,41,42,43]. None of the studies measured the uptake of lactate by the myocardium directly.
Lactate infusion in children (n = 1/34 articles)
One study investigated the infusion of hyperosmolar lactate in children with dengue shock syndrome [44]. For fluid resuscitation in dengue shock syndrome, the World Health Organization guideline requires large volumes of Ringer lactate, which might induce fluid overload. The effectiveness of the recommended volume of Ringer lactate versus a smaller volume of hyperosmolar lactate solution were compared and a similar hemodynamic shock recovery and plasma expansion were achieved in both groups, with a significantly lower fluid intake and fluid accumulation in the hyperosmolar lactate solution group, without major side effects. Unfortunately, the authors did not investigate whether lactate was used as an alternative energy source.
Possible adverse effects of sodium lactate infusion (n = 9/34 articles)
In 1967, Pitts and McClure noticed so-called exercise-induced attacks of anxiety in patients with “anxiety neurosis.” They found that during standard workouts, the level of lactate in blood significantly increased, and they postulated that the high concentration of lactate in blood provoked panic attacks [45]. This induced a curiosity for the neurobiological genesis of panic [46]. Up until now, the panic-inducing effect of lactate infusion has been demonstrated in animals and patients with panic attacks. Besides lactate, researchers developed a variety of other means to evoke panic attacks under controlled circumstances [47]. Studies infusing lactate to induce panic attacks generally used the same protocol as the original study of Pitts and McClure, resulting in blood lactate concentrations up to approximately 10 mmol/L [48,49,50,51,52,53,54,55,56]. However, panic attacks only occurred in studies set up to induce these attacks. No other studies with lactate concentrations up to 15 mmol/L reported panic attacks as an adverse event [42, 57].
An overview of noted adverse effects is shown in Table 1. These adverse effects include changes in the equilibrium of electrolytes, and change in pH using sodium lactate, especially an increase of serum sodium concentration might be a concern. However, only a few studies reported sodium increases in their patients or study participants (see Table 1). We found that all adverse effects, including increase of serum sodium concentrations were minor, none of which requiring treatment or interruption of the lactate infusion [19, 25,26,27,28, 32, 33, 40,41,42,43,44].
Summary of infusion protocols
Reviewing the included studies, we closely paid attention to the used study protocols. A summary of used doses, time of administration, and concentration of lactate in blood can be found in Table 1.
When evaluating the infusion protocols, we excluded the studies of Gallagher et al. and of Jalloh et al. since they did not infuse lactate intravenously [11, 33]. First, studies used an infusion fluid with a concentration of lactate between 400 and 1000 mmol/L (for comparison: Ringer’s lactate has a lactate concentration of 28 mmol/L). The lactate solutions used in the studies were either prepared by the pharmacy department of the investigating hospital, or purchased from an external company in the requested concentration. Second, the infusion rate and time differed greatly between protocols. The median infusion rate was 0.05 mmol of lactate per kg bodyweight per minute (mmol/kg/min) (mean 0.13 mmol/kg/min), with the lowest rate at 0.00417 mmol/kg/min and the highest rate at 0.76 mmol/kg/min. The median infusion time was 60 min (mean 235 min), with the shortest infusion time at 15 min and the longest infusion time 2880 min (48 h). In addition, there was a large variability in infusion rate and time in different studies investigating similar patient populations. Finally, due to the different infusion rate and time, the total amounts of infused lactate and the peak concentration of lactate in blood also varied among studies. The median total amount of lactate infused was 5.0 mmol/kg (mean 5.8 mmol/kg), with the lowest total amount of lactate of 0.15 mmol/kg and the highest total amount of lactate of 29.34 mmol/kg. The mean peak concentration of lactate in blood was 4.9 mmol/L (median 4.5 mmol/L), with the lowest peak concentration at 1.5 mmol/L and the highest at 14.8 mmol/L. None of the protocols explained the chosen lactate concentration, infusion rate or duration. The most reported side effects were changes in the equilibrium of electrolytes. None of the studies reported panic attacks. All side effects were minor, none of the patients needed treatment and the protocol of lactate infusion could continue in all cases.
Protocol for future studies
The protocols of the included studies (see Table 1) can help when drafting a protocol for a future study, but it is impossible to provide a protocol that would fit all future trials in pediatric patients. Besides the fact that children are not little adults, the details of such a protocol, e.g., the duration of infusion and the monitoring of specific parameters, would obviously depend on the disorder involved, and the aim of the study.
Previous studies have shown that the concentration of lactate in the brain increased rapidly following the raise of its blood concentration [9, 10]. For that reason it is suggested, when conducting a study to investigate lactate as an alternative energy source for the brain, to start the infusion with a bolus of sodium lactate for priming, followed by a continuous (but lower) infusion rate for the remainder of the study. In children, when infusing lactate for a limited duration of time, we consider it safe to target concentrations of lactate in blood that are normally reached during strenuous but physiological exercise, i.e., between 7.5 and 10 mmol/L [58].
When infusing lactate in children in a future study, we would advise to monitor vital signs including heartrate, respiratory rate, and blood pressure and specific parameters depending on the condition that is investigated. In relation to the possible adverse effect, we suggest to take frequent blood samples at baseline, during the infusion time and after infusion of lactate, to monitor pH and base excess, as well as concentrations of sodium, potassium, chloride, bicarbonate, lactate, and glucose in blood. Because of the sodium load, we would not include children with renal failure or chronic diarrhea / dehydration.
Discussion
This review shows that sodium lactate can be safely administrated to humans with insulin-induced hypoglycemia, TBI, Alzheimer’s disease, or heart failure. One study infused lactate in children, with the same minimal adverse effects as studies in adult populations. There are clear suggestions that the brain can use lactate as an energy source to compensate for the shortage of energy under conditions of hypoglycemia or as a potential therapeutic agent to limit the adverse consequences of TBI.
Nine studies that used lactate to induce panic attacks were included, although these studies did not use lactate infusion in a clinical setting. We decided to include these studies because of the possible risk to provoke panic attacks in future studies. However, these studies state that only patients with an anxiety disorder are susceptible to this provocation and a review of these studies reveals serious methodologic problems and the failure to consider cognitive variables. This renders the validity of these studies questionable [59]. Fortunately, panic attacks have not been reported in later studies involving healthy volunteers [60] and additionally, none of the other studies included in this review have reported panic attacks. It thus seems that the risk of panic attacks during lactate administration is small and, if at all present, restricted to patients with panic disorders.
Lactate infusion is not incorporated in standard care for any patient population. However, lactate infusion may be a possible treatment to improve the outcome of patients with TBI in the future [61,62,63,64,65]. To our knowledge, this review is the first to provide an overview of the therapeutic, albeit still experimental, use of lactate in clinical context. Previously, reviews outlining the basal metabolism of lactate have been published, these are beyond the scope of the current review [5, 7, 66,67,68,69,70,71]. Our review adds a focus on the clinical application of lactate infusion in humans by providing an overview of protocols used in literature to facilitate designing a protocol for future studies.
Additionally, it would be interesting to see if lactate could be used as an alternative energy source in children with a metabolic disorder, for example, Glut1DS, since the studies in this review suggest that lactate can be used by the adult brain as an alternative energy source. Difficulties in setting up a future study could be caused by the fact that so far only one study infused lactate in children. Little is known about the metabolism of lactate in children, and children are not little adults. So far, studies investigating the pharmacokinetic and pharmacodynamic properties of lactate infusion in pediatric patients have not been conducted. Obviously, such studies are of great importance to ensure safety of future clinical trials in children.
In conclusion, this review shows that lactate is an energy source for the adult brain. So far, one study investigated the infusion of lactate in children without major side effects. Additional studies to assess the safety of lactate infusion in children are needed, to allow future studies on its potential therapeutic effects, especially in children with rare inborn errors of metabolism. It will be also interesting to see if lactate infusion has beneficial effects in case of hypoglycemia (neuroglycopenia), TBI, or cardiac failure in pediatric populations. With this review, we hope to contribute to the discussion of lactate as potential therapeutic agent in children, and to facilitate future research.
Abbreviations
- ANLS:
-
Astrocyte neuron lactate shuttle
- Glut1DS:
-
Glucose transporter 1 deficiency syndrome
- GSD1:
-
Glycogen storage disease 1
- TBI:
-
Traumatic brain injury
References
Taher M, Leen WG, Wevers RA, Willemsen MA (2016) Lactate and its many faces. Eur J Paediatr Neurol 20:3–10. https://doi.org/10.1016/j.ejpn.2015.09.008
Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB (2018) Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 118:691–728. https://doi.org/10.1007/s00421-017-3795-6
Brooks GA, Gladden LB (2003) The metabolic systems: anaerobic metabolism (glycolytic and phosphagen). In: T CM (ed) In Exercise physiol. People and Ideas. Oxford University Press, New York, p. 322–360. https://doi.org/10.1016/B978-019512527-6.50009-X
Fletcher WM (1907) Lactic acid in amphibian muscle. J Physiol 35:247–309. https://doi.org/10.1113/jphysiol.1907.sp001194
Gladden LB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558:5–30. https://doi.org/10.1113/jphysiol.2003.058701
Brooks GA (1985) Lactate: Glycolytic product and oxidative substrate during sustained exercise in mammals – the ‘lactate shuttle.’ In: Gilles R (ed) Comparative physiology and biochemistry: current topics and trends, respiration-metabolism-circulation. Springer, Berlin, pp 208–218
van Hall G (2010) Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 199:499–508. https://doi.org/10.1111/j.1748-1716.2010.02122.x
Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B (2011) In vivo evidence for lactate as a neuronal energy source. J Neurosci 31:7477–7485. https://doi.org/10.1523/jneurosci.0415-11.2011
van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB (2009) Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29:1121–1129. https://doi.org/10.1038/jcbfm.2009.35
Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL (2010) The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 30:13983–13991. https://doi.org/10.1523/jneurosci.2040-10.2010
Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, Timofeev I, Menon DK, Kirkpatrick PJ, Pickard JD, Sutherland GR, Hutchinson PJ (2009) The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132:2839–2849. https://doi.org/10.1093/brain/awp202
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166. https://doi.org/10.1038/jcbfm.2011.149
Barros LF (2013) Metabolic signaling by lactate in the brain. Trends Neurosci 36:396–404. https://doi.org/10.1016/j.tins.2013.04.002
Bergersen LH (2015) Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab 35:176–185. https://doi.org/10.1038/jcbfm.2014.206
Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86:883–901. https://doi.org/10.1016/j.neuron.2015.03.035
Steinman MQ, Gao V, Alberini CM (2016) The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci 10:10. https://doi.org/10.3389/fnint.2016.00010
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D et al (2018) PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/m18-0850
Maran A, Cranston I, Lomas J, Macdonald I, Amiel SA (1994) Protection by lactate of cerebral function during hypoglycaemia. Lancet 343:16–20
Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J (1994) Effect of hyperketonemia and hyperlacticacidemia on symptoms, cognitive dysfunction, and counterregulatory hormone responses during hypoglycemia in normal humans. Diabetes 43:1311–1317
King P, Kong MF, Parkin H, MacDonald IA, Barber C, Tattersall RB (1998) Intravenous lactate prevents cerebral dysfunction during hypoglycaemia in insulin-dependent diabetes mellitus. Clin Sci (Lond) 94:157–163
King P, Parkin H, Macdonald IA, Barber C, Tattersall RB (1997) The effect of intravenous lactate on cerebral function during hypoglycaemia. Diabet Med 14:19–28. https://doi.org/10.1002/(sici)1096-9136(199701)14:1%3c19::Aid-dia289%3e3.0.Co;2-0
Maran A, Crepaldi C, Trupiani S, Lucca T, Jori E, Macdonald IA, Tiengo A, Avogaro A, Del Prato S (2000) Brain function rescue effect of lactate following hypoglycaemia is not an adaptation process in both normal and type I diabetic subjects. Diabetologia 43:733–741. https://doi.org/10.1007/s001250051371
De Feyter HM, Mason GF, Shulman GI, Rothman DL, Petersen KF (2013) Increased brain lactate concentrations without increased lactate oxidation during hypoglycemia in type 1 diabetic individuals. Diabetes 62:3075–3080. https://doi.org/10.2337/db13-0313
Wiegers EC, Rooijackers HM, Tack CJ, Philips BW, Heerschap A, van der Graaf M, de Galan BE (2018) Effect of lactate administration on brain lactate levels during hypoglycemia in patients with type 1 diabetes. J Cereb Blood Flow Metab 271678x18775884. https://doi.org/10.1177/0271678x18775884
Ichai C, Armando G, Orban JC, Berthier F, Rami L, Samat-Long C, Grimaud D, Leverve X (2009) Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med 35:471–479. https://doi.org/10.1007/s00134-008-1283-5
Ichai C, Payen JF, Orban JC, Quintard H, Roth H, Legrand R, Francony G, Leverve XM (2013) Half-molar sodium lactate infusion to prevent intracranial hypertensive episodes in severe traumatic brain injured patients: a randomized controlled trial. Intensive Care Med 39:1413–1422. https://doi.org/10.1007/s00134-013-2978-9
Bouzat P, Sala N, Suys T, Zerlauth JB, Marques-Vidal P, Feihl F, Bloch J, Messerer M, Levivier M, Meuli R, Magistretti PJ, Oddo M (2014) Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med 40:412–421. https://doi.org/10.1007/s00134-013-3203-6
Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda DA, Vespa P, Brooks GA (2015) Lactate: Brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma 32:820–832. https://doi.org/10.1089/neu.2014.3483
Bisri T, Utomo BA, Fuadi I (2016) Exogenous lactate infusion improved neurocognitive function of patients with mild traumatic brain injury. Asian J Neurosurg 11:151–159. https://doi.org/10.4103/1793-5482.145375
Quintard H, Patet C, Zerlauth JB, Suys T, Bouzat P, Pellerin L, Meuli R, Magistretti PJ, Oddo M (2016) Improvement of neuroenergetics by hypertonic lactate therapy in patients with traumatic brain injury is dependent on baseline cerebral lactate/pyruvate ratio. J Neurotrauma 33:681–687. https://doi.org/10.1089/neu.2015.4057
Carteron L, Solari D, Patet C, Quintard H, Miroz JP, Bloch J, Daniel RT, Hirt L, Eckert P, Magistretti PJ, Oddo M (2018) Hypertonic lactate to improve cerebral perfusion and glucose availability after acute brain injury. Crit Care Med 46:1649–1655. https://doi.org/10.1097/ccm.0000000000003274
Jalloh I, Helmy A, Howe DJ, Shannon RJ, Grice P, Mason A, Gallagher CN, Murphy MP, Pickard JD, Menon DK, Carpenter TA, Hutchinson PJ, Carpenter KLH (2018) A comparison of oxidative lactate metabolism in traumatically injured brain and control brain. J Neurotrauma 35:2025–2035. https://doi.org/10.1089/neu.2017.5459
Wolahan SM, Mao HC, Real C, Vespa PM, Glenn TC (2018) Lactate supplementation in severe traumatic brain injured adults by primed constant infusion of sodium L-lactate. J Neurosci Res 96:688–695. https://doi.org/10.1002/jnr.24085
Jardanhazy A, Jardanhazy T, Kalman J (2008) Sodium lactate differently alters relative EEG power and functional connectivity in Alzheimer’s disease patients’ brain regions. Eur J Neurol 15:150–155. https://doi.org/10.1111/j.1468-1331.2007.02016.x
Kalman J, Palotas A, Bodi N, Kincses TZ, Benedek G, Janka Z, Antal A (2005) Lactate infusion fails to improve semantic categorization in Alzheimer’s disease. Brain Res Bull 65:533–539. https://doi.org/10.1016/j.brainresbull.2005.03.009
Kalman J, Palotas A, Kis G, Boda K, Turi P, Bari F, Domoki F, Doda I, Argyelan M, Vincze G, Sera T, Csernay L, Janka Z, Pavics L (2005) Regional cortical blood flow changes following sodium lactate infusion in Alzheimer’s disease. Eur J Neurosci 21:1671–1678. https://doi.org/10.1111/j.1460-9568.2005.03924.x
Wiegers EC, Rooijackers HM, Tack CJ, Heerschap A, de Galan BE, van der Graaf M (2016) Brain lactate concentration falls in response to hypoglycemia in patients with type 1 diabetes and impaired awareness of hypoglycemia. Diabetes 65:1601–1605. https://doi.org/10.2337/db16-0068
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741. https://doi.org/10.1016/s1474-4422(08)70164-9
Boom CE, Herdono P, Koto CG, Hadi S, Permana IM (2013) Effect of hyperosmolar sodium lactate infusion on haemodynamic status and fluid balance compared with hydroxyethyl starch 6% during the cardiac surgery. Indian J Anaesth 57:576–582. https://doi.org/10.4103/0019-5049.123330
Leverve XM, Boon C, Hakim T, Anwar M, Siregar E, Mustafa I (2008) Half-molar sodium-lactate solution has a beneficial effect in patients after coronary artery bypass grafting. Intensive Care Med 34:1796–1803. https://doi.org/10.1007/s00134-008-1165-x
Mustafa I, Leverve XM (2002) Metabolic and hemodynamic effects of hypertonic solutions: Sodium-lactate versus sodium chloride infusion in postoperative patients. Shock 18:306–310
Nalos M, Leverve X, Huang S, Weisbrodt L, Parkin R, Seppelt I, Ting I, McLean A (2014) Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: a pilot randomised controlled clinical trial. Crit Care 18:R48. https://doi.org/10.1186/cc13793
Somasetia DH, Setiati TE, Sjahrodji AM, Idjradinata PS, Setiabudi D, Roth H, Ichai C, Fontaine E, Leverve XM (2014) Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium-lactate: a randomized single-blind clinical trial of efficacy and safety. Crit Care 18:466. https://doi.org/10.1186/s13054-014-0466-4
Pitts FN Jr, McClure JN Jr (1967) Lactate metabolism in anxiety neurosis. N Engl J Med 277:1329–1336. https://doi.org/10.1056/nejm196712212772502
Kellner M (2011) Experimental panic provocation in healthy man-a translational role in anti-panic drug development? Dialogues Clin Neurosci 13:485–493
Nutt D, Lawson C (1992) Panic attacks. A neurochemical overview of models and mechanisms. Br J Psychiatry 160:165–178
Dager SR, Friedman SD, Heide A, Layton ME, Richards T, Artru A, Strauss W, Hayes C, Posse S (1999) Two-dimensional proton echo-planar spectroscopic imaging of brain metabolic changes during lactate-induced panic. Arch Gen Psychiatry 56:70–77
Dager SR, Marro KI, Richards TL, Metzger GD (1992) Localized magnetic resonance spectroscopy measurement of brain lactate during intravenous lactate infusion in healthy volunteers. Life Sci 51:973–985
Dager SR, Marro KI, Richards TL, Metzger GD (1994) Preliminary application of magnetic resonance spectroscopy to investigate lactate-induced panic. Am J Psychiatry 151:57–63. https://doi.org/10.1176/ajp.151.1.57
Dager SR, Richards T, Strauss W, Artru A (1997) Single-voxel 1H-MRS investigation of brain metabolic changes during lactate-induced panic. Psychiatry Res 76:89–99
Friedman SD, Dager SR, Richards TL, Petropoulos H, Posse S (2000) Modeling brain compartmental lactate response to metabolic challenge: a feasibility study. Psychiatry Res 98:55–66
Layton ME, Friedman SD, Dager SR (2001) Brain metabolic changes during lactate-induced panic: Effects of gabapentin treatment. Depress Anxiety 14:251–254
Otte C, Kellner M, Arlt J, Jahn H, Holsboer F, Wiedemann K (2002) Prolactin but not ACTH increases during sodium lactate-induced panic attacks. Psychiatry Res 109:201–205
van der Molen GM, van den Hout MA (1988) Expectancy effects on respiration during lactate infusion. Psychosom Med 50:439–443
Keck PE Jr, Taylor VE, Tugrul KC, McElroy SL, Bennett JA (1993) Valproate treatment of panic disorder and lactate-induced panic attacks. Biol Psychiatry 33:542–546
Wiegers EC, Rooijackers HM, Tack CJ, Groenewoud H, Heerschap A, de Galan BE, van der Graaf M (2017) Effect of exercise-induced lactate elevation on brain lactate levels during hypoglycemia in patients with type 1 diabetes and impaired awareness of hypoglycemia. Diabetes 66:3105–3110. https://doi.org/10.2337/db17-0794
Smith EW, Skelton MS, Kremer DE, Pascoe DD, Gladden LB (1997) Lactate distribution in the blood during progressive exercise. Med Sci Sports Exerc 29:654–660. https://doi.org/10.1097/00005768-199705000-00011
Margraf J, Ehlers A, Roth WT (1986) Sodium lactate infusions and panic attacks: a review and critique. Psychosom Med 48:23–51
Schenberg LC (2010) Towards a translational model of panic attack. Psychol Neurosci 3:9–37. https://doi.org/10.3922/j.psns.2010.1.003
Brooks GA, Martin NA (2014) Cerebral metabolism following traumatic brain injury: New discoveries with implications for treatment. Front Neurosci 8:408. https://doi.org/10.3389/fnins.2014.00408
Bouzat P, Oddo M (2014) Lactate and the injured brain: Friend or foe? Curr Opin Crit Care 20:133–140. https://doi.org/10.1097/mcc.0000000000000072
Gantner D, Moore EM, Cooper DJ (2014) Intravenous fluids in traumatic brain injury: What’s the solution? Curr Opin Crit Care 20:385–389. https://doi.org/10.1097/mcc.0000000000000114
Carpenter KL, Jalloh I, Hutchinson PJ (2015) Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci 9:112. https://doi.org/10.3389/fnins.2015.00112
Bouzat P, Sala N, Payen JF, Oddo M (2013) Beyond intracranial pressure: Optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann Intensive Care 3:23. https://doi.org/10.1186/2110-5820-3-23
Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587:5591–5600. https://doi.org/10.1113/jphysiol.2009.178350
Gladden LB (2008) A lactatic perspective on metabolism. Med Sci Sports Exerc 40:477–485. https://doi.org/10.1249/MSS.0b013e31815fa580
Quistorff B, Secher NH, Van Lieshout JJ (2008) Lactate fuels the human brain during exercise. Faseb j 22:3443–3449. https://doi.org/10.1096/fj.08-106104
Dalsgaard MK (2006) Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab 26:731–750. https://doi.org/10.1038/sj.jcbfm.9600256
Rabinowitz JD, Enerbäck S (2020) Lactate: the ugly duckling of energy metabolism. Nat Metab 2:566–571. https://doi.org/10.1038/s42255-020-0243-4
Goodwin ML, Gladden LB, Nijsten MWN (2020) Lactate-protected hypoglycemia (LPH). Front Neurosci 14:920. https://doi.org/10.3389/fnins.2020.00920
Author information
Authors and Affiliations
Contributions
LVG collected the data, drafted the initial manuscript, and reviewed and revised the manuscript. BDG, RW, and RTH advised on conceptualizing the study and critically reviewed the manuscript for important intellectual content. MW conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Consent for publication
The authors have approved the manuscript and agree with its submission to European Journal of Pediatrics.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Gemert, L.A., de Galan, B.E., Wevers, R.A. et al. Lactate infusion as therapeutical intervention: a scoping review. Eur J Pediatr 181, 2227–2235 (2022). https://doi.org/10.1007/s00431-022-04446-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04446-3