Abstract
Contemporary criminal investigations are based on the statements made by the victim and the eyewitnesses. They also rely on the physical evidences found in the crime scene. These evidences, and more particularly biological ones, have a great judicial value in the courtroom. They are usually used to revoke the suspect’s allegations and confirm or refute the statements made by the victim and the witnesses. Stains of body fluids are biological evidences highly sought by forensic investigators. In many criminal cases, the success of the investigation relies on the correct identification and classification of these stains. Therefore, the adoption of reliable and accurate forensic analytical methods seems to be of vital importance to attain this objective. Attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) is a modern and universal analytical technique capable of fingerprint recognition of the analyte using minimal amount of the test sample. The current systematic review aims to through light on the fundamentals of this technique and to illustrate its wide range of applications in forensic investigations. ATR-FTIR is a nondestructive technique which has demonstrated an exceptional efficiency in detecting, identifying and discriminating between stains of various types of body fluids usually encountered in crime scenes. The ATR-FTIR spectral data generated from bloodstains can be used to deduce a wealth of information related to the donor species, age, gender, and race. These data can also be exploited to discriminate between stains of different types of bloods including menstrual and peripheral bloods. In addition, ATR-FTIR has a great utility in the postmortem investigations. More particularly, in estimating the postmortem interval and diagnosing death caused by extreme weather conditions. It is also useful in diagnosing some ambiguous death causes such as fatal anaphylactic shock and diabetic ketoacidosis.
Similar content being viewed by others
Introduction
The reliability of a criminal justice system relies on its ability to solve crimes in a timely and efficient manner. This endeavor is vital for establishing a successful law enforcement policy by providing justice to victims and convicting criminals. Effective crime solving requires the intervention of competent team of forensic scientists and investigators. Those professionals must be able to perform a careful and meticulous examination of the crime scene to identify, collect, document and preserve all types of physical evidences in a proper manner. They should also be able to create hypotheses based on the available evidences to reconstruct the crime scene in a realistic way. Solving crimes also requires the adoption of reliable and accurate forensic techniques to ensure a precise and faultless analysis of the physical evidences and, by consequence, correct interpretation of the analyses results [1, 2]. Physical evidences collected from crime scenes are the essential foundations for criminal investigations. They include a wide range of items such as weapon, bullets and electronic devices. They also involve observations of environmental conditions in the crime scene such as the presence of smoke and the temperature level. Physical evidence also covers a wide range of biological evidences such as stains of body fluids, hair and tissues [1, 3, 4]. Biological evidences, and more particularly stains of biofluids, are of utmost importance in criminal investigations. They can provide a wealth of information about the identity of the victim and the suspect. In addition, in certain homicide cases, biological evidences can help in predicting the cause of death and defining the postmortem interval. Therefore, sensitive detection and correct identification of biological evidences are essential for a successful outcome in criminal investigations [2, 5,6,7,8,9,10,11]. These tasks can only be achieved by adopting reliable and accurate analytical techniques and working protocols. In this connection, vibrational spectroscopes are modern analytical techniques with promising applications in the analysis of biological evidences commonly found in crime scenes [12,13,14].
Vibrational spectroscopes, including Raman spectroscopy and Fourier transform infrared spectroscopy (FTIR), are increasingly popular in forensic analyses. The characteristic spectral data produced by these techniques are generally defined by the chemical structure of the analyte and by its molecular vibrations [15]. Raman spectroscopy is an optical sensing technique based on Stokes Raman scattering. It uses monochromatic laser beam as incident light. When laser hits the molecule being analysed, the molecule will absorb the energy of incident photons and, by consequence, will be promoted to a higher vibrational state. The new vibrational state is transitional and the molecule will soon return to a lower state different from its original one. As a result, the molecule will emit light (photons) with a wavelength dependent on the energy difference between the transitional and the last vibrational states of the molecule. This phenomenon is called inelastic light scattering or Stokes Raman scattering [16, 17]. Raman spectroscopy is gaining increasing popularity in forensic analyses especially those related to biological evidences [18,19,20]. FTIR is based on the ability of vibrating molecules to absorb infrared (IR) electromagnetic radiation and convert it to molecular vibrations. Therefore, this technique uses IR radiation as incident light. The spectral data produced by FTIR demonstrate the IR absorption bands of the analyte. The IR absorption spectrum of a given molecule is generally defined by the chemical structure of the molecule and more specifically by the vibrations of its chemical groups. Hence, FTIR provides fingerprint recognition of the analyte based on the molecular vibrations of its functional groups [21,22,23]. FTIR, and more particularly ATR-FTIR, is gaining increasing importance in forensic investigations. This vibrational spectroscopic technique has a wide range of applications in the analysis of biological evidences and postmortem investigations. The combined use of ATR-FTIR and chemometrics in forensic analyses has been highlighted in multiple studies [24,25,26,27,28,29,30,31].
When using ATR-FTIR for detecting and identifying forensic evidences, the IR absorption spectrum of the analyte is usually compared with standard chemometric models. The comparison process enables the analyst to reach a decision with a defined limit of confidence. Therefore, chemometrics provide the statistical basis and the degree of confidence for the ATR-FTIR-based analyses. More precisely, chemometrics are the statistical tools used to develop the ATR-FTIR standard models and to define the prediction errors for each type of forensic evidences. Multiple chemometric tests can be used for this purpose depending on the problem under consideration. Among those pattern recognition techniques, principal component analysis-linear discriminant analysis (PCA-LDA) and Partial least squares-discriminant analysis (PLS-DA) are the most popular [14]. The current systematic review aims to cast light on the potential applications of ATR-FTIR in forensic investigations. We reported here the vast majority of studies which investigated the use of ATR-FTIR in the analysis of biological evidences and in the postmortem investigations. We discussed in detail the use of this technique in the detection and classification of stains of body fluids. The main focus has been given to the ATR-FTIR-based analyses of blood and semen stains which are commonly found in crime scenes. We also reviewed in-depth the use of ATR-FTIR in the estimation of postmortem interval and in the prediction of the death cause. For better understanding of this review, we will start with a concise presentation focused on molecular vibrations and FTIR.
Molecular vibrations and absorption of electromagnetic radiation
Consider a molecule composed of covalently linked atoms. The molecule as a whole can move in three-dimensional space in two distinct types of motions: a translational motion and a rotational one. The translation motion allows the molecule to move in three directions or axes (x, y, and z). Meanwhile, the rotational motion allows the molecule to rotate around any of these three axes [32]. When zooming inside the molecule itself, we can say that the chemical bonds within the molecule are not rigid, which makes the molecule itself flexible. Therefore, atoms within the molecule are subject to periodic vibrational motions that make the angles and the distances between the atoms changes continuously while the mass center of the molecule remains stable. These internal periodic motions are generally called molecular vibrations. In the scientific literature, they are also named fundamental vibrations or normal modes of vibration [32,33,34,35,36].
The normal modes of vibrations involve two cardinal types of vibrational motions that are generally named as stretching and bending. Stretching can be defined as a periodic change in the distance between two bonded atoms. More precisely, it can be described as a periodic change in the bond’s length between the two atoms. This normal mode of vibration can be further classified into symmetric stretching and anti-symmetric stretching. To elucidate these two subclasses, let us consider the water molecule H–O-H. This molecule has a nonlinear structure with two single covalent bonds between the oxygen atom and the hydrogen atoms. In the symmetric stretching, the length of the single bonds increases and decreases simultaneously. In other words, the hydrogen atoms move to and away from the oxygen atom in a simultaneous way. While in the anti-symmetric stretching, one hydrogen atom moves towards the central oxygen, meanwhile the other one moves away from it. Bending can be described as a periodic change in the angles between two bonds while the bonds’ length remains stable. Bending is also divided into four subclasses including rocking, scissoring, wagging, and twisting. To clarify these vibrational motions, let us consider the ethylene molecule H2C = CH2. In this molecule, a double covalent bond exists between the two central carbon atoms. Meanwhile, each carbon atom shares two single bonds with two hydrogen atoms. Rocking occurs when the two hydrogen atoms move clockwise or anticlockwise simultaneously and in the same plane around the central carbon atom. Therefore, the length of the single bonds remains stable. In addition, in exception to the previous bending rule, the angle between the two hydrogen atoms also remains unchanged. Scissoring happens when the hydrogen atoms move towards each other or away from each other in one plane. While in wagging, the hydrogen atoms move towards each other or away from each other out of planes. Finally, in twisting, a hydrogen atom moves forward while the other moves backward simultaneously and out of plane. Thus, in scissoring, wagging and twisting, the angle between the single bonds changes while their length remains constant [33, 36, 37].
The number of the normal modes of vibration that can be observed in a molecule varies depending on the number of atoms in the molecule (N) on the one hand, and on the molecule linearity on the other hand. As a rule, there are 3 N-5 normal modes of vibration in linear molecules. Meanwhile, 3 N-6 vibrational motions can be described in nonlinear molecules. This difference is due to the fact that nonlinear molecules, such as H2O, have two identical bending modes that happen at the same frequency. Thus, no energy difference can be observed between them [35, 36].
For a given molecule, each normal mode of vibration requires a defined level of energy. Therefore, a molecule can vibrate in a particular mode if it absorbs the energy necessary for it. This energy might be in form of heat or electromagnetic radiation. When the molecule is provided with high level of energy, it will vibrate at a higher frequency (faster) or at higher vibrational level. The level of energy required to incite a particular mode of vibration in a molecule varies depending on three factors. The first one is the mode of vibration itself. For example stretching requires a higher level of energy when compared with bending. The second factor is the type of bonds involved in the vibration. Generally, single bonds are weaker than double ones and, therefore, they require a lower level of energy to vibrate. Finally, the masses of atoms involved in the vibration are also a defining factor of the level of energy needed to induce this vibration [34, 36]. The vibration frequency is also dependent on these factors. In addition, it is highly influenced by other ones related to the environment of the vibrating atoms. More precisely, the presence of a functional group in the vicinity of the vibrating atoms, and the type of this group, can influence the vibration frequency. Besides that, the electric charge of the surrounding atoms can also affect it. Moreover, the involvement of vibrating atoms in hydrogen bonds with neighbouring atoms is also a decisive factor. It should be noted that hydrogen interactions reduce the vibration frequency by weakening the strength of the bond involved in the vibration [38, 39].
Molecular vibrations have a frequency range between 1013 and 1014 Hz approximately. A vibrating molecule can interact with the oscillating electromagnetic field of a light wave in a frequency-dependent manner. The strongest interactions are observed when the electromagnetic frequency of the light wave is very close or identical to the vibration frequency of the molecule [40]. The outcome of this interaction is the absorption of the electromagnetic radiation by the vibrating molecule which converts it into molecular vibrations. It is important to outline the fact that only molecular vibrations associated with a change in the dipole moment in the molecule can absorb electromagnetic radiation [41]. The electromagnetic spectrum involves seven types of radiations that can be arranged in increasing energy level (increasing frequencies) as the following: radio wave, microwaves, IR radiation, visible light, ultraviolet light, X-rays, and gamma rays. Among these, the IR radiation is the only type that can interact with vibrating molecules. The IR spectrum is divided into three regions: far IR, mid IR and near IR. It should be noted that molecular vibrations can be incited by the absorption of mid-IR radiation. This phenomenon can be attributed to the fact that mid-IR radiation has a frequency range between 1013 and 1014, which matches the above-mentioned frequencies of molecular vibrations. In other words, mid-IR radiation has the suitable energy levels to excite a molecule to move from a vibrational energy level to a higher one. It should be added that the vibrational IR radiation is usually measured by the number of waves per centimeter (or frequency in wavenumber). This later is the reciprocal of the wavelength in centimeters. Therefore, the frequencies of vibrational IR radiation in wavenumber range between 4000 and 400 cm−1 [34, 36].
Finally, it is important to emphasize the fact that each functional group has a defined range of vibration frequencies and absorbs a defined region (band) of IR frequencies. The IR absorption band for each functional group is dependent on the chemical bonds and the masses of atoms in this group. Besides that, it is influenced by the hydrogen bond interactions and the neighbouring functional groups [34, 38, 42, 43]. This can be clearly exemplified by the IR absorption bands of the functional groups of amino acids. For example, the IR absorption band of the stretching vibration of the group C = O in the side chain of Glutamic acid in water is 1712–1788 cm−1 [38]. In the meantime, the same group (C = O) in Glutamine has a stretching vibration IR absorption band of 1668–1687 cm−1 [38]. In conclusion, the IR absorption spectrum of a chemical compound is defined primarily by its functional groups and by the molecular vibrations of these groups. Therefore, this spectrum can be used as identifying fingerprint for this compound [43,44,45,46,47].
Principles of FTIR
FTIR is a vibrational spectroscopic technique based on the ability of vibrating molecules to absorb IR electromagnetic radiation and transform it into molecular vibrations. To absorb IR radiation by a vibrating molecule, the vibration must be associated with a change in the dipole moment in the molecule. In FTIR spectroscopy, the molecule to be analysed is formulated into a test sample and, thereafter, is exposed to vibrational IR spectrum between 4000 and 400 cm−1. As a result, the molecule absorbs certain IR frequencies and moves from a lower vibrational state to a higher one. The IR frequencies absorbed by the molecule are defined by the characteristic molecular vibrations of its functional groups. It is important to mention that FTIR measures the amount and the frequencies of IR radiation absorbed by the molecule to produce an absorption spectrum. In this spectrum, the vertical axe represents the percentage of IR radiation transmitted through the sample being analysed. While the horizontal axe represents the IR frequencies range to which the sample was exposed (400–400 cm−1). It should be added that most functional groups absorb IR frequencies between 3500 and 1500 cm−1. The IR absorption spectrum is highly specific for each molecule and it is defined by the functional groups present in the molecule. Therefore, it can be used for identification and quantification purposes. More precisely, FTIR provides fingerprint recognition of the molecule based on the molecular vibrations of its functional groups [21, 22, 34, 37, 41, 48].
The earliest version of IR spectroscopy was a rudimentary spectrophotometer based on the use of a dispersive device named monochromator. This device separates the IR spectrum into continuous bands with defined wavelengths. Therefore, the tested sample is sequentially exposed to IR radiation with defined frequencies and its absorption is measured. The absorbance spectrum of the sample is then developed basing on the collected spectral data. The current version of IR spectroscopy (FTIR) was developed in the years 1960s. This version consists of an IR radiation source that produces an IR beam with a defined wavelength. It also comprises a detector and a computer system. In addition, this version includes an interferometer that substitutes the dispersive monochromator used in the old version [36, 37]. The interferometer used in FTIR was originally developed by Albert Abraham Michelson. It is composed of a beam splitter and a configuration of a stationary mirror and a moving one. When the moving mirror changes its position in a scanning movement, a continuous change in the wavelength of the IR beam will be produced. As a result, the sample being tested will be exposed to IR beam with continuously changing wavelength and its absorbance will be recorded. The spectral data are then processed by the computer system to obtain the IR absorbance spectrum of the sample being tested. More precisely, the stationary mirror and the moving one with its scanning motion allow two identical IR beams, which have travelled two different distances, to recombine and generate a new beam with a continuously changing wavelength. By consequence, the tested sample will be exposed to an IR beam with a frequency defined by the scanning motion of the moving mirror [22, 23, 37].
ATR-FTIR is the standard technique for FTIR measurement. In this technique, an ATR crystal with a low critical angle (or a high refractive index) is used as a sample support. ATR crystals are usually made of IR transparent materials such as zinc selenide (ZnSe), germanium (Ge) or diamond. The IR radiation penetrates the sample to reach the ATR crystal where it will be totally reflected. Total internal reflection of the IR radiation occurs only if the angle of the incident IR beam is greater than the critical angle of the ATR crystal. Other modalities of FTIR have also been developed. These include transmission FTIR, photoacoustic FTIR (FTIR–PAS) and diffuse reflection IR Fourier-transform (DRIFT) [22, 36, 49, 50].
ATR-FTIR and forensic investigations
Two categories of forensic evidences can be distinguished in each criminal investigation. The first one is called direct evidences and it is based on the declarations made by the witnesses and the victim [3]. The second category is named physical evidences and it involves various types of items, electronic devices and even photograph of the crime scene [1]. This category also entails a wide range of biological evidences such as hair, tissue and stains of body fluids that might belong to the victim or to the culprit [1, 3]. Physical evidences usually found in the crime scene are of vital importance in criminal investigations. They are considered as conclusive evidences by jurors in the courtroom [2, 5, 51]. Besides their role in guiding the investigation, they can provide indisputable identification of the culprit, or exonerate an innocent suspect. In addition, the declarations of the victim, suspects and witnesses can be affirmed or revoked by these evidences. In many criminal cases, physical evidences can be irrefutable proof on whether an offence has occurred or not.
ATR-FTIR is a universal and highly accurate analytical method that can be used for a quick in situ screening and identification of a wide range of materials and stains in criminal investigations [11]. This technique has several advantages over other techniques used in forensic analyses. Firstly, FTIR spectroscopy is a small sized handheld apparatus that can be easily transported and used in various types of work fields. It can be used to directly identify and discriminate between stains of body fluids in crime scenes [52, 53]. In contrast to other analytical methods, ATR-FTIR does not require any specific preparations to be carried out on the sample prior to testing. Besides that, only a small amount of the test sample is needed to perform the measurement. Moreover, no necessary reagents are required for the ATR-FTIR–based analysis. It should be added that ATR-FTIR is a nondestructive technique that does not cause any alterations in the tested sample. This fact means that the sample can still be used in further forensic analyses after being examined by ATR-FTIR spectroscopy [52, 54, 55].
ATR-FTIR and analysis of biological evidences
Biological evidences are the most important type of evidences that can be recovered from the crime scene. They can provide a valuable source of information about the identity of the victim and the culprit [56]. This type of evidences involves biotissue, epithelial cells, hair, and bones. In addition, body fluids such as blood, semen, vaginal fluid, urine, sweat, and saliva are also biological evidences of a great judicial value [57]. ATR-FTIR is a reliable and cost-effective analytical method capable of identifying and differentiating between stains of all body fluids [53, 54, 58]. The differences between ATR-FTIR spectra of various types of biofluids were firstly reported in 2011 by Elkins and colleagues [54]. This observation was further confirmed by Orphanou et al. in 2015 [59]. Nevertheless, the decisive discrimination between body fluids using ATR-FTIR was demonstrated in 2018 by Takamura and co-workers. They showed that ATR-FTIR can be used to discriminate between stains of five body fluids including blood, semen, urine, saliva and sweat. Three types of samples were used in this study. The first one involved spots of body fluids on glass slides which were incubated at room conditions overnight. The second type involved spots of body fluids on glass slides which were incubated at ambient room conditions for different periods ranging from 1 day up to 8 months. Besides that, fourteen other samples, such as milk, soy sauce, toothpaste, cotton cloth …etc., were also included in the study and treated in the same manner as the previous two types. The ATR-FTIR spectra generated from the spots of all the tested samples were subjected to a chemometric analysis using PLS-DA. As a result, the author developed a dichotomous model based on Q-statistics testing to discriminate between these spectra. The proposed model demonstrated a high discrimination power between the spectra of the spots of body fluids incubated at the room temperature overnight. It also successfully differentiated between the spectra of spots aged 1 day or more. In addition, the spectra of all nonbody-fluid samples were successfully excluded by the model. These results highlighted the potential application of ATR-FTIR in differentiating between stains of various types of body fluids [53]. Therefore, extensive researches have been carried out to further investigate the use of this technique in the forensic analysis of stains of blood, semen, vaginal secretions, and urine.
Blood
Blood is considered the most informative biological evidence that can be recovered from crime scenes. Besides being a source of DNA, blood can be used to obtain valuable information about the age, gender, and race of the person from whom it originated [11, 55, 60]. Therefore, sensitive and accurate detection of bloodstains is of extreme importance in forensic investigations. Commercial kits used in routine for forensic identification of human bloodstains are based on the detection of primate hemoglobin by immunoassays. However, hemoglobin of animal sources can interfere in these tests causing false positive results (Table 1) [61]. Differentiation between human and animal bloodstains is of paramount importance in criminal investigations of traffic accidents and more particularly hit and run incidents. Moreover, the previous immunological tests are destructive. This fact makes impossible the use of bloodstains in further analyses. ATR-FTIR has emerged as an accurate and nondestructive analytical method for the detection and differentiation between stains of blood of different species. The potential applications of this technique in the forensic analysis of bloodstains have been outlined in several studies [52, 55, 58, 60, 62,63,64,65,66]. Here below, we presented a detailed review of these studies based on the type of information that can be deduced from the analysis.
Detection and discrimination between bloodstains of different species
The use of ATR-FTIR in the analysis of bloodstains for forensic purposes was firstly reported by De Wael and colleagues in 2008. They showed that vibrational spectroscopes, including Raman and ATR-FTIR, can be used to detect blood microparticles recovered from clothes of homicide suspects. However, the author concluded that the spectra generated from human and animal bloodstains cannot be differentiated by visual examination [62]. Later on, it has been demonstrated that ATR-FTIR can detect bloodstains on different types of fabrics with an impressive sensitivity. The highest sensitivity levels were observed in case of bloodstains on cotton fabric (0.0010 µg) and polyesters (0.0066 µg) [67].
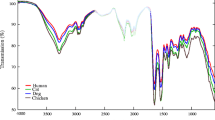
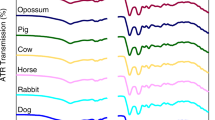
Despite the conclusion made by De Wael and his team, research continued to investigate the potential use of ATR-FTIR in differentiating between stains of bloods of different species. Multiple studies used ATR-FTIR in combination with chemometric to achieve this purpose [52, 63, 64, 66]. In 2015, Mistek et al. showed that ATR-FTIR spectroscopy can be used jointly with PLS-DA to differentiate between bloodstains prepared from human and animal bloods. This combined use demonstrated an accuracy of 100% in discriminating between stains of bloods of three species including human, dog and cat [52]. Later on, these findings were validated by the same research team on a wider group of species. The team tested stains of bloods of twelve species including human, domestic and wildlife animals [66]. The potential application of ATR-FTIR in discriminating between stains of bloods of different species was further confirmed in a relatively recent study carried out in 2018. Six species, including human and domestic animal, were included in this study. It has been demonstrated that ATR-FTIR in combination with chemometrics is able to differentiate between stains of bloods of all the tested species with high accuracy. In addition, the study showed that ATR-FTIR can identify bloodstains kept in outdoor conditions as blood after a period of 107 days [64].
In a recently published work, Sahrma et al. investigated the use of ATR-FTIR in the detection of bloodstains more closely and in conditions mimicking those usually encountered in crime scenes. In addition, the author examined the ability of this analytical method to discriminate between bloodstains and stains of other biofluids. As a result, it has been demonstrated that ATR-FTIR can differentiate between bloodstains and stains of other body fluids used in the study with an accuracy of 100%. Besides that, this analytical method has also showed 100% specificity in discriminating between bloodstains and stains of other substances that look like blood such as tomato ketchup, red wine and red lipstick. Concerning the detection sensitivity, stains of dilutions of blood as high as 1:64 could be detected by ATR-FTIR. Furthermore, 15-day-old bloodstains have been successfully detected by this technique. However, this analytical method has failed to detect bloodstains after washing and chemical treatment. These data highlighted the potential of ATR-FTIR as a nondestructive method for in situ analysis of bloodstains [63]. Table 1 demonstrates the advantages and limitations of this technique compared to the standard forensic methods used in the detection of bloodstains.
Interestingly, analysis of blood samples of animals of different species by visible light spectroscopy generates different spectra. Therefore, a recent study compared the performance of this technique with that of ATR-FTIR. Using PCA-LDA for chemometric analysis of spectra, the study demonstrated that ATR-FTIR has a higher discrimination power than visible light spectroscopy [68].
Estimation of the age of bloodstains
Precise evaluation of the age of bloodstains is very informative in criminal investigations. It can help investigators in defining the time when a crime was committed or when an accident had happened. This information can reduce the number of suspects and narrow the investigation. It can also assist the police forces in defining the chronological sequence of events. Besides its ability to discriminate between stains of blood of different species, the potential use of ATR-FTIR in estimating the age of bloodstains has also been explored in multiple studies [29,30,31, 69, 70]. The earliest reports indicated changes in the ATR-FTIR spectra of bloodstains over time [69, 70]. These reports were validated by Lin and co-workers who used ATR-FTIR in combination with chemometrics to study the age of bloodstains incubated in conditions which mimic the crime scene conditions. The study pointed out that ATR-FTIR jointly with chemometric is an excellent tool to discriminate between fresh bloodstains (aging up to 1 day) and old ones (aging more than 1 day) [31]. In a relatively recent study, stains prepared from bloods of animal and human donors were incubated for a period of 175 days in indoor conditions. The stains were examined by ATR-FTIR every 24 h during the first week and every 7 days during the remaining time. The obtained spectra were analysed by three advanced chemometrics tests. The multiple linear regression test (MLR) produced the most accurate model for estimating the age of bloodstains. Using this model, the age of bloodstains could be estimated with an error of ~ 3 ± 1 days from their actual age [30]. Although the chemometric models developed in the study did not predict the actual age of the bloodstains with a high accuracy, the result obtained by the MLR model remains encouraging and subject to improvements in future studies.
Discrimination between different types of human blood
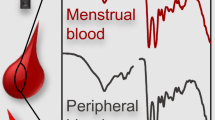
In criminal cases involving rape and sexual assault, defining the biological origin of bloodstains is of crucial importance for establishing the facts and verifying the claims made by the victim. In this connection, discriminating between menstrual and venous bloods seems to be a decisive step. The biochemical methods used in this type of analyses, such as immunochromatography and Real-Time PCR, are sophisticated and can only be carried out in a forensic laboratory [71, 72]. To overcome these challenges, some researchers explored the potential use of ATR-FTIR in differentiating between stains of various types of blood [55, 58, 73, 74]. The earliest study that examined the difference between ATR-FTIR spectra of stains of menstrual and venous bloods was carried out in 2017. The study reported that a peak at 1039 cm−1 can be used to differentiate between the spectra of these two types of blood. This peak is generally observed in the spectra of stains of menstrual blood. The peak is attributed to phosphoric acid normally present in this type of blood. The report also concluded that the intensity of this peak varies depending on the porosity and knit of the fabric carrying the stain [58]. This remark was examined more profoundly later in 2020. Sharma et al. investigated the ability of ATR-FTIR in combination with chemometrics to discriminate between menstrual blood and peripheral blood, and between menstrual blood on the one hand and vaginal and seminal fluids on the other hand. It has been demonstrated that ATR-FTIR jointly with PCA-LDA can discriminate with 100% accuracy between menstrual blood and all other types of body fluids included in the study. The same result was obtained in case of substances that look like blood [73]. These results have been recently confirmed by Mistek-Morabito et al. who demonstrated that ATR-FTIR jointly with PLS-DA can differentiate between menstrual and peripheral blood with 100% accuracy [74].
Interestingly, ATR-FTIR together with chemometrics also demonstrated a great potential in differentiating between stains of postmortem and antemortem bloods. In 2017, Takamura and co-workers used an innovative chemometric method, named multivariate spectral processing, to analyze spectra of stains of postmortem and antemortem bloods. The analysis allowed the development of a differentiation model that demonstrated a high accuracy in discriminating between stains of the two types of blood. The discrimination process was based on three steps. Firstly, ATR-FTIR spectra of bloodstains deposited on different types of fabric substrates were measured. Thereafter, the signal due to the fabric substrate was extracted from the bloodstains spectra. In the final step, the signal obtained after the extraction was analysed by the previous model to define whether the origin of the stains is postmortem or antemortem blood [65].
Phenotype profiling
Homicide criminal investigations require phenotype profiling of the victim by a certified professional. This process involves physical examination of the victim’s corpse or skeleton by a forensic anthropologist to determine the victim’s age, race, and gender. However, this task becomes challenging if the corpse of the victim is physically absent [75]. Therefore, researchers studied phenotype profiling of subjects basing on the analysis of their bloodstains by ATR-FTIR [55, 60]. They relied on the fact that the biochemical content of blood, and more particularly its content of proteins, lipids, glucose and other metabolites, varies depending on age, gender, and ethnicity [76,77,78]. In a relatively recent study, stains prepared from bloods of donors of different age groups, including adults, adolescents and newborns, were dried overnight and examined by ATR-FTIR. The obtained spectra were then analysed by PLS-DA. As a result, a differentiation model has been developed. The model demonstrated 92% accuracy in classifying donors according to their age groups [60]. Finally, in 2019, Mistek et al. described the development of other models based on PLS-DA analysis of ATR-FTIR spectra of stains of bloods collected from Caucasian, African American, and Hispanic donors. These models demonstrated a high level of accuracy in classifying donors according to their ethnicity and gender [55].
Semen and vaginal fluid
Semen is considered the most valuable forensic evidence in criminal investigations of rape and sexual assault. The presence of a suspect’s semen in the crime scene is deemed to be irrefutable evidence of his presence and that an ejaculation occurred. However, it proves neither the occurrence of sexual intercourse, nor the non-consensual sex act. These facts remain to be established by forensic experts [79]. Detection of semen in a crime scene can be performed by examination with UV light. Seminal stains fluoresce when exposed to UV light at a wavelength of 455 nm. Semen detection can also be performed by biochemical methods such as analysing the activity of alkaline phosphatase by colorimetric methods (Table 1) [80]. ATR-FTIR has been proven to be an efficient method for detecting semen stains and discriminating between them and stains of other body fluids [27, 28, 81, 82]. When used in combination with chemometrics such as partial least square regression (PLSR) or PCA-LDA, ATR-FTIR can discriminate with 100% accuracy between semen stains and stains of other body fluids including vaginal secretions. In addition, this technique can also differentiate between semen and non-biological substances that look like semen [81]. Furthermore, it has been demonstrated that ATR-FTIR can be used as a reliable tool to detect semen stains deposited on fabric substrates and dried at room conditions for 3 h. It can also detect semen stains even when they are in a mixed state with urine and saliva. ATR-FTIR spectra of seminal stains are distinguished by two peaks at 1635.8 cm−1 and 1537.8 cm−1. These two peaks can still be observed even when semen is mixed with equal volume of the previous body fluids [28]. It should be noted that ATR-FTIR can also detect semen stains on highly absorbent napkin. However, in case of mixed samples of urine and semen, urine could be detected in most cases, while semen was detected only in a few ones. In addition, in case of mixed samples of semen and vaginal fluid, visual discrimination between the spectra of these two types of body fluids is impossible [27]. Further investigations have been carried out using ATR-FTIR and chemometric to study the ability of this analytical method to detect semen in mixed stains of semen and vaginal secretions [81]. Table 1 demonstrates the advantages and limitations of this technique compared to the standard forensic methods used in the detection of semen stains.
Concerning species identification basing on ATR-FTIR analysis of semen stains, species including human, bull, rabbit, and dog could be identified with 100% accuracy using ATR-FTIR jointly with PLS-DA. Species identification by this analytical method can be achieved even in case of azoospermia [83].
Defining the age of seminal stains can be very informative in criminal investigations of rape and sexual assault. Therefore, the time-dependent changes in the ATR-FTIR spectra of seminal stains have been investigated to explore the potential of this technique in estimating the age of semen stains [84, 85]. These changes have been divided in two phases basing on the speed of water evaporation. However, since water evaporation varies depending on the substrate porosity, estimation of the age of seminal stains must be studied using various substrates [85]. Zha and colleagues used ATR-FTIR to estimate the age of seminal stains deposited on three types of substrates including tissues, glass and cellulose fibers within a period of 6 days after deposition. PLSR-based analysis of the spectral data demonstrated that this technique can predict the age of seminal stains within this period with a prediction error of 1 day approximately [84].
Besides semen, vaginal fluids are also considered valuable evidence in criminal investigations of rape and sexual assault. Identification of vaginal fluids in the crime scenes of such cases is always sought by forensic investigators. In contrast to other body fluids, no characteristic proteins can be identified in the vaginal secretions. This fact makes the detection of vaginal fluids in a crime scene a difficult task [86]. In a proof of concept study, Sharma et al. investigated the use of ATR-FTIR in conjunction with chemometrics in this type of analyses. As a result, this analytical method succeeded in detecting the stains of vaginal fluids deposited on all the nonporous substrates used in the study. However, detection of the stains deposited on porous substrates revealed to be challenging. In addition, the author proposed a PCA-LDA classification model. The model demonstrated 100% accuracy in discriminating between the stains of vaginal fluids and the stains of all other fluids and substances that look like it which were included in the study [24].
Urine
Urine is a widely used biofluid in forensic investigations, more particularly those involving doping and drugs intoxication. Compared to blood, urine is considered the biological sample of choice in such cases. This can be ascribed to the fact that drugs’ metabolites can be found at higher concentrations in urine rather than in blood. In addition, collection of urine samples is a simple process that does not require the intervention of a trained nurse. Furthermore, repeating the sampling process for confirmation purposes is also easier in case of urine [87, 88]. The potential application of ATR-FTIR in the detection and quantification of doping agents in urine samples has been investigated [89, 90]. Terbutaline and its precursor bambuterol are tow drugs used in asthma treatment in more than 25 countries [91]. These two drugs are classified as doping agents by the World Anti-Doping Agency [92]. Detection of these two drugs in the urine samples can be performed by gas chromatography jointly with mass spectrophotometer [93, 94]. However, these techniques are costly and can be operated only by skilled.
forensic scientists. In 2020, Algethami et al. explored the potential use of ATR-FTIR in combination with chemometrics in this type of analyses. It has been demonstrated that this technique can detect and quantify these two drugs in volunteers’ urine with a high level of accuracy [89]. Moreover, MT-45 is a synthetic opioid that has been developed in the years 1970s by a Japanese pharmaceutical company to be used as an alternative painkiller for morphine. However, the use of this drug as an illicit psychoactive drug has been reported in the USA and Europe [109, 110]. McKenzie et al. have demonstrated that ATR-FTIR can be used to identify MT-45 metabolites in the urine samples of mice. In addition, this technique has been successfully used to detect these metabolites in the urine samples of two human addicts who were confirmed of having ingested MT-45 [90].
Other biological evidences
Hair is one of the most important biological evidences commonly found in crime scenes. Discrimination between human and animal hair is usually performed by microscopic examination. Although this method is considered as the reference method for forensic hair analysis, errors might occur due to the examiner’s incompetence. Therefore, analysis of mitochondrial DNA has been proposed as a validation and confirmation method. However, analysis of mitochondrial DNA is a destructive method that can be performed in a specialized forensic laboratory only [111]. Interestingly, ATR-FTIR in combination with chemometrics demonstrated a high accuracy in discriminating between animal and human hairs. Therefore, this technique was also proposed as a fast and nondestructive confirmatory test besides microscopic examination [112].
ATR-FTIR and postmortem investigations
The purpose of forensic postmortem investigations, including forensic autopsy, is to establish the identity of the deceased person and to define his gender, age, and race. Forensic autopsy has also been for the purpose to estimate the postmortem interval (PMI) and to determine the cause of death [113]. Earlier in this review, we described the use of ATR-FTIR in phenotype profiling of subjects basing on the analysis of stains of blood and semen. Here below, we will cast the light on the application of this universal analytical method in the evaluation of PMI and in the prediction of the death cause.
Estimation of PMI
Defining the time interval since death is a frequent task in criminal investigations. Accurate estimation of PMI can provide valuable insight into the chronological sequence of events. It can also help in reducing the number of suspects and verifying the witnesses’ statements. Estimation of PMI can be performed by various methods including physical examination (ex. cold body, rigor, lividity….etc.), molecular method (ex. DNA degradation) and others (Table 1.). Despite the multitude of these methods, PMI estimation remains a challenging task for forensic pathologists due to the limitations associated with each one of them (Table 1) [99, 114,115,116,117]. To overcome these limitations, the potential use of ATR-FTIR in PMI prediction has been investigated in several studies [25, 26, 103,104,105,106]. Table 1 demonstrates the advantages and limitations of this technique compared to the standard forensic methods used in PMI estimation.
PMI prediction using soft tissues
The postmortem decomposition of organs and tissues is a natural process that occurs in all organisms after death. This phenomenon is generally associated with biochemical changes in the composition of tissues as they gradually decay. From a scientific standpoint, these changes should be reflected in variations in the ATR-FTIR spectra of the degrading tissues [118, 119]. Therefore, in the aim of exploiting this technique in PMI estimation, ATR-FTIR has been used to study the postmortem biochemical changes in the lung, liver, kidney and adipose tissues [25, 26, 103, 104].
ATR-FTIR was firstly used to investigate the decomposition process in the lung tissues of rats. The time-dependent variations observed in the spectra of the lung tissues indicated the potential use of this technique in PMI determination [104]. Later on, this observation was confirmed by Wang et al. who used ATR-FTIR and chemometrics to study the postmortem biochemical changes in the liver tissues of rats. The author used three groups of rats which were scarified and incubated in environmentally controlled conditions at different temperatures. Samples of liver tissues were removed from each group at defined intervals and examined by ATR-FTIR. Concerning the group of rats which was kept at 20 °C, PLS-DA-dependent analysis of spectra collected from this group allowed the development of a chemometric model. The developed model divided these spectra in three time-dependent groups: 120–168 h, 48–96 h and 0–24 h. The model demonstrated a 90% accuracy in PMI prediction [103]. Another model was developed basing on the analysis of kidney tissues of rats during a period of 72 h after death. The model demonstrated an adequate accuracy in PMI prediction [120].
Furthermore, ATR-FTIR was also used to study in vitro the decomposition process of adipose tissues collected from human cadavers. The tissues were incubated in environmentally controlled conditions at a temperature of 25 °C for a period of 14 days. Samples were removed from the tissues every 2 days and analysed by ATR-FTIR. Chemometric analysis of the obtained spectra indicated a time-dependent increase in the concentration of free fatty acids due to the hydrolysis process occurring in these tissues. To better mimic the in vivo conditions, the postmortem biochemical changes in adipose tissues were also studied in vivo in a mouse model. A group of mice were scarified and kept in the same controlled conditions for the same period of time. Samples of adipose tissues were collected from the mice at defined intervals and analysed by ATR-FTIR. As a result, a PLS model has been proposed for PMI determination. The model demonstrated a relatively accurate PMI estimation with a prediction error of 2 days approximately [26]. It should be noted that the rate of the postmortem degradation of adipose tissues is highly influenced by the ambient temperature. Therefore, ATR-FTIR in combination with chemometrics was used to study this process at two temperatures: 25 °C and 5 °C. As a result, two temperature-related models have been developed to predict PMI. The prediction error varied depending on the model, or more precisely depending on the incubation temperature [25]. Finally, it should be noted that estimation of PMI by ATR-FTIR using soft tissues is not affected by the cause of death [120]. However, humidity and temperature seem to play a considerable role in this process [25, 121].
PMI prediction using body fluids
Two body fluids, including the vitreous humor (VH) and the pericardial fluid (PF), were investigated to explore the possibility of using them in PMI estimation [105, 106]. VH is a gelatinous fluid occupying the posterior cavity of the eye between the retina and the lens. The privileged site of this body fluid makes it well protected against microbial activities. In addition, VH is characterized by the absence of many conventional enzymes usually found in other body fluids. For these reasons, VH is resistant to decomposition after death and can be used as an alternative to blood and urine in postmortem forensic analyses [122, 123]. In a study carried out in 2018, Zhang and co-workers explored the potential use of VH in PMI determination. The author investigated the variations in the ATR-FTIR spectra of VH in rabbits during 48 h after death. As a result, the study proposed three chemometrics models. The artificial neural network (ANN) model demonstrated the highest level of accuracy with a prediction error of 2 h. These data suggest that analysis of VH by ATR -FTIR might be useful in PMI prediction [105]. In addition, PF has also been the main focus of some research groups who investigated its potential use in PMI estimation [106, 124, 125]. The earliest studies indicated that the postmortem changes in electrolytes concentrations in PF could be used to predict PMI [124, 125]. In 2017, Zhang and co-workers used ATR-FTIR to further explore the biochemical changes in rabbits’ PF during 48 h after death. The variations observed in ATR-FTIR spectra were attributed to the time-dependent protein degradation. In addition, the chemometric analysis of these variations allowed the development of two models. The nu-support vector machine (nu-SVM) model showed the highest level of accuracy with a prediction error of 2.5 h approximately [106].
PMI estimation using cartridges and bones
Cartridges and bones are more resistant to petrifaction and decay than soft tissues and body fluids. Therefore, ATR-FTIR was used to study the biochemical changes in human annular cartridges during a period of 30 days after death. Analysis of spectral changes observed during this period allowed the development of a chemometric model for PMI determination. The modSel demonstrated a satisfactory PMI estimation with an approximate prediction error of 1.5 day [7]. In addition, ATR-FTIR was used to study the postmortem mineral variation in human bones. Analysis of mineral content of samples of femur and humerus by this technique revealed a decrease in the crystallinity index and in the ratio between the two types of carbonate A and B. These tow markers have been proposed as a useful mean for PMI determination [107]. Further ATR-FTIR-based researches have been carried out on human bones to investigate their use in the long-term prediction of PMI [6, 126].
PMI estimation using entomological evidences
It is important to mention that PMI can also be determined basing on the analysis of entomological evidences that can be found in the crime scene. More precisely, this method is based on defining the age and the species of larvae found on the victim’s corpse to predict PMI. Despite being a historical method dating back to the thirteenth century, the principle of this method is still used in modern forensics. However, the inaccuracy of techniques used to determine the larvae age and species is the main limitation for this method [100, 101]. In a proof of concept study, Pickering et al. demonstrated that ATR-FTIR in combination with chemometrics can be exploited in the analysis of entomological evidences. ATR-FTIR was successfully used to identify the larvae species and determine their life cycle stage with 100% sensitivity and specificity. By consequence, this technique can be a time-saving tool for a fast and reliable PMI estimation based on entomological evidences found in the crime scene [102].
Identification of the cause of death
Defining the cause of death is an essential step in deciding whether the death was a homicide, suicide or accident. In other words, the legal pathologist must be able to identify the direct cause of death and describe the circumstances that led to it to decide whether a crime had been committed or not. Occasionally, this task might be challenging especially in cases associated with multiple organ dysfunction. ATR-FTIR revealed to be useful in such cases, more particularly in the diagnosis of weather related death and death caused by anaphylactic shock and diabetic ketoacidosis.
Postmortem diagnosis of weather related death
Extreme weather conditions might have severe consequences on the human body. In the USA, 2000 weather-related deaths had been reported every year between 2006 and 2010 [127]. Death caused by extreme temperatures is generally ascribed to dysfunction of multiple organs in the human body. Therefore, forensic diagnosis of death due to hypothermia or hyperthermia is a challenging task to legal pathologists [108, 128,129,130]. In a proof of concept study, Lin and co-workers investigated the potential use of ATR-FTIR in the postmortem diagnosis of these two causes of death. The author used ATR-FTIR to examine plasmas of five groups of rats whose death was induced by lethal hypothermia, lethal hyperthermia, drowning, asphyxiation and brain injury. PCA-based analysis of spectral data generated from the rats’ plasmas allowed the identification of specific bands for each group. To predict the death caused by hypothermia or hyperthermia, two binary classification models had been developed using PLS-DA. The models demonstrated 100% accuracy in predicting the death related to these two causes when used to analyze the spectra of 42 unknown samples of rat’s plasma [131].
The same research group also investigated the use of ATR-FTIR in the diagnosis of lethal hypothermia basing on the analysis of human pulmonary autopsies. The analysis demonstrated that the pulmonary edema associated with fatal hypothermia is characterized by increased concentrations of proteins with B-sheet conformation in the edema fluid. Therefore, this specific biomarker has been exploited in discriminating between fatal hypothermia and other causes of death. The study used pulmonary autopsies harvested from 54 cadavers with different death causes (including 14 cases of hypothermia). PLS-DA-based analysis of spectral data generated from the previous autopsies allowed the development of a chemometric model. The model demonstrated 100% accuracy in predicting fatal hypothermia as a cause of death after analysing the spectra of 8 pulmonary autopsies including 3 ones collected from fatal hypothermia cases [8].
Furthermore, it has been reported that ATR-FTIR can be used to diagnose the death caused by hypothermia and heatstroke in rabbits basing on the analysis of rabbits’ VH [9, 10]. Concerning lethal hypothermia, chemometric analysis of spectral data generated from VH of hypothermic rabbits indicated a significant increase in the concentrations of glucose and nucleic acids. Therefore, a classification model was developed to predict the death caused by lethal hypothermia. The model demonstrated 100% accuracy in identifying hypothermia as the cause of death in unknown VH donors [10]. Finally, in heatstroke rabbits, the spectral changes observed in the rabbits’ VH were attributed to structural and compositional changes in a wide range of VH biomolecules. Thus, a prediction model was developed basing on the chemometric analysis of these spectral variations. The model demonstrated 100% specificity and sensitivity in diagnosing heatstroke as a cause of death in rabbits [9].
Postmortem diagnosis of other ambiguous death causes
The use of ATR-FTIR in the postmortem diagnosis of ambiguous death causes, such as fatal anaphylactic shock (FAS) and diabetic ketoacidosis (DKA), has been explored in a few studies [132, 133]. FAS is a severe systemic syndrome triggered by allergic reaction to some foods, drugs, or venoms. If untreated, this hypersensitivity immune reaction can be life threatening [134]. Diagnosis of death caused by FAS is an exceptionally difficult task. In such cases, forensic pathologists resort to excluding all other causes of death [135, 136]. To overcome this challenge, researchers investigated the possibility of using ATR-FTIR in the diagnosis of FAS. In a study published in 2018, Lin and colleagues examined the fluids of pulmonary edema due FAS by ATR-FTIR. The study was performed using pulmonary autopsies harvested from 20 cadavers with different death causes including 8 cases of FAS. Analysis of the obtained spectral data revealed a significant increase in protein concentration in FAS cases. This increase was ascribed to the high level of proteins with α-helix structure. Meanwhile, the level of proteins rich in tyrosine was less than the normal range. Chemometric analysis of these spectral data allowed the development of a classification model. The model demonstrated a satisfactory discrimination between the FAS cases and other cases included in the study. This proof of concept study highlighted the potential use of ATR-FTIR in the postmortem diagnosis of FAS [132].
Similarly to FAS, diagnosis of death caused by DKA is also challenging for legal pathologists. This can be attributed to the fact that the level of blood glucose decreases after death. The reason behind this decrease is the spontaneous short-term cellular activity observed after death. In addition, the medical reanimation process is usually associated with an increase in the level of blood glucose. Thus, blood glucose cannot be used as a reliable marker for postmortem diagnosis of DKA [137]. Therefore, researchers explored the use of ATR-FTIR for this purpose. Wu et al. analysed pulmonary autopsies collected from confirmed cases of fatal DKA and other causes of death by this analytical method. Chemometric analysis of obtained data pointed out the potential application of this technique in the postmortem diagnosis of DKA [133].
Moreover, in case of cadavers recovered from seawater, it is often challenging for forensic pathologists to decide whether the death was caused by drowning in sea or the corpse was thrown in water after death. This task becomes more difficult in case of decomposed cadavers. ATR-FTIR-based analysis of pulmonary autopsies revealed that this technique, in combination with chemometrics, can discriminate between drowning and postmortem immersion with an accuracy up 100% [138].
Conclusion
In this review, we explained the fundamentals of the normal modes of vibration and the ability of vibrating molecules to absorb IR electromagnetic radiation and convert it to molecular vibrations. We also briefly discussed the principles of FTIR and the main advantages of this analytical method. In addition, we reviewed in depth the use of ATR-FTIR in the analysis of biological evidences usually found in crime scenes with particular focus on body fluids. The data presented here demonstrate the potential applications of this nondestructive technique in detecting, identifying and discriminating between stains of different types of biofluids. Bloodstains are commonly found in crime scenes especially those involving homicide. In addition, blood is considered the most informative biological evidence in forensic investigations. Spectral data produced by examining bloodstains by ATR-FTIR can be used to deduce a wealth of information related to the donor species, age, sex, and race. These data can also be used to estimate the age of bloodstains and to differentiate between those belonging to different types of blood including menstrual and peripheral bloods. Similar information can also be deduced from the IR absorption spectra of seminal fluid. Furthermore, we discussed in detail the potential applications of this analytical method in PMI estimation. We explained how ATR-FTIR can be used to predict PMI basing on the analysis of postmortem biochemical changes in adipose tissues, cartridges and VH. The ATR-FTIR-based analysis of entomological evidences found in the crime scene is also useful in PMI estimation. Moreover, this review provided a comprehensive description about the use of ATR-FTIR in the diagnosis of weather related death and some ambiguous death causes such as FAS and DKA.
As it has been demonstrated above in the “ATR-FTIR and analysis of biological evidences” section, a considerable amount of research data related to the use of ATR-FTIR in the detection and discrimination between body fluids has been published in the past decade. These data constitute a solid base for the introduction of ATR-FTIR in the routine forensic practice to analyze stains of biofluids that can be found in crime scenes. However, the majority of the studies, which investigated the use of ATR-FTIR in PMI estimations, have been carried out on animal models or on limited numbers of human cadavers. Therefore, further studies involving a statistically significant numbers of human cadavers need to be performed before extrapolation on humans and before introducing ATR-FTIR in forensic practice for PMI prediction purposes. It should also be emphasized that comparative studies should also be carried out to evaluate the performance of this technique compared to the gold standard methods currently followed in forensic investigations. Besides that, it should be outlined that there is a dire need for a collective, if not international, agreement in the scientific community on the adoption of defined standard chemometric models for each type of analyses. Added to that is the need for standard chemometric models specific for defined environmental conditions to be used in PMI estimation. Once such standard models have been developed and agreed, a suitable commercialization campaign by the manufacturing companies of ATR-FTIR might lead to the introduction of this technique in forensic investigations. Once introduced and its efficiency is proven in forensic practice, ATR-FTIR can then be considered whether it can be a standard forensic method or not.
It should be noted that the applications of ATR-FTIR in forensic analyses are not limited to these areas. Several studies have outlined the potential use of this technique in the analysis of other types of physical evidences. This includes analysis of vehicles’ coating and splinters of car bumpers which are commonly found in hit and run traffic incidents [139, 140]. ATR-FTIR is also useful in analysing traces of explosives [141, 142] and drugs [143,144,145], which might extend the use of this technique to airports and border checkpoints. Interestingly, this vibrational spectroscopic technique has also found its way to other analytical fields. For example, ATR-FTIR is widely used in the pharmaceutical industry for quality control purposes. More precisely, it is used to determine the concentration of active pharmaceutical ingredients in solid and semi-solid formulations. It is also used in biopharmaceutics to monitor drug release from pharmaceutical dosage forms. In addition, this analytical technique has demonstrated a great utility in identifying polymers and copolymers which are widely used in pharmaceutical formulations [146,147,148]. Moreover, ATR-FTIR has been successfully used in bioimaging including tissue and cellular imaging [149, 150]. The great potential of this technique in the medical diagnosis of diseases has also been demonstrated in multiples studies. ATR-FTIR has been successfully used to diagnose cancers such as ovarian and breast cancers [151, 152]. It has also been used to diagnose metabolic diseases such as diabetes [153, 154], and infectious diseases such as coronavirus disease 2019 (COVID-19) and hepatitis B and C [155, 156]. It is important to mention that the wide range of applications of ATR-FTIR can hardly be summarized in this section. Nevertheless, we believe that this brief summary will give the reader a quick glance about the potentials of this outstanding analytical method.
Raman spectroscopy is another vibrational spectroscopic technique that has also demonstrated a great utility in forensic analyses. The use of this optical sensing method in the detection and identification of stains of body fluids has been highlighted in multiple studies. Similar to ATR-FTIR, this technique can also be used to analyze stains of blood and semen and deduce important information related to donors’ race, gender and age [157,158,159,160,161,162,163,164]. Compared to Raman spectroscopy, ATR-FTIR can produce a stronger signal and, therefore, can detect lower concentrations of the analyte. In addition, ATR-FTIR–based analysis eliminates all nonspecific interactions especially those caused by fluorescence. The main issue when using Raman as analytical method is interference due to fluorescence emitted by the analyte itself or by concomitant impurities. Moreover, when using Raman spectroscopy, increasing the laser power might expose the sample being analysed to overheating and deterioration. For this reason, ATR-FTIR seems to be less destructive than Raman spectroscopy and can ensure a better stability for the sample. However, although it is considered a nondestructive analytical technique, ATR-FTIR requires close contact with the test sample. It should be added that IR absorption spectra can be interpreted easily, while Raman spectra are commonly less understood. When considering analyte in aqueous solutions, Raman spectroscopy seems to surpass ATR-FTIR in this condition. The ability of water molecules to absorb IR radiation makes Raman-based analysis more precise than the spectral data produced by ATR-FTIR [165,166,167].
Finally, it is important to emphasize the fact that ATR-FTIR is a universal analytical technique that offers many advantages over other analytical methods used in forensic analyses. The commercial availability of handheld ATR-FTIR spectroscopy makes the use of this analytical device in crime scenes an easy task. In addition, this nondestructive analytical method provides fingerprint recognition of the analyte using minimal amount of the test sample. Extensive researches have been carried out to investigate the wide range of applications of ATR-FTIR in forensic analyses. However, additional researches are needed to explore the potential use of this technique in the diagnosis of drug abuse and addiction. The ability of this analytical method to detect traces of stimulants and psychoactive substances in saliva or sweat samples would be a breakthrough in the lute against this type of modern crimes. In addition, the great utility of this technique in predicting some death causes such as diabetic ketoacidosis has opened the door for another field of applications that requires a substantial amount of researches and investigations. The astounding application of ATR-FTIR in medical diagnosis of metabolic and infectious diseases is also a vast scope for future researches that might extends over the next five decades.
References
Lee HC, Pagliaro EM (2013) Forensic evidence and crime scene investigation. J Forensic Investig 1:1–5
Peterson JL, Hickman MJ, Strom KJ, Johnson DJ (2013) Effect of forensic evidence on criminal justice case processing. J Forensic Sci 58:S78–S90. https://doi.org/10.1111/1556-4029.12020
Magalhaes T, Dinis-Oliveira RJ, Silva B, Corte-Real F, Nuno Vieira D (2015) Biological evidence management for DNA analysis in cases of sexual assault. Sci World J 2015:365674. https://doi.org/10.1155/2015/365674
Maras MH, Miranda MD (2017) Overlooking forensic evidence? A review of the 2014 International Protocol on the Documentation and Investigation of Sexual Violence in Conflict. Glob Secur: Health Sci Policy 2:10–21. https://doi.org/10.1080/23779497.2017.1281088
Pearson JM, Law JR, Skene JAG, Beskind DH, Vidmar N, Ball DA et al (2018) Modelling the effects of crime type and evidence on judgments about guilt. Nat Hum Behav 2:856–866. https://doi.org/10.1038/s41562-018-0451-z
Leskovar T, Pajnič IZ, Jerman I, Črešnar M (2020) Preservation state assessment and post-mortem interval estimation of human skeletal remains using ATR-FTIR spectra. Aust J Forensic Sci 54:511–532. https://doi.org/10.1080/00450618.2020.1836254
Li Z, Huang J, Wang Z, Zhang J, Huang P (2019) An investigation on annular cartilage samples for post-mortem interval estimation using Fourier transform infrared spectroscopy. Forensic Sci Med Pathol 15:521–527. https://doi.org/10.1007/s12024-019-00146-x
Lin H, Guo X, Luo Y, Chen Y, Zhao R, Guan D et al (2020) Postmortem diagnosis of fatal hypothermia by fourier transform infrared spectroscopic analysis of edema fluid in formalin-fixed, paraffin-embedded lung tissues. J Forensic Sci 65:846–854. https://doi.org/10.1111/1556-4029.14260
Tuo Y, Zhang K, Wang L, Luo Y, Sun Q, Lin H et al (2020) Characterization and postmortem diagnosis of fatal heatstroke using attenuated total reflectance Fourier transform infrared spectroscopy combined with chemometrics. Spectrosc Lett 53:1–11. https://doi.org/10.1080/00387010.2020.1759104
Zhang Z, Lin H, Li Z, Luo Y, Wang L, Chen L et al (2021) Identification of fatal hypothermia via attenuated total reflection Fourier transform infrared spectroscopy of rabbit vitreous humour. Aust J Forensic Sci 53:27–39. https://doi.org/10.1080/00450618.2019.1629021
Mistek E, Lednev IK (2018) FT-IR spectroscopy for identification of biological stains for forensic purposes. IR Spectrosc Today’s Spectros 33:8–19
Muro CK, Doty KC, Bueno J, Halamkova L, Lednev IK (2015) Vibrational spectroscopy: recent developments to revolutionize forensic science. Anal Chem 87:306–327. https://doi.org/10.1021/ac504068a
Zapata F, Gregório I, García-Ruiz C (2015) Body fluids and spectroscopic techniques in forensics: a perfect match? J Forensic Med 1:1–7. https://doi.org/10.4172/2472-1026.1000101
Silva CS, Brazb A, Pimentel MF (2019) Vibrational spectroscopy and chemometrics in forensic chemistry: critical review, current trends and challenges. J Braz Chem Soc 30:2259–2290. https://doi.org/10.21577/0103-5053.20190140
Suzuki EM, Buzzing P (2018) Applications of Raman spectroscopy in forensic science. I: Principles, comparison to infrared spectroscopy, and instrumentation. Forensic Sci Rev 30:111–135
Rostron P, Gaber S, Gaber D (2016) Raman spectroscopy, review. IJETR 6:50–64
Jones RR, Hooper DC, Zhang L, Wolverson D, Valev VK (2019) Raman techniques: fundamentals and frontiers. Nanoscale Res Lett 14:231–245. https://doi.org/10.1186/s11671-019-3039-2
Fikiet MA, Khandasammy SR, Mistek E, Ahmed Y, Halámková L, Bueno J et al (2019) Forensics: evidence examination via Raman spectroscopy. Phys Sci Rev 4:49–60. https://doi.org/10.1515/psr-2017-0049
Virkler K, Lednev IK (2009) Blood species identification for forensic purposes using Raman spectroscopy combined with advanced statistical analysis. Anal Chem 81:7773–7777. https://doi.org/10.1021/ac901350a
Suzuki EM, Buzzing P (2018) Applications of Raman spectroscopy in forensic science. II: analysis considerations, spectral interpretation, and examination of evidence. Forensic Sci Rev 30:137–169
Berthomieu C, Hienerwadel R (2009) Fourier transform infrared (FTIR) spectroscopy. Photosynth Res 101:157–170. https://doi.org/10.1007/s11120-009-9439-x
Dole MN, Patel PA, Sawant SD, Shedpure PS (2011) Advance applications of Fourier transform infrared spectroscopy. Review Article. Int J Pharm Sci Rev Res 7:159–166
Levin IW, Bhargava R (2005) Fourier transform infrared vibrational spectroscopic imaging: integrating microscopy and molecular recognition. Annu Rev Phys Chem 56:429–474. https://doi.org/10.1146/annurev.physchem.56.092503.141205
Sharma S, Singh R (2020) Detection of vaginal fluid stains on common substrates via ATR FT-IR spectroscopy. Int J Legal Med 134:1591–1602. https://doi.org/10.1007/s00414-020-02333-w
Yu K, Zhang H, Liu Y, Wu H, Cai W, Wei X et al (2021) Adipose tissue estimates the postmortem interval based on ATR-FTIR spectroscopy. Microchem J 164:105977. https://doi.org/10.1016/j.microc.2021.105977
Zhang H, Wang Q, Zhang K, Liu R, Fan S, Wang Z (2019) Estimation of postmortem interval using attenuated total reflectance: Fourier transform infrared spectroscopy in adipose tissues. J Forensic Sci Med 5:7–12. https://doi.org/10.4103/jfsm.jfsm_47_18
Gregorio I, Zapata F, Garcia-Ruiz C (2017) Analysis of human bodily fluids on superabsorbent pads by ATR-FTIR. Talanta 162:634–640. https://doi.org/10.1016/j.talanta.2016.10.061
Kaushik A, Verma P, Jain S (2021) FTIR analysis for identification of semen in mixed biological fluids. Int J Forens Sci 6:246–257. https://doi.org/10.23880/ijfsc-16000246
Edelman G, Manti V, van Ruth SM, van Leeuwen T, Aalders M (2012) Identification and age estimation of blood stains on colored backgrounds by near infrared spectroscopy. Forensic Sci Int 220:239–244. https://doi.org/10.1016/j.forsciint.2012.03.009
Kumar R, Sharma K, Sharma V (2020) Bloodstain age estimation through infrared spectroscopy and Chemometric models. Sci Justice 60:538–546. https://doi.org/10.1016/j.scijus.2020.07.004
Lin H, Zhang Y, Wang Q, Li B, Huang P, Wang Z (2017) Estimation of the age of human bloodstains under the simulated indoor and outdoor crime scene conditions by ATR-FTIR spectroscopy. Sci Rep 7:13254–13268. https://doi.org/10.1038/s41598-017-13725-1
Hynes JT (2015) Molecules in motion: chemical reaction and allied dynamics in solution and elsewhere. Annu Rev Phys Chem 66:1–20. https://doi.org/10.1146/annurev-physchem-040214-121833
Wu G (2005) Molecular vibration. In: Wu G (ed) Nonlinearity and chaos in molecular vibrations, 1st edn. Elsevier, New York, pp 1–14. https://doi.org/10.1016/B978-044451906-1/50001-6
Brown WH, Iverson BL, Anslyn EV, Foote CS (2018) Organic Chemistry. Cengage Learning, Boston
Monnier GF (2018) A review of infrared spectroscopy in microarchaeology: methods, applications, and recent trends. J Archaeol Sci Rep 18:806–823. https://doi.org/10.1016/j.jasrep.2017.12.029
El-Azazy M (2019) Infrared spectroscopy - a synopsis of the fundamentals and applications. In: El-Azazy M (ed) Infrared Spectroscopy - Principles, Advances, and Applications, 1st edn. IntechOpen, London, pp 1–10. https://doi.org/10.5772/intechopen.82210
Pawar AR, Khade PD, Sabale SK (2020) Infrared spectroscopy: a review. World J Pharm Res 9:465–478. https://doi.org/10.20959/wjpr20205-17298
Barth A (2000) The infrared absorption of amino acid side chains. Prog Biophys Mol Biol 74:141–173. https://doi.org/10.1016/s0079-6107(00)00021-3
Deng H, Callender R (1999) Raman spectroscopic studies of the structures, energetics, and bond distortions of substrates bound to enzymes. Methods Enzymol 308:176–201. https://doi.org/10.1016/s0076-6879(99)08010-6
Barth A (2013) Molecular vibrations and their interaction with electromagnetic radiation. In: Roberts GCK (ed) Encyclopedia of Biophysics, 2013 edn. Springer, Berlin, pp 16712–16716. https://doi.org/10.1007/978-3-642-16712-6_301
Hackshaw KV, Miller JS, Aykas DP, Rodriguez-Saona L (2020) Vibrational spectroscopy for identification of metabolites in biologic samples. Molecules 25:4725–4737. https://doi.org/10.3390/molecules25204725
Venyaminov S, Kalnin NN (1990) Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolymers 30:1243–1257. https://doi.org/10.1002/bip.360301309
Wolpert M, Hellwig P (2006) Infrared spectra and molar absorption coefficients of the 20 alpha amino acids in aqueous solutions in the spectral range from 1800 to 500 cm(-1). Spectrochim Acta A Mol Biomol Spectrosc 64:987–1001. https://doi.org/10.1016/j.saa.2005.08.025
Bellisola G, Sorio C (2012) Infrared spectroscopy and microscopy in cancer research and diagnosis. Am J Cancer Res 2:1–21
Mihrin D, Li M, Sanchez MA, Hoeck C, Larsen RW, Feilberg KL (2020) Spectroscopic fingerprinting of organic material extracted from tight chalk core samples of the North Sea. ACS Omega 5:31753–31764. https://doi.org/10.1021/acsomega.0c04431
Bergamaschi M, Cipolat-Gotet C, Cecchinato A, Schiavon S, Bittante G (2020) Chemometric authentication of farming systems of origin of food (milk and ripened cheese) using infrared spectra, fatty acid profiles, flavor fingerprints, and sensory descriptions. Food Chem 305:125480. https://doi.org/10.1016/j.foodchem.2019.125480
Matei A, Drichko N, Gompf B, Dressel M (2005) Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. Chem Phys 316:61–71. https://doi.org/10.1016/j.chemphys.2005.04.033
Bunaciu AA, Aboul-Enein HY, Fleschin S (2011) Recent applications of Fourier transform infrared spectrophotometry in herbal medicine analysis. Appl Spectrosc Rev 46:251–260. https://doi.org/10.1080/05704928.2011.565532
Volkov DS, Krivoshein PK, Proskurnin MA (2020) Detonation nanodiamonds: a comparison study by photoacoustic, diffuse reflectance, and attenuated total reflection FTIR spectroscopies. Nanomaterials (Basel) 10:2501. https://doi.org/10.3390/nano10122501
Volkov DS, O.B., R, M.A., P, (2021) Organic matter and mineral composition of silicate soils: FTIR comparison study by photoacoustic, diffuse reflectance, and attenuated total reflection modalities. Agronomy 11:1879–1909. https://doi.org/10.3390/agronomy11091879
Ling S, Kaplan J, Berryessa CM (2021) The importance of forensic evidence for decisions on criminal guilt. Sci Justice 61:142–149. https://doi.org/10.1016/j.scijus.2020.11.004
Mistek E, Lednev IK (2015) Identification of species’ blood by attenuated total reflection (ATR) Fourier transform infrared (FT-IR) spectroscopy. Anal Bioanal Chem 407:7435–7442. https://doi.org/10.1007/s00216-015-8909-6
Takamura A, Watanabe K, Akutsu T, Ozawa T (2018) Soft and robust identification of body fluid using Fourier transform infrared spectroscopy and chemometric strategies for forensic analysis. Sci Rep 8:8459–8462. https://doi.org/10.1038/s41598-018-26873-9
Elkins KM (2011) Rapid presumptive “fingerprinting” of body fluids and materials by ATR FT-IR spectroscopy. J Forensic Sci 56:1580–1587. https://doi.org/10.1111/j.1556-4029.2011.01870.x
Mistek E, Halámková L, Lednev IK (2019) Phenotype profiling for forensic purposes: nondestructive potentially on scene attenuated total reflection Fourier transform-infrared (ATR FT-IR) spectroscopy of bloodstains. Forensic Chem 16:100176. https://doi.org/10.1016/j.forc.2019.100176
Virkler K, Lednev IK (2009) Analysis of body fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int 188:1–17. https://doi.org/10.1016/j.forsciint.2009.02.013
Lee HC, Ladd C (2001) Preservation and collection of biological evidence. Croat Med J 42:225–228
Quinn AA, Elkins KM (2017) The differentiation of menstrual from venous blood and other body fluids on various substrates using ATR FT-IR spectroscopy. J Forensic Sci 62:197–204. https://doi.org/10.1111/1556-4029.13250
Orphanou CM, Walton-Williams L, Mountain H, Cassella J (2015) The detection and discrimination of human body fluids using ATR FT-IR spectroscopy. Forensic Sci Int 252:10–16. https://doi.org/10.1016/j.forsciint.2015.04.020
Giuliano S, Mistek-Morabito E, Lednev IK (2020) Forensic phenotype profiling based on the attenuated total reflection Fourier transform-infrared spectroscopy of blood: chronological age of the donor. ACS Omega 5:27026–27031. https://doi.org/10.1021/acsomega.0c01914
Horjan I, Barbaric L, Mrsic G (2016) Applicability of three commercially available kits for forensic identification of blood stains. J Forensic Leg Med 38:101–105. https://doi.org/10.1016/j.jflm.2015.11.021
De Wael K, Lepot L, Gason F, Gilbert B (2008) In search of blood–detection of minute particles using spectroscopic methods. Forensic Sci Int 180:37–42. https://doi.org/10.1016/j.forsciint.2008.06.013
Sharma S, Chophi R, Jossan JK, Singh R (2021) Detection of bloodstains using attenuated total reflectance-Fourier transform infrared spectroscopy supported with PCA and PCA-LDA. Med Sci Law 61:292–301. https://doi.org/10.1177/00258024211010926
Lin H, Zhang Y, Wang Q, Li B, Fan S, Wang Z (2018) Species identification of bloodstains by ATR-FTIR spectroscopy: the effects of bloodstain age and the deposition environment. Int J Legal Med 132:667–674. https://doi.org/10.1007/s00414-017-1634-2
Takamura A, Watanabe K, Akutsu T, Ikegaya H, Ozawa T (2017) Spectral mining for discriminating blood origins in the presence of substrate interference via attenuated total reflection Fourier transform infrared spectroscopy: postmortem or antemortem blood? Anal Chem 89:9797–9804. https://doi.org/10.1021/acs.analchem.7b01756
Mistek-Morabito E, Lednev IK (2020) Discrimination between human and animal blood by attenuated total reflection Fourier transform-infrared spectroscopy. Commun Chem 3:178. https://doi.org/10.1038/s42004-020-00424-8
Lu Z, DeJong SA, Cassidy BM, Belliveau RG, Myrick ML, Morgan SL (2017) Detection limits for blood on fabrics using attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy and derivative processing. Appl Spectrosc 71:839–846. https://doi.org/10.1177/0003702816654154
Sandran DD, Zakaria Y, Muslim NZM, Hassan NFN (2020) Species determination and discrimination of animal blood: a multi-analytical spectroscopic-chemometrics approach in forensic science. Malays J Med Health Sci 16:162–169
Zhang Y, Wang Q, Li B, Wang Z, Li C, Yao Y et al (2017) Changes in attenuated total reflection Fourier transform infrared spectra as blood dries out. J Forensic Sci 62:761–767. https://doi.org/10.1111/1556-4029.13324
Zou Y, Xia P, Yang F, Cao F, Ma M, Mi Z et al (2016) Whole blood and semen identification using mid-infrared and Raman spectrum analysis for forensic applications. Anal Methods 8:3763–3767. https://doi.org/10.1039/C5AY03337C
Holtkotter H, Dias Filho CR, Schwender K, Stadler C, Vennemann M, Pacheco AC et al (2018) Forensic differentiation between peripheral and menstrual blood in cases of alleged sexual assault-validating an immunochromatographic multiplex assay for simultaneous detection of human hemoglobin and D-dimer. Int J Legal Med 132:683–690. https://doi.org/10.1007/s00414-017-1719-y
Sauer E, Reinke AK, Courts C (2016) Differentiation of five body fluids from forensic samples by expression analysis of four microRNAs using quantitative PCR. Forensic Sci Int Genet 22:89–99. https://doi.org/10.1016/j.fsigen.2016.01.018
Sharma S, Chophi R, Singh R (2020) Forensic discrimination of menstrual blood and peripheral blood using attenuated total reflectance (ATR)-Fourier transform infrared (FT-IR) spectroscopy and chemometrics. Int J Legal Med 134:63–77. https://doi.org/10.1007/s00414-019-02134-w
Mistek-Morabito E, Lednev IK (2021) Discrimination of menstrual and peripheral blood traces using attenuated total reflection Fourier transform-infrared (ATR FT-IR) spectroscopy and chemometrics for forensic purposes. Anal Bioanal Chem 413:2513–2522. https://doi.org/10.1007/s00216-021-03206-w
Klepinger LL (2013) Towards personal identification. In: Siegel JA, Saukko PJ, Houck MM (ed) Fundamentals of Forensic Anthropology. 2ed edn. Elsevier BV, New Jersey, pp 68–75. https://doi.org/10.4324/9781315528939
Duggan C, Watkins JB, Koletzko B, Walker WA (2016) Nutrition in Pediatrics: basic science, clinical applications. People’s Medical Publishing House, Shelton
Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L et al (2008) Analysis of the adult human plasma metabolome. Pharma 9:383–397. https://doi.org/10.2217/14622416.9.4.383
Ozcurumez MK, Haeckel R (2018) Biological variables influencing the estimation of reference limits. Scand J Clin Lab Invest 78:337–345. https://doi.org/10.1080/00365513.2018.1471617
Johnson D, Peterso J, Sommers I, Baskin D (2012) Use of forensic science in investigating crimes of sexual violence: contrasting its theoretical potential with empirical realities. Violence Against Women 18:193–222. https://doi.org/10.1177/1077801212440157
Awasthi A, Jain B, Mishra G (2020) Semen stain detection: a study on the different fabrics for effects of washing. Int J Forensic Sci 5:207–211. https://doi.org/10.23880/ijfsc-16000207
Sharma S, Singh R (2019) Detection and discrimination of seminal fluid using attenuated total reflectance Fourier transform infrared (ATR FT-IR) spectroscopy combined with chemometrics. Int J Legal Med 134:411–432. https://doi.org/10.1007/s00414-019-02222-x
Gregorio I, Zapata F, Torre M, Garcia-Ruiz C (2017) Statistical approach for ATR-FTIR screening of semen in sexual evidence. Talanta 174:853–857. https://doi.org/10.1016/j.talanta.2017.07.016
Wei X, Yu K, Wu D, Huang P, Sun Q, Wang Z (2021) Species identification of semen stains by ATR-FTIR spectroscopy. Int J Legal Med 135:73–80. https://doi.org/10.1007/s00414-020-02367-0
Zha S, Wei X, Fang R, Wang Q, Lin H, Zhang K et al (2020) Estimation of the age of human semen stains by attenuated total reflection Fourier transform infrared spectroscopy: a preliminary study. Forensic Sci Res 5:119–125. https://doi.org/10.1080/20961790.2019.1642567
Das T, Harshey A, Srivastava A, Nigam K, Yadav VK, Sharma K et al (2021) Analysis of the ex-vivo transformation of semen, saliva and urine as they dry out using ATR-FTIR spectroscopy and chemometric approach. Sci Rep 11:11855–11865. https://doi.org/10.1038/s41598-021-91009-5
Sakurada K, Watanabe K, Akutsu T (2020) Current methods for body fluid identification related to sexual crime: focusing on saliva, semen, and vaginal fluid. Diagnostics (Basel) 10:693–705. https://doi.org/10.3390/diagnostics10090693
Lindon JC, Holmes E, Bollard ME, Stanley EG, Nicholson JK (2004) Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers 9:1–31. https://doi.org/10.1080/13547500410001668379
Maurer HH (1992) Systematic toxicological analysis of drugs and their metabolites by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 580:3–41. https://doi.org/10.1016/0378-4347(92)80526-V
Algethami FK, Eid SM, Kelani KM, Elghobashy MR, Abd El-Rahman MK (2020) Chemical fingerprinting and quantitative monitoring of the doping drugs bambuterol and terbutaline in human urine samples using ATR-FTIR coupled with a PLSR chemometric tool. RSC Adv 10:7146–7154. https://doi.org/10.1039/c9ra10033d
McKenzie C, Sutcliffe OB, Read KD, Scullion P, Epemolu O, Fletcher D et al (2018) Chemical synthesis, characterisation and in vitro and in vivo metabolism of the synthetic opioid MT-45 and its newly identified fluorinated analogue 2F-MT-45 with metabolite confirmation in urine samples from known drug users. Forensic Toxicol 36:359–374. https://doi.org/10.1007/s11419-018-0413-1
Sitar DS (1996) Clinical pharmacokinetics of bambuterol. Clin Pharmacokinet 31:246–256. https://doi.org/10.2165/00003088-199631040-00002
Thevis M, Kuuranne T, Walpurgis K, Geyer H, Schanzer W (2016) Annual banned-substance review: analytical approaches in human sports drug testing. Drug Test Anal 8:7–29. https://doi.org/10.1002/dta.1928
Van Gansbeke W, Polet M, Hooghe F, Devos C, Van Eenoo P (2015) Improved sensitivity by use of gas chromatography-positive chemical ionization triple quadrupole mass spectrometry for the analysis of drug related substances. J Chromatogr B Analyt Technol Biomed Life Sci 1001:221–240. https://doi.org/10.1016/j.jchromb.2015.07.052
Van Eenoo P, Van Gansbeke W, De Brabanter N, Deventer K, Delbeke FT (2011) A fast, comprehensive screening method for doping agents in urine by gas chromatography-triple quadrupole mass spectrometry. J Chromatogr A 1218:3306–3316. https://doi.org/10.1016/j.chroma.2010.09.082
Almulhim A.M, Menezes R.G (2022) Evaluation of postmortem changes. StatPearls [Internet]. Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK554464/
Shrestha R, Kanchan T, Krishan K (2022) Methods of estimation of time since death. StatPearls [Internet]. Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK549867/
Lodha A, Ansari N, Prajapati T, Rao MV, Menon SK (2017) Novel approach to determine post-mortem interval from ATPase activity. Aust J Forensic Sci 49:22–30. https://doi.org/10.1080/00450618.2015.1115128
Giri SS, Tingne CV, Dixit PG (2017) Estimation of postmortem interval in early postmortem period using serum enzymes and its correlation with rigor mortis and postmortem lividity. J Indian Acad Forensic Med 39:378–383 https://doi.org/10.5958/0974-0848.2017.00074.4
Li C, Wang Q, Zhang Y, Lin H, Zhang J, Huang P et al (2016) Research progress in the estimation of the postmortem interval by Chinese forensic scholars. Forensic Sci Res 1:3–13. https://doi.org/10.1080/20961790.2016.1229377
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJR (2007) Best practice in forensic entomology–standards and guidelines. Int J Legal Med 121:90–104. https://doi.org/10.1007/s00414-006-0086-x
Ames C, Turner B (2003) Low temperature episodes in development of blowflies: implications for postmortem interval estimation. Med Vet Entomol 17:178–186. https://doi.org/10.1046/j.1365-2915.2003.00421.x
Pickering CL, Hands JR, Fullwood LM, Smith JA, Baker MJ (2015) Rapid discrimination of maggots utilising ATR-FTIR spectroscopy. Forensic Sci Int 249:189–196. https://doi.org/10.1016/j.forsciint.2015.01.036
Wang L, Lind H, Luo Y, Sun Q, Li Z, Chen Y et al (2018) Post-mortem interval estimation in rat liver tissues using attenuated total reflection Fourier transform infrared spectroscopy combined with chemometrics. Aust J Forensic Sci 51:527–537. https://doi.org/10.1080/00450618.2018.1429016
Huang P, Tian WP, Yang GD, Tuo Y, Wang ZY (2007) Study on estimation of postmortem interval using FTIR in rat lung. Guang Pu Xue Yu Guang Pu Fen Xi 27:1962–1965
Zhang J, Wei X, Huang J, Lin H, Deng K, Li Z et al (2018) Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectral prediction of postmortem interval from vitreous humor samples. Anal Bioanal Chem 410:7611–7620. https://doi.org/10.1007/s00216-018-1367-1
Zhang J, Li B, Wang Q, Wei X, Feng W, Chen Y et al (2017) Application of Fourier transform infrared spectroscopy with chemometrics on postmortem interval estimation based on pericardial fluids. Sci Rep 7:18013. https://doi.org/10.1038/s41598-017-18228-7
Baptista A, Pedrosa M, Curate F, Ferreira MT, Marques MPM (2021) Estimation of the post-mortem interval in human bones by infrared spectroscopy. Int J Legal Med 136:309–317. https://doi.org/10.1007/s00414-021-02641-9
Palmiere C, Mangin P (2013) Hyperthermia and postmortem biochemical investigations. Int J Legal Med 127:93–102. https://doi.org/10.1007/s00414-012-0722-6
Zawilska JB (2017) An Expanding world of novel psychoactive substances: opioids. Front Psychiatry 8:110. https://doi.org/10.3389/fpsyt.2017.00110
Prekupec MP, Mansky PA, Baumann MH (2017) Misuse of novel synthetic opioids: a deadly new trend. J Addict Med 11:256–265. https://doi.org/10.1097/ADM.0000000000000324
Houck MM (2005) Forensic human hair examination and comparison in the 21st Century. Forensic Sci Rev 17:51–66
Manheim J, Doty KC, McLaughlin G, Lednev IK (2016) Forensic hair differentiation using attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy. Appl Spectrosc 70:1109–1117. https://doi.org/10.1177/0003702816652321
Menezes RG, Monteiro FN (2021) Forensic autopsy. StatPearls [Internet]. Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK539901/
Alibegovic A (2014) Cartilage: a new parameter for the determination of the postmortem interval? J Forensic Leg Med 27:39–45. https://doi.org/10.1016/j.jflm.2014.08.005
Alaeddini R, Walsh SJ, Abbas A (2010) Forensic implications of genetic analyses from degraded DNA–a review. Forensic Sci Int Genet 4:148–157. https://doi.org/10.1016/j.fsigen.2009.09.007
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJ (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7:379–392. https://doi.org/10.1007/s12024-010-9209-2
Coe JI (1993) Postmortem chemistry update. Emphasis on forensic application. Am J Forensic Med Pathol 14:91–117. https://doi.org/10.1097/00000433-199306000-00001
Byrne HJ, Knief P, Keating ME, Bonnier F (2016) Spectral pre and post processing for infrared and Raman spectroscopy of biological tissues and cells. Chem Soc Rev 45:1865–1878. https://doi.org/10.1039/c5cs00440c
Almulhim AM, Menezes RG (2022) Evaluation of postmortem changes. StatPearls [Internet]. Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK554464/
Zhang K, Wang Q, Liu R, Wei X, Li Z, Fan S et al (2020) Evaluating the effects of causes of death on postmortem interval estimation by ATR-FTIR spectroscopy. Int J Legal Med 134:565–574. https://doi.org/10.1007/s00414-019-02042-z
Ke Y (2019) The changes of attenuated total reflection-Fourier transform infrared after rat’s death under different humidity conditions. Aust J Forensic Sci 51:549–556. https://doi.org/10.1080/00450618.2018.1434831
Skopp G (2004) Preanalytic aspects in postmortem toxicology. Forensic Sci Int 142:75–100. https://doi.org/10.1016/j.forsciint.2004.02.012
Harper DR (1989) A comparative study of the microbiological contamination of postmortem blood and vitreous humour samples taken for ethanol determination. Forensic Sci Int 43:37–44. https://doi.org/10.1016/0379-0738(89)90120-5
Balasooriya BA, St Hill CA, Williams AR (1984) The biochemical changes in pericardial fluid after death. An investigation of the relationship between the time since death and the rise or fall in electrolyte and enzyme concentrations and their possible usefulness in determining the time of death. Forensic Sci Int 26:93–102. https://doi.org/10.1016/0379-0738(84)90065-3
Singh D, Prashad R, Sharma SK, Pandey AN (2006) Estimation of postmortem interval from human pericardial fluid electrolytes concentrations in Chandigarh zone of India: log transformed linear regression model. Leg Med (Tokyo) 8:279–287. https://doi.org/10.1016/j.legalmed.2006.06.004
Wang Q, Zhang Y, Lin H, Zha S, Fang R, Wei X et al (2017) Estimation of the late postmortem interval using FTIR spectroscopy and chemometrics in human skeletal remains. Forensic Sci Int 281:113–120. https://doi.org/10.1016/j.forsciint.2017.10.033
Berko J, Ingram DD, Saha S, Parker JD (2014) Deaths attributed to heat, cold, and other weather events in the United States 2006–2010. Natl Health Stat Report 76:1–15
Turk EE (2010) Hypothermia. Forensic Sci Med Pathol 6:106–115. https://doi.org/10.1007/s12024-010-9142-4
Byard RW, Bright FM (2018) Lethal hypothermia - a sometimes elusive diagnosis. Forensic Sci Med Pathol 14:421–423. https://doi.org/10.1007/s12024-017-9916-z
Doberentz E, Madea B (2017) Microscopic examination of pituitary glands in cases of fatal accidental hypothermia. Forensic Sci Res 2:132–138. https://doi.org/10.1080/20961790.2017.1330804
Lin H, Zou D, Luo Y, Wang L, Zhang Z, Zhang J et al (2019) Postmortem diagnosis of fatal hypothermia/hyperthermia by spectrochemical analysis of plasma. Forensic Sci Med Pathol 15:332–341. https://doi.org/10.1007/s12024-019-00111-8
Lin H, Luo Y, Wang L, Deng K, Sun Q, Fang R et al (2018) Identification of pulmonary edema in forensic autopsy cases of fatal anaphylactic shock using Fourier transform infrared microspectroscopy. Int J Legal Med 132:477–486. https://doi.org/10.1007/s00414-017-1721-4
Wu D, Luo YW, Zhang J, Luo B, Zhang K, Yu K et al (2021) Fourier-transform infrared microspectroscopy of pulmonary edema fluid for postmortem diagnosis of diabetic ketoacidosis. Spectrochim Acta A Mol Biomol Spectrosc 258:119882. https://doi.org/10.1016/j.saa.2021.119882
Reber LL, Hernandez JD, Galli SJ (2017) The pathophysiology of anaphylaxis. J Allergy Clin Immunol 140:335–348. https://doi.org/10.1016/j.jaci.2017.06.003
Da Broi U, Moreschi C (2011) Post-mortem diagnosis of anaphylaxis: a difficult task in forensic medicine. Forensic Sci Int 204:1–5. https://doi.org/10.1016/j.forsciint.2010.04.039
Cecchi R (2016) Diagnosis of anaphylactic death in forensics: review and future perspectives. Leg Med (Tokyo) 22:75–81. https://doi.org/10.1016/j.legalmed.2016.08.006
Palmiere C (2015) Postmortem diagnosis of diabetes mellitus and its complications. Croat Med J 56:181–193. https://doi.org/10.3325/cmj.2015.56.181
Xiong H, Wanga Q, Zhao M, Zheng Z, Zhu S, Zhu Y et al (2021) Drowning and postmortem immersion identification using attenuated total reflection-Fourier transform infrared spectroscopy. Microchem J 167:106310. https://doi.org/10.1016/j.microc.2021.106310
Chen R, Lv J, Feng J (2015) Characterization of paint by Fourier-transform infrared spectroscopy, Raman microscopy, and scanning electron microscopy-energy dispersive X-ray spectroscopy. Anal Lett 48:1502–1510. https://doi.org/10.1080/00032719.2014.984190
Wei C, Wang J (2021) A rapid and nondestructive approach for forensic identification of car bumper splinters using attenuated total reflectance Fourier transform infrared spectroscopy and chemometrics. J Forensic Sci 66:583–593. https://doi.org/10.1111/1556-4029.14606
Mou Y, Rabalais JW (2009) Detection and identification of explosive particles in fingerprints using attenuated total reflection-Fourier transform infrared spectromicroscopy. J Forensic Sci 54:846–850. https://doi.org/10.1111/j.1556-4029.2009.01060.x
He N, Ni Y, Teng J, Li H, Yao L, Zhao P (2019) Identification of inorganic oxidizing salts in homemade explosives using Fourier transform infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 221:117164. https://doi.org/10.1016/j.saa.2019.117164
Materazzi S, Gregori A, Ripani L, Apriceno A, Risoluti R (2017) Cocaine profiling: implementation of a predictive model by ATR-FTIR coupled with chemometrics in forensic chemistry. Talanta 166:328–335. https://doi.org/10.1016/j.talanta.2017.01.045
Coelho Neto J (2015) Rapid detection of NBOME’s and other NPS on blotter papers by direct ATR-FTIR spectrometry. Forensic Sci Int 252:87–92. https://doi.org/10.1016/j.forsciint.2015.04.025
Pereira LSA, Lisboa FLC, Neto JC, Valladão FN, Sena MM (2017) Direct classification of new psychoactive substances in seized blotter papers by ATR-FTIR and multivariate discriminant analysis. Microchem J 133:96–103. https://doi.org/10.1016/j.microc.2017.03.032
Fahelelbom KMS, Saleh A, Mansour R, Sayed S (2020) First derivative ATR-FTIR spectroscopic method as a green tool for the quantitative determination of diclofenac sodium tablets. F1000Res 9:1–14. https://doi.org/10.12688/f1000research.22274.2
Bunaciu AA, Aboul Enein HY, Fleschin S (2010) Application of Fourier transform infrared spectrophotometry in pharmaceutical drugs analysis. Appl Spectrosc Rev 45:206–219. https://doi.org/10.1080/00387011003601044
Dole MN, Patel PA, Sawant SD, Shedpure PS (2011) Advance applications of Fourier transform infrared spectroscopy. Int J Pharm Sci Rev Res 7:159–166
Kazarian SG, Chan KL (2013) ATR-FTIR spectroscopic imaging: recent advances and applications to biological systems. Analyst 138:1940–1951. https://doi.org/10.1039/c3an36865c
Kazarian SG, Chan KL (2006) Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim Biophys Acta 1758:858–867. https://doi.org/10.1016/j.bbamem.2006.02.011
Sitnikova VE, Kotkova MA, Nosenko TN, Kotkova TN, Martynova DM, Uspenskaya MV (2020) Breast cancer detection by ATR-FTIR spectroscopy of blood serum and multivariate data-analysis. Talanta 214:120857. https://doi.org/10.1016/j.talanta.2020.120857
Giamougiannis P, Morais CLM, Rodriguez B, Wood NJ, Martin-Hirsch PL, Martin FL (2021) Detection of ovarian cancer (+/- neo-adjuvant chemotherapy effects) via ATR-FTIR spectroscopy: comparative analysis of blood and urine biofluids in a large patient cohort. Anal Bioanal Chem 413:5095–5107. https://doi.org/10.1007/s00216-021-03472-8
Coopman R, Van de Vyver T, Kishabongo AS, Katchunga P, Van Aken EH, Cikomola J et al (2017) Glycation in human fingernail clippings using ATR-FTIR spectrometry, a new marker for the diagnosis and monitoring of diabetes mellitus. Clin Biochem 50:62–67. https://doi.org/10.1016/j.clinbiochem.2016.09.001
Yang X, Fang T, Li Y, Guo L, Li F, Huang F et al (2019) Pre-diabetes diagnosis based on ATR-FTIR spectroscopy combined with CART and XGBoots. Optik 180:189–198. https://doi.org/10.1016/j.ijleo.2018.11.059
Martinez-Cuazitl A, Vazquez-Zapien GJ, Sanchez-Brito M, Limon-Pacheco JH, Guerrero-Ruiz M, Garibay-Gonzalez F et al (2021) ATR-FTIR spectrum analysis of saliva samples from COVID-19 positive patients. Sci Rep 11:19980. https://doi.org/10.1038/s41598-021-99529-w
Roy S, Perez-Guaita D, Bowden S, Heraud P, Wood BR (2019) Spectroscopy goes viral: diagnosis of hepatitis B and C virus infection from human sera using ATR-FTIR spectroscopy. Clinical Spectroscopy 1:100001–100015. https://doi.org/10.1016/j.clispe.2020.100001
Muro CK, Lednev IK (2017) Race differentiation based on Raman spectroscopy of semen traces for forensic purposes. Anal Chem 89:4344–4348. https://doi.org/10.1021/acs.analchem.7b00106
McLaughlin G, Lednev IK (2015) In situ identification of semen stains on common substrates via Raman spectroscopy. J Forensic Sci 60:595–604. https://doi.org/10.1111/1556-4029.12708
Sikirzhytskaya A, Sikirzhytski V, Lednev IK (2017) Determining gender by Raman spectroscopy of a bloodstain. Anal Chem 89:1486–1492. https://doi.org/10.1021/acs.analchem.6b02986
Mistek E, Halamkova L, Doty KC, Muro CK, Lednev IK (2016) Race differentiation by Raman spectroscopy of a bloodstain for forensic purposes. Anal Chem 88:7453–7456. https://doi.org/10.1021/acs.analchem.6b01173
Doty KC, Lednev IK (2018) Differentiating donor age groups based on Raman spectroscopy of bloodstains for forensic purposes. ACS Cent Sci 4:862–867. https://doi.org/10.1021/acscentsci.8b00198
Muro CK, Doty KC, Fernandes LS, Lednev IK (2016) Forensic body fluid identification and differentiation by Raman spectroscopy. Forensic Chem 1:31–38. https://doi.org/10.1016/j.forc.2016.06.003
Schlagetter T, Kammrath BW, Glynn CL (2017) The use of Raman spectroscopy for the identification of forensically relevant body fluid stains. Spectroscopy 32:19–24
Rosenblatt R, Halámková L, Doty KC, de Oliveira EAC, Lednev IK (2019) Raman spectroscopy for forensic bloodstain identification: method validation vs environmental interferences. Forensic Chem 16:100175. https://doi.org/10.1016/j.forc.2019.100175
Lin S-Y, Li M-J, Cheng W-T (2007) FT-IR and Raman vibrational microspectroscopies used for spectral biodiagnosis of human tissues. Spectroscopy 21:1–30. https://doi.org/10.1155/2007/278765
Byrne H, Sockalingum G, Stone N (2011) Raman Spectroscopy complement or competitor? In: Biomedical applications of synchrotron infrared microspectroscopy. Moss D Royal Soc Chem RCS Anal Spectroscopy Monographs no 11:105–142. https://doi.org/10.1039/9781849731997-00105
Wellner N (2013) 6 - Fourier transform infrared (FTIR) and Raman microscopy: principles and applications to food microstructures. In: Morris VJ, Groves K (ed), Woodhead Publishing Series in Food Science, Technology and Nutrition, 2013 edn. Woodhead Publishing, pp 163–191. https://doi.org/10.1533/9780857098894.1.163
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alkhuder, K. Attenuated total reflection-Fourier transform infrared spectroscopy: a universal analytical technique with promising applications in forensic analyses. Int J Legal Med 136, 1717–1736 (2022). https://doi.org/10.1007/s00414-022-02882-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-022-02882-2