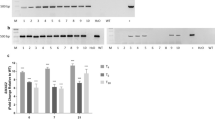
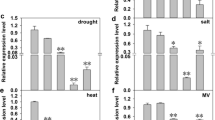
Abstract
Main conclusion
Overexpression of the citrus CsTIP2;1 improves plant growth and tolerance to salt and drought stresses by enhancing cell expansion, H 2 O 2 detoxification and stomatal conductance.
Tonoplast intrinsic proteins (TIPs) are a subfamily of aquaporins, belonging to the major intrinsic protein family. In a previous study, we have shown that a citrus TIP isoform, CsTIP2;1, is highly expressed in leaves and also transcriptionally regulated in leaves and roots by salt and drought stresses and infection by ‘Candidatus Liberibacter asiaticus’, the causal agent of the Huanglongbing disease, suggesting its involvement in the regulation of the flow of water and nutrients required during both normal growth and stress conditions. Here, we show that the overexpression of CsTIP2;1 in transgenic tobacco increases plant growth under optimal and water- and salt-stress conditions and also significantly improves the leaf water and oxidative status, photosynthetic capacity, transpiration rate and water use efficiency of plants subjected to a progressive soil drying. These results correlated with the enhanced mesophyll cell expansion, midrib aquiferous parenchyma abundance, H2O2 detoxification and stomatal conductance observed in the transgenic plants. Taken together, our results indicate that CsTIP2;1 plays an active role in regulating the water and oxidative status required for plant growth and adaptation to stressful environmental conditions and may be potentially useful for engineering stress tolerance in citrus and other crop plants.









Similar content being viewed by others
Abbreviations
- DAB:
-
3,3′-Diaminobenzidine
- MIP:
-
Major intrinsic protein
- RWC:
-
Relative water content
- WT:
-
Wild type
- WUE:
-
Water use efficiency
References
Azad AK, Yoshikawa N, Ishikawa T, Sawa Y, Shibata H (2012) Substitution of a single amino acid residue in the aromatic/arginine selectivity filter alters the transport profiles of tonoplast aquaporin homologs. Biochim Biophys Acta 1818:1–11
Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125:1206–1215
Cidade LC, de Oliveira TM, Mendes AFS, Macedo AF, Floh EIS, Gesteira AS, Soares-Filho WS, Costa MGC (2012) Ectopic expression of a fruit phytoene synthase from Citrus paradisi Macf. promotes abiotic stress tolerance in transgenic tobacco. Mol Biol Rep 39:10201–10209
Dynowski M, Schaaf G, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414:53–61
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Fleurat-Lessard P, Michonneau P, Maeshima M, Drevon J-J, Serraj R (2005) The distribution of aquaporin subtypes (PIP1, PIP2 and g-TIP) is tissue dependent in soybean (Glycine max) root nodules. Ann Bot 96:457–460
Flexas J, Ribas-Carbo M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48:427–439
Fouquet R, Léon C, Ollat N, Barrieu F (2008) Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep 27:1541–1550
Fraysse LC, Wells B, McCann MC, Kjellbom P (2005) Specific plasma membrane aquaporins of the PIP1 subfamily are expressed in sieve elements and guard cells. Biol Cell 97:519–534
Gomes D, Agasse A, Thiébaud P, Delrot S, Gerós H, Chaumont F (2009) Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta 1788:1213–1228
Gupta AB, Sankararamakrishnan R (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9:134
Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45:521–529
Higuchi T, Suga S, Tsuchiya T, Hisada H, Morishima S, Okada Y, Maeshima M (1998) Molecular cloning, water channel activity and tissue specific expression of two isoforms of radish vacuolar aquaporin. Plant Cell Physiol 39:905–913
Hove RM, Bhave M (2011) Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol Biol 75:413–430
Hu W, Yuan Q, Wang Y, Cai R, Deng X, Wang J, Zhou S, Chen M, Chen L, Huang C, Ma Z, Yang G, He G (2012) Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol 53:2127–2141
Jauh GY, Fischer AM, Grimes HD, Ryan CA, Rogers JC (1998) δ-Tonoplast intrinsic protein defines unique plant vacuole functions. Proc Natl Acad Sci USA 95:12995–12999
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Johansen DA (1940) Plant microtechnique. MacGraw Hill, New York
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126:1358–1369
Kaldenhoff R, Kolling A, Meyers J, Karmann U, Ruppel G, Richter G (1995) The blue light-responsive AthH2 gene of Arabidopsis thaliana is primarily expressed in expanding as well as in differentiating cells and encodes a putative channel protein of the plasmalemma. Plant J 7:87–95
Kawase M, Hanba YT, Katsuhara M (2013) The photosynthetic response of tobacco plants overexpressing ice plant aquaporin McMIPB to a soil water deficit and high vapor pressure deficit. J Plant Res 126:517–527
Lin WL, Peng YH, Li GW, Arora R, Tang ZC, Su WA, Cai WM (2007) Isolation and functional characterization of PgTIP1, a hormone autotrophic cells-specific tonoplast intrinsic protein in ginseng. J Exp Bot 58:947–956
Loqué D, Ludewig U, Yuan L, von Wirén N (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137:671–680
Martins CPS, Pedrosa AM, Du D, Gonçalves LP, Yu Q, Gmitter FG Jr, Costa MGC (2015) Genome-wide characterization and expression analysis of major intrinsic proteins during abiotic and biotic stresses in sweet orange (Citrus sinensis L. Osb.). PLoS One 10(9):e0138786
Maurel C, Tacnet F, Güclü J, Guern J, Ripoche P (1997) Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. Proc Natl Acad Sci USA 94:7103–7108
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Maurel C, Santoni V, Luu D-T, Wudick MM, Verdoucq L (2009) The cellular dynamics of plant aquaporin expression and functions. Curr Opin Plant Biol 12:690–698
Okubo-Kurihara E, Sano T, Higaki T, Kutsuna N, Hasezawa S (2009) Acceleration of vacuolar regeneration and cell growth by overexpression of an aquaporin NtTIP1;1 in tobacco BY-2 cells. Plant Cell Physiol 50:151–160
Otto B, Kaldenhoff R (2000) Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 211:167–172
Peng Y, Lin W, Cai W, Arora R (2007) Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 226:729–740
Prado K, Maurel C (2013) Regulation of leaf hydraulics: from molecular to whole plant levels. Front Plant Sci 4:255
Prado K, Boursiac Y, Tournaire-Roux C, Monneuse JM, Postaire O, Da Ines O, Schäffner AR, Hem S, Santoni V, Maurel C (2013) Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell 25:1029–1039
Reisen D, Leborgne-Castel N, Özalp C, Chaumont F, Marty F (2003) Expression of a cauliflower tonoplast aquaporin tagged with GFP in tobacco suspension cells correlates with an increase in cell size. Plant Mol Biol 52:387–400
Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, Moshelion M (2010) The role of tobacco aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol 152:245–254
Sakr S, Alves G, Morillon R, Maurel K, Decourteix M, Guilliot A, Fleurat-Lessard P, Julien JL, Chrispeels MJ (2003) Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiol 133:630–641
Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analyses of their expression and function. Plant Cell Physiol 45:1568–1577
Sarda X, Tousch D, Ferrare K, Legrand E, Dupuis JM, CasseDelbart F, Lamaze T (1997) Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J 12:1103–1111
Secchi F, Zwieniecki MA (2010) Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ 33:1285–1297
Slater A, Scott NW, Fowler MR (2008) Plant biotechnology: the genetic manipulation of plants. Oxford University Press, New York
Sun MH, Wen X, Zhu YF, Su WA, Tang ZC (2007) A simple method for in situ hybridization to RNA in guard cells of Vicia faba L.: the expression of aquaporins in guard cells. Plant Mol Biol Rep 19:129–135
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425:734–737
Wang X, Li Y, Ji W, Bai X, Cai H, Zhu D, Sun X-L, Chen L-J, Zhu Y-M (2011) A novel Glycine soja tonoplast intrinsic protein gene responds to abiotic stress and depresses salt and dehydration tolerance in transgenic Arabidopsis thaliana. J Plant Physiol 168:1241–1248
Wudick MM, Luu D-T, Maurel C (2009) A look inside: localization patterns and functions of intracellular plant aquaporins. New Phytol 184:289–302
Zhou S, Hu W, Deng X, Ma Z, Chen L, Huang C, Wang C, Wang J, He Y, Yang G, He G (2012) Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS One 7(12):e52439
Acknowledgements
This work was supported by research grants from Embrapa (Macroprograma 2), CNPq (Brasília, Brazil) and FAPESP (São Paulo, Brazil). We gratefully acknowledge the Ph.D. Degree scholarship to C.P.S. Martins by CAPES (Brasília, Brazil).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martins, C.P.S., Neves, D.M., Cidade, L.C. et al. Expression of the citrus CsTIP2;1 gene improves tobacco plant growth, antioxidant capacity and physiological adaptation under stress conditions. Planta 245, 951–963 (2017). https://doi.org/10.1007/s00425-017-2653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2653-4