Abstract
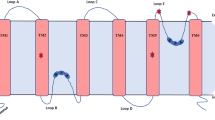
Research in recent years on plant Major Intrinsic Proteins (MIPs), commonly referred to as ‘aquaporins’, has seen a vast expansion in the substrates found to be transported via these membrane channels. The diversity in sizes, chemical nature and physiological significance of these substrates has meant a need to critically analyse the possible structural and biochemical properties of MIPs that transport these, in order to understand their roles. In this work we have undertaken a comprehensive analysis of all plant MIPs, coming from different families, that have been proven to transport ammonia, boron, carbon dioxide, hydrogen peroxide, silicon and urea. The sequences were analysed for all primary selectivity-related motifs (NPA motifs, ar/R filter, P1–P5 residues). In addition, the putative regulatory phosphorylation and glycosylation sites and mechanistic regulators such as loop lengths have been analysed. Further, nine specificity-determining positions (SDPs) were predicted for each group. The results show the ar/R filter residues, P2–P4 positions and some of the SDPs are characteristic for certain groups, and O-glycosylation sites are unique to a subgroup while N-glycosylation was characteristic of the other MIPs. Certain residues, especially in loop C, and structural parameters such as loop lengths also contribute to the uniqueness of groups. The comprehensive analysis makes significant inroads into appraising the intriguing diversity of plant MIPs and their roles in fundamental life processes, and provides tools for plant selections, protein engineering and transgenics.




Similar content being viewed by others
Abbreviations
- AEF:
-
Alanine-glutamic acid-phenyl alanine
- GIP:
-
GlpF-like intrinsic proteins
- HIP:
-
Hybrid intrinsic proteins
- MIP:
-
Major intrinsic proteins
- NIP:
-
NOD26-like intrinsic proteins
- NPA:
-
Asparagine-proline-alanine
- ar/R:
-
Aromatic/arginine
- P1–P5:
-
Residues at P1 to P5 positions
- PIP:
-
Plasma membrane intrinsic proteins
- TIP:
-
Tonoplast intrinsic proteins
- TM:
-
Transmembrane helix
- SIP:
-
Small, basic intrinsic proteins
- XIP:
-
Uncategorized X intrinsic proteins
References
Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15:439–447
Alleva K, Niemietz CM, Maurel C, Parisi M, Tyerman SD, Amodeo G (2006) Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J Exp Bot 57:609–621
Baker D, Sali A (2001) Protein structure prediction and structural genomics. Science 294:93–96
Baumgarten R, Van De Pol MH, Wetzels JF, Van Os CH, Deen PM (1998) Glycosylation is not essential for vasopressin dependent routing of aquaporin-2 in transfected Madin–Darby canine kidney cells. J Am Soc Nephrol 9:1553–1559
Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T (2006) Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 103:269–274
Benkert P, Tosatto SCE, Schomburg D (2008) QMEAN: a comprehensive scoring function for model quality assessment. Proteins Struct Funct Bioinform 71:261–277
Bertl A, Kaldenhoff R (2007) Function of a separate NH3-pore in Aquaporin TIP2;2 from wheat. FEBS Lett 581:5413–5417
Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18:565–570
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta Biomembranes 1758:994–1003
Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192
Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijo JA, Becker JD (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol 148:1168–1181
Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ (2001) Futile transmembrane NH4 + cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA 98:4255–4258
Buck TM, Wagner J, Grund S, Skach WR (2007) A novel tripartite motif involved in aquaporin topogenesis, monomer folding and tetramerization. Nat Struct Mol Biol 14:762–769
Bykova NV, Rampitsch C, Krokhin O, Standing KG, Ens W (2006) Determination and characterization of site-specific N-glycosylation using MALDI–Qq–TOF tandem mass spectrometry: case study with a plant protease. Anal Chem 78:1093–1103
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Chiba Y, Mitani N, Yamaji N, Ma JF (2009) HvLsi1 is a silicon influx transporter in barley. Plant J 57:810–818
Choi WG, Roberts DM (2007) Arabidopsis NIP2;1: a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem 282:24209–24218
Danielson JA, Johanson U (2008) Unexpected complexity of the Aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol 8:45
de Groot BL, Frigato T, Helms V, Grubmuller H (2003) The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol 333:279–293
Dean RM, Rivers RL, Zeidel ML, Roberts DM (1999) Purification and functional reconstitution of soybean nodulin 26. An aquaporin with water and glycerol transport properties. Biochem 38:347–353
Dell B, Huang L (1997) Physiological response of plants to low boron. Plant Soil 193:103–120
Dordas C, Chrispeels MJ, Brown PH (2000) Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiol 124:1349–1362
Dynowski M, Mayer M, Moran O, Ludewig U (2008a) Molecular determinants of ammonia and urea conductance in plant aquaporin homologs. FEBS Lett 582:2458–2462
Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008b) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414:53–61
Eckert M, Biela A, Siefritz F, Kaldenhoff R (1999) New aspects of plant aquaporin regulation and specificity. J Exp Bot 50:1541–1545
Engel A, Fujiyoshi Y, Agre P (2000) The importance of aquaporin water channel protein structures. EMBO J 19:800–806
Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16:215–228
Fitzpatrick KL, Reid R (2009) The involvement of aquaglyceroporins in transport of boron in barley root. Plant Cell Environ 32:1357–1365
Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48:427–439
Forrest KL, Bhave M (2007) Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Funct Integr Genomics 7:263–289
Froger A, Tallur B, Thomas D, Delamarche C (1998) Prediction of functional residues in water channels and related proteins. Protein Sci 7:1458–1468
Fu D, Libson A, Miercke LJW, Weitzman C, Nollert P, Krucinski J, Stroud RM (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290:481–486
Gaspar M, Bousser A, Sissoëff I, Roche O, Hoarau J, Mahé A (2003) Cloning and characterization of ZmPIP1–5b, an aquaporin transporting water and urea. Plant Sci 165:21–31
Gerbeau P, Güçlü J, Pierre R, Maurel C (1999) Aquaporin Nt-TIPa can account for the high permeability of tobacco cell vacuolar membrane to small neutral solutes. Plant J 18:577–587
Guenther JF, Roberts DM (2000) Water-selective and multifunctional aquaporins from Lotus japonicus nodules. Planta 210:741–748
Guenther JF, Chanmanivone N, Galetovic MP, Wallace IS, Cobb JA, Roberts DM (2003) Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 15:981–991
Gupta AB, Sankararamakrishnan R (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9:134–162
Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45:521–529
Hedfalk K, Bill RM, Mullins JG, Karlgren S, Filipsson C, Bergstrom J, Tamas MJ, Rydstrom J, Hohmann S (2004) A regulatory domain in the C-terminal extension of the yeast glycerol channel Fps1p. J Biol Chem 279:14954–14960
Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot 60:2971–2985
Hendriks G, Koudijs M, van Balkom BW, Oorschot V, Klumperman J, Deen PM, van der Sluijs P (2004) Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J Biol Chem 279:2975–2983
Henzler T, Steudle E (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 51:2053–2066
Heymann JB, Engel A (2000) Structural clues in the sequences of the aquaporins. J Mol Biol 295:1039–1053
Holm LM, Jahn TP, Moller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T (2005) NH3 and NH4 + permeability in aquaporin-expressing Xenopus oocytes. Pflüg Arch 450:415–428
Hub JS, de Groot BL (2006) Does CO2 permeate through Aquaporin-1? Biophys J 93:842–848
Jahn TP, Møller ALB, Zeuthen T, Holm LM, Klærke DA, Mohsin B, Kühlbrandt W, Schjoerring JK (2004) Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett 574:31–36
Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10:451–459
Johnson KD, Chrispeels MJ (1992) Tonoplast-bound protein kinase phosphorylates tonoplast intrinsic protein. Plant Physiol 100:1787–1795
Kalinina OV, Novichkov PS, Mironov AA, Gelfand MS, Rakhmaninova AB (2004) SDPpred: a tool for prediction of amino acid residues that determine differences in functional specificity of homologous proteins. Nucleic Acids Res 32:W424–W428
Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, Fujiwara T (2009) NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem 284:2114–2120
Kato Y, Miwa K, Takano J, Wada M, Fujiwara T (2009) Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant Cell Physiol 50:58–66
Katsuhara M, Hanba YT (2008) Barley plasma membrane intrinsic proteins (PIP aquaporins) as water and CO2 transporters. Pflugers Arch Eur J Physiol 456:687–691
Katsuhara M, Shibasaka M (2007) Barley root hydraulic conductivity and aquaporins expression in relation to salt tolerance. Soil Sci Plant Nutr 53:466–470
Klebl F, Wolf M, Sauer N (2003) A defect in the yeast plasma membrane urea transporter Dur3p is complemented by CpNIP1, a Nod26-like protein from zucchini (Cucurbita pepo L.), and by Arabidopsis thaliana delta-TIP or gamma-TIP. FEBS Lett 547:69–74
Kong Y, Ma G (2001) Dynamic mechanisms of the membrane water channel aquaporin-1 (AQP1). Proc Natl Acad Sci USA 98:14345–14349
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291
Litman T, Sogaard R, Zeuthen T (2009) Ammonia and urea permeability of mammalian aquaporins. In: Aquaporins, Beitz E (ed) Handbook of experimental pharmacology. Springer, Berlin, 190: 327–358
Liu L-H, Ludewig U, Gassert B, Frommer WB, von Wiren N (2003) Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiol 133:1220–1228
Loque D, Ludewig U, Yuan LX, von Wiren N (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137:671–680
Ludewig U, Dynowski M (2009) Plant aquaporin selectivity: where transport assays, computer simulations and physiology meet. Cell Mol Life Sci 66:3161–3175
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, Zhao F-J (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935
Maurel C, Kado RT, Guern J, Chrispeels MJ (1995) Phosphorylation regulates the water channel activity of the seed-specific aquaporin -TIP. EMBO J 14:3028–3035
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Miao GH, Hong Z, Verma DPS (1992) Topology and phosphorylation of soybean nodulin-26, an intrinsic protein of the peribacteroid membrane. J Cell Biol 118:481–490
Mitani N, Yamaji N, Ma JF (2008) Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch 456:679–686
Mitani N, Yamaji N, Ma JF (2009) Identification of maize silicon influx transporters. Plant Cell Physiol 50:5–12
Newby ZE, O’Connell J III, Robles-Colmenares Y, Khademi S, Miercke LJ, Stroud RM (2008) Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol 15:619–625
Niemietz CM, Tyerman SD (2000) Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett 465:110–114
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88:11110–11114
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Bio 50:2–18
Ramachandran GN, Ramakrishnan C, Sasisekharan V (1963) Stereochemistry of polypeptide chain configurations. J Mol Biol 7:95–99
Rougé P, Barre A (2008) A molecular modeling approach defines a new group of Nodulin 26-like aquaporins in plants. Biochem Biophys Res Commun 367:60–66
Santoni V, Verdoucq L, Sommerer N, Vinh J, Pflieger D, Maurel C (2006) Methylation of aquaporins in plant plasma membrane. Biochem J 400:189–197
Schnurbusch T, Hayes J, Hrmova M, Baumann U, Ramesh S, Tyerman S, Langridge P, Sutton T (2010) Boron toxicity tolerance through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiol 153:1706–1715
Soto G, Alleva K, Mazella MA, Amodeo G, Muschietti JP (2008) AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett 582:4077–4082
Sui H, Han B-G, Lee JK, Walian P, Jap BK (2001) Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878
Tajkhorshid E, Nollert P, Jensen MO, Miercke LJW, O’Connell J, Stroud RM, Schulten K (2002) Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296:525–530
Takano J, Wada M, Ludewig U, Schaaf G, von Wiren N, Fujiwara T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18:1498–1509
Tamura K, Dudley J, Nei M, Kumar S (2007) Mega4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T (2008) NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20:2860–2875
Terashima I, Ono K (2002) Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol 43:70–78
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354
Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P (2006) Structural mechanism of plant aquaporin gating. Nature 439:688–694
Tyerman S, Bohnert H, Maurel C, Steudle E, Smith J (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Expt Bot 50:1055–1071
Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425:734–737
Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R (2008) Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20:648–657
Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (2004) Novel regulation of aquaporins during osmotic stress. Plant Physiol 135:2318–2329
Wallace IS, Roberts DM (2004) Homology modelling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiol 135:1059–1068
Wallace IS, Roberts DM (2005) Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44:16826–16834
Wallace IS, Wills DM, Guenther JF, Roberts DM (2002) Functional selectivity for glycerol of the nodulin 26 subfamily of plant membrane intrinsic proteins. FEBS Lett 523:109–112
Wallace IS, Choi WG, Roberts DM (2006) The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim Biophys Acta 1758:1165–1175
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. J Exp Bot 55:411–422
Wang Y, Cohen J, Boron WF, Schulten K, Tajkhorshid E (2007) Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J Struct Biol 157:534–544
Wang W-H, Kohler B, Cao F-Q, Liu L-H (2008) Molecular and physiological aspects of urea transport in higher plants. Plant Sci 175:467–477
Wu B, Beitz E (2007) Aquaporins with selectivity for unconventional permeants. Cell Mol Life Sci 64:2413–2421
Yamaji N, Mitani N, Ma JF (2008) A transporter regulating silicon distribution in rice shoot. Plant Cell 20:1381–1389
Ye R, Verkman AS (1989) Simultaneous optical measurement of osmotic and diffusional water permeability in cells and liposomes. Biochemistry 28:824–829
Ye Q, Wiera B, Steudle E (2004) A cohesion/tension mechanism explains the gating of water channels (aquaporins) in Chara internodes by high concentration. J Exp Bot 55:449–461
Zardoya R, Villalba S (2001) A phylogenetic framework for the aquaporin family in eukaryotes. J Mol Evol 52:391–404
Acknowledgments
The authors are most grateful to the two anonymous reviewers whose critical comments and suggestions helped greatly in enhancing the quality of this submission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hove, R.M., Bhave, M. Plant aquaporins with non-aqua functions: deciphering the signature sequences. Plant Mol Biol 75, 413–430 (2011). https://doi.org/10.1007/s11103-011-9737-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-011-9737-5