Abstract
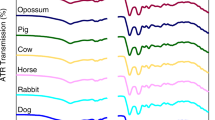
In this study, we investigated the potential of attenuated total reflection (ATR) Fourier transform infrared (FTIR) spectroscopy combined with advanced chemometrics for species identification of bloodstains similar to evidence obtained from real crime scenes. Two partial least squares-discriminant analysis classification models (a human-mammal-domestic fowl trilateral model and a species-specific model) were established. The models demonstrated complete separation among the three classes (human, mammal, and domestic fowl) and distinguished six species (human, rat, rabbit, dog, chicken, and duck). Validation was subsequently conducted to evaluate the robustness of these two models, which resulted in 100 and 94.2% accuracy; even human bloodstains placed in an outdoor environment for up to 107 days were successfully identified. Additionally, all bloodstains were positively identified as blood using the squared Euclidean cosine method by comparing the spectra with those of non-blood substances that had a similar appearance or easily produced false positives. These results demonstrate that ATR-FTIR spectroscopy combined with chemometrics can be a powerful tool for species identification of bloodstains.




Similar content being viewed by others
References
James SH, Kish PE, Sutton TP, Eckert WG (2016) Principles of bloodstain pattern analysis: theory and practice. CRC Press, Florida
Lee HC, Gaensslen RE, Pagliaro EM, Guman MB, Berka KM, Keith TP (1989) The effect of presumptive test, latent fingerprint and some other reagents and materials on subsequent serological identification, genetic marker and DNA testing in bloodstains. J Forensic Identif 39(6):339–358
Li R (2015) Forensic biology. CRC Press, Florida
Kobilinsky L (2012) Forensic chemistry handbook. Wiley, New Jersey
James SH, Nordby JJ (2016) Forensic science: an introduction to scientific and investigative techniques. CRC Press, Florida
Daeid NN, Houck M (2012) Interpol’s forensic science review 2010. CRC Press, Florida
McLaughlin G, Doty KC, Lednev IK (2014) Raman spectroscopy of blood for species identification. Anal Chem 86(23):11628–11633. doi:10.1021/ac5026368
Zapata F, García-Ruiz C (2015) Emerging spectrometric techniques for the forensic analysis of body fluids. TrAC, Trends Anal Chem 64:53–63
Virkler K, Lednev IK (2009) Blood species identification for forensic purposes using Raman spectroscopy combined with advanced statistical analysis. Anal Chem 81(18):7773–7777
Mclaughlin G, Doty KC, Lednev IK (2014) Discrimination of human and animal blood traces via Raman spectroscopy. Forensic Sci Int 238C(5):91–95
Mistek E, Lednev IK (2015) Identification of species’ blood by attenuated total reflection (ATR) Fourier transform infrared (FT-IR) spectroscopy. Anal Bioanal Chem 407(24):7435–7442. doi:10.1007/s00216-015-8909-6
Zhang L, Zhang S, Sun M, Wang Z, Li H, Li Y, Li G, Lin L (2016) Blood species identification using near-infrared diffuse transmitted spectra and PLS-DA method. Infrared Phys Technol 76:587–591
Doty KC, Mclaughlin G, Lednev IK (2016) A Raman “spectroscopic clock” for bloodstain age determination: the first week after deposition. Anal Bioanal Chem 408(15):1–9
Barnes RJ, Dhanoa MS, Lister SJ (1989) Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl Spectrosc 43(5):772–777
Zimmermann B, Kohler A (2013) Optimizing Savitzky-Golay parameters for improving spectral resolution and quantification in infrared spectroscopy. Appl Spectrosc 67(8):892–902
Li J, Hibbert DB, Fuller S (2007) Numerical methods for comparing fresh and weathered oils by their FTIR spectra. Analyst 132(8):792–800
Cotrim AR, Ferraz A, Gonçalves AR, Silva FT, Bruns RE (1999) Identifying the origin of lignins and monitoring their structural changes by means of FTIR-PCA and -SIMCA. Bioresour Technol 68(1):29–34
Jain AK (2010) Data clustering: 50 years beyond K-means. Pattern Recogn Lett 31(8):651–666. doi:10.1016/j.patrec.2009.09.011
Napoleon D, Pavalakodi S (2010) A new method for dimensionality reduction using KMeans clustering algorithm for high dimensional data set. Int J Comput Appl 13(7):41–46
Ballabio D, Consonni V (2013) Classification tools in chemistry. Part 1: linear models. PLS-DA. Anal Methods 5(16):3790. doi:10.1039/c3ay40582f
Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JPM, van Dorsten FA (2008) Assessment of PLSDA cross validation. Metabolomics 4(1):81–89. doi:10.1007/s11306-007-0099-6
Trevisan J, Angelov PP, Carmichael PL, Scott AD, Martin FL (2012) Extracting biological information with computational analysis of Fourier-transform infrared (FTIR) biospectroscopy datasets: current practices to future perspectives. Analyst 137(14):3202–3215
Baker MJ, Hussain SR, Lovergne L, Untereiner V, Hughes C, Lukaszewski RA, Thiefin G, Sockalingum GD (2016) Developing and understanding biofluid vibrational spectroscopy: a critical review. Chem Soc Rev 45(7):1803–1818. doi:10.1039/c5cs00585j
SC, Singh BR (2004) A distinct utility of the amide III infrared band for secondary structure estimation of aqueous protein solutions using partial least squares methods†. Biochemistry 43(9):2541–2549
Caine S, Heraud P, Tobin MJ, McNaughton D, Bernard CC (2012) The application of Fourier transform infrared microspectroscopy for the study of diseased central nervous system tissue. NeuroImage 59(4):3624–3640. doi:10.1016/j.neuroimage.2011.11.033
Kochan K, Heraud P, Kiupel M, Yuzbasiyangurkan V, Mcnaughton D, Baranska M, Wood BR (2015) Comparison of FTIR transmission and transfection substrates for canine liver cancer detection. Analyst 140(7):2402–2411
Polyanichko A, Chikhirzhina E (2013) Interaction between DNA and chromosomal proteins HMGB1 and H1 studied by IR/VCD spectroscopy. J Mol Struct 1044(29):167–172
Fujioka N, Morimoto Y, Arai T, Kikuchi M (2004) Discrimination between normal and malignant human gastric tissues by Fourier transform infrared spectroscopy. Cancer detect. Prevention 28(1):32–36
Dovbeshko GI, Gridina NY, Kruglova EB, Pashchuk OP (2000) FTIR spectroscopy studies of nucleic acid damage. Talanta 53(1):233–246
Petibois C, Déléris G (2005) Erythrocyte adaptation to oxidative stress in endurance training. Arch Med Res 36(5):524–531
Kamezawa S (1955) A contribution to the morphological study of nucleated erythrocytes in man and various animals; the 24th report of histochemical study of peroxidase. Okajimas Folia Anat Jpn 27(6):383–400
Bremmer RH, de Bruin KG, van Gemert MJ, van Leeuwen TG, Aalders MC (2012) Forensic quest for age determination of bloodstains. Forensic Sci Int 216(1–3):1–11
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81471819).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, H., Zhang, Y., Wang, Q. et al. Species identification of bloodstains by ATR-FTIR spectroscopy: the effects of bloodstain age and the deposition environment. Int J Legal Med 132, 667–674 (2018). https://doi.org/10.1007/s00414-017-1634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-017-1634-2