Abstract
Introduction
Calprotectin is a protein endowed with antimicrobial properties, rendering it a distinctive marker for infection. Two methods are currently available for the assay of calprotectin: the enzyme-linked immunosorbent assay (ELISA) and the lateral flow test (LFT). We aimed to assess the diagnostic accuracy of synovial fluid calprotectin and to compare the accuracy of the laboratory-based test and the qualitative assessment for the diagnosis of hip and knee prosthetic infection.
Materials and methods
We searched (from inception to November 2023) MEDLINE, Scopus, EMBASE, Web of Science, and Cochrane for studies on calprotectin in the diagnosis of periprosthetic joint infection (PJI). Sensitivity, specificity, positive and negative likelihood ratio (LR), and diagnostic odds ratio were analyzed. The receiver-operating curve for each method was calculated.
Results
We included 14 articles in our meta-analysis, including 902 patients who underwent total hip and knee arthroplasties revision; 331 (37%) had a joint infection according to MSIS, MSIS-modified criteria, ICM 2018 and EBJIS 2021. Considering the false-positive result rate of 6% and false-negative result rate of 7%, pooled sensitivity and specificity were 0.92 (95% CI 0.89–0.94) and 0.93 (0.91–0.95), respectively. The area under the curve (AUC) was 0.93 (95% CI 0.91–0.94). No statistical differences in terms of sensitivity and specificity were found between ELISA and LFT. The pooled sensitivity and specificity of the two calprotectin assessment methods were: LFT 0.90 (95% CI 0.869–0.935) and 0.92 (95% CI 0.894–0.941), respectively; ELISA 0.96 (95% CI 0.914–0.986) and 0.97 (95% CI 0.934–0.988), respectively. The diagnostic odds ratio of the ELISA was superior to that of the LFT (906.6667, 95% CI 271.2686–3030.3712 versus 113.8886, 95% CI 70.4001-184.2414; p < 0.001). The AUC for ELISA and LFT was 0.968 (95% CI 0.944–0.984) and 0.915 (95% CI 0.895–0.933), respectively.
Conclusions
Detection of synovial calprotectin is an accurate test for diagnosis of hip and knee prosthetic infections. The diagnostic accuracy of the two calprotectin assessment methods is almost comparable. The LFT is a valid, rapid, and more available diagnostic tool, particularly to rule out PJI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periprosthetic joint infection (PJI) is one of the most serious complications after total hip arthroplasty (THA) and total knee arthroplasty (TKA). It is currently the most common indication for early revision TKA and the second indication for late revision TKA (31.3% and 22.2% respectively) and the fourth most common indication for revision total hip arthroplasty (16% of all hip revisions) worldwide [1,2,3,4]. Although a definite preoperative diagnosis of septic failure is imperative for proper treatment and management, the diagnosis of PJI remains a serious clinical challenge [5,6,7,8]. Unfortunately, no gold standard exists, and no single test is available with 100% diagnostic accuracy to detect an infection. Serological markers such as CRP, D-dimer, and ESR have been widely used in diagnosing PJI; they are highly influenced by various systemic and confounding factors [9,10,11]. The emergence of new diagnostic modalities has made synovial biomarkers of particular interest, including synovial WBC, leukocyte esterase, and Alpha-Defensin [11,12,13,14], which have shown promising potential as diagnostic tools in PJI. Since then, other synovial biomarkers have been investigated. Among them, synovial calprotectin, secreted by neutrophilic granulocytes and monocytes at sites of local inflammation, plays a role in leukocyte migration and stimulation [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31], thus making calprotectin an intriguing biomarker for PJI [32]. There are two available methods for measuring calprotectin in synovial fluid. The calprotectin ELISA Immunoassay is based on colorimetric detection using monoclonal and polyclonal antibodies against calprotectin, while the calprotectin lateral flow test (LFT) is a quantitative detection of synovial calprotectin, which has the advantage of immediate availability of results, so it is useful for intra-operative diagnosis of PJI. Recently, three meta-analyses analyzed the accuracy of this marker for the diagnosis of PJI and concluded that calprotectin has an excellent diagnostic accuracy [29,30,31]. Since then, further published studies have evaluated the accuracies of the LFT and the ELISA one, so a meta-analysis that includes these emerging studies is needed to verify the accuracy of the previous results and to compare the two methods. Furthermore, we designed the present meta-analysis because the available evidence on these two tests has not been investigated exclusively on hip and knee prosthetic infections.
We therefore asked: (1) What is the role of synovial fluid calprotectin as a biomarker for infection of joint prostheses? (2) What is its reliability and validity in terms of sensitivity, specificity, diagnostic odds ratio (DOR), positive predictive value, negative predictive value, and area under the curve (AUC)? (3) Which method, the LFT or ELISA, offers more advantages?
Materials and methods
Calprotectin assessment methods
Two calprotectin assessment methods are available. For the calprotectin LFT, 20 µl of each joint fluid aspirate was added to 2 ml of dilution buffer. Subsequently, 80 µl of the mix was pipetted onto a well in the test cartridge. Calprotectin is bound by a specific antibody complex on the membrane, resulting in a visible test line for colorimetric detection. The remaining antibody complexes flow laterally and are immobilized on a control line. The color intensity of the test line is proportional to the calprotectin concentration. After 15 min, the test results were photometrically assessed using a smartphone application provided by Lyfstone. Three categories were defined when measuring the calprotectin concentration: < 14 mg/ml or low risk, 14–50 mg/ml or moderate risk, and 50– > 300 mg/ml or high risk for infection [26]. In the laboratory-based test, aliquots for calprotectin testing were subjected to centrifugation. The immunoassay for synovial fluid calprotectin was generated using monoclonal and polyclonal antibodies against calprotectin adsorbed to the surface of plastic wells [17]. Most studies have used 50 mg/L as the threshold value [15,16,17, 21,22,23].
Data sources and search strategy
We searched for studies investigating diagnostic accuracy of synovial fluid calprotectin in patients with periprosthetic infections in the MEDLINE, Scopus, EMBASE, Web of Science, and Cochrane databases from inception to November 2023. The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) methodology guidance was employed [33]. The search strategy used a combination of the following key words: calprotectin AND PJI OR periprosthetic joint infection OR prosthetic infection, with no language restrictions. The reference lists of selected articles were also hand-searched for any additional articles that were not identified from the database search.
Eligibility criteria
Longitudinal studies (retrospective and prospective) and randomized controlled trials evaluating the diagnostic accuracy of these ratios in PJI were finally selected. Studies evaluated the diagnostic accuracy of synovial fluid calprotectin measured either by the immunoassay and lateral flow test in the diagnosis of PJI were included. Papers considered MSIS or later modified MSIS criteria, or ICM 2018, or EBJIS 2021 as reference standard for the diagnosis of PJI were also included. The exclusion criteria included: case reports, expert opinions, previous metanalysis and systematic reviews, letters to the editor, studies that did not report quantitative values of sensitivity, specificity or likelihood ratios, or diagnostic accuracy.
Study assessment and data extraction
Initial screening of titles and abstracts was performed by two pairs of independent reviewers. The full text was obtained for all abstracts that appeared to meet the inclusion criteria or where there was any uncertainty. Each study was assessed by two independent reviewers using the inclusion criteria and any discrepancies regarding the eligibility of an article were resolved with a third author. Relevant data were extracted from each included study. Two authors performed quality assessment of each study using the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) tool [34]. The QUADAS score consists of four domains: (1) patient selection, (2) index test, reference standard, (3) flow, and (4) timing. The risk of bias assessment of the four domains and the clinical applicability of the first three domains were assessed with signaling questions. Questions were answered “yes” for low risk of bias/concerns, “no” for high risk of bias/concerns, or “unclear”.
Results
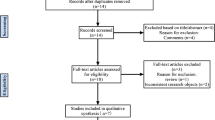
The flow diagram of our search strategy is reported in Fig. 1. Computer search and manual screening of reference lists of relevant studies identified 234 potentially relevant citations. After initial screening of titles and abstracts, the full text of 124 articles was evaluated. After detailed assessment, we excluded 110 references. The remaining 14 articles were included in our meta-analysis. Table 1 summarizes the characteristics of the included studies. In total, 902 patients who underwent total joint arthroplasties revision were evaluated, among whom 331 (37%; range 22–53%) were confirmed to have a joint infection according to MSIS, MSIS-modified criteria., ICM 2018 and EBJIS 2021. The number of joints included was explained in all papers and the site of infection was described in all paper but one [28]. The sensitivity, specificity, positive and negative likelihood ratio, and DOR of included studies, and their corresponding pooled indices, are shown in Table 2. The area under the curve (AUC) was 0.93 (95% CI 0.91–0.94). The results of the QUADAS-2 are in Fig. 2.
Diagnostic accuracy of LFT vs. ELISA of calprotectin in synovial fluid
The pooled sensitivity of the LFT and ELISA was 0.90 (95% CI 0.869–0.935) and 0.96 (95% CI 0.914–0.986), respectively. The pooled specificity for LFT was 0.92 (95% CI 0.894–0.941). Better results in term of specificity were reported for ELISA with a value of 0.97 (95% CI 0.934–0.988). No statistical differences in terms of sensitivity and specificity were found between the two assays. The ELISA had a superior DOR value to that of the LFT (906.6667, 95% CI 271.2686–3030.3712 versus 113.8886, 95% CI 70.4001-184.2414; p < 0.001). Furthermore, the ELISA had a higher positive likelihood ratio 33.115 (95% IC 15.044–72.897) versus 11.446 (95% IC 8.602–15.229. A lower value of negative LR was retrieved for the ELISA 0.036 (95% IC 0.015–0.086) versus 0.100 (95% IC 0.071–0.14). In addition, the AUC for the LFT was 0.915 (95% CI 0.895–0.933), whereas the AUC for the ELISA was 0.968 (95% CI 0.944–0.984). Utilizing the DeLong’s test, we found a statistical difference between the two accuracy values (p < 0.005) [35]. Furthermore, we used the fixed-effect model for both tests [36]. The analysis obtained a risk ratio (RR) of 7.55 (95% IC 5.66–10.08) and 24.90 (95% IC 12.06–51.41), respectively (Figs. 3 and 4).
Discussion
The main findings of this study are that calprotectin shows high diagnostic accuracy in the diagnostic workup of painful total joint arthroplasty independently from the assessment methods. This would position it among the emerging biomarkers for diagnosing PJIs, alongside fibrinogen and others [37, 38]. Recent meta-analyses reported the high diagnostic accuracy of synovial calprotectin [29,30,31]. Although further studies have been published, the pooled estimation of the 14 studies included in our study indicates a similar specificity and sensitivity rate result. Considering the false-positive result rate of 11% (50/440) and false-negative result rate of 5% (36/711), the pooled sensitivity and specificity were 0.924 (95% CI 0.895–0.945) and 0.934 (95% CI 0.913–0.950), respectively. The studies included did not clarify why patients with joint infection report average levels of calprotectin in synovial fluid. Bloody and clotted aspirates could explain negative calprotectin results, as described in different papers [24]. Another possible reason could be attributed to low-grade infections, generally sustained by less virulent bacteria such as P. acnes or coagulase-negative Staphylococci, which are a condition where clinical and laboratory criteria may misdiagnose PJI [39, 40]. Micro-organisms of low virulence rapidly adhere to implants, evading host defense and resulting in a weak immune response [38,39,41]. The dampened inflammation could explain the increase of false-negative results of serum and synovial fluid biomarkers in low-grade PJI. Antibiotic treatment does not appear to give false-negative results [42, 43]. Zhang et al. analyzed the concentration of calprotectin in two groups. In the antibiotic treatment group, this marker was 663 (IQR, 480 to 1,106) µg/ml, while in the non-antibiotic treatment group was 792 (IQR, 577 to 1,203) µg/ml. This difference was not statistically significant (p = 0.343). However, regardless of the use of antibiotics, the concentration of calprotectin in the PJI group was significantly higher than that in the aseptic failure group, and the difference was statistically significant (p < 0.001) [18].
Regarding the false positives, the ability of calprotectin to role in PJI can be modified by inflammatory non-infectious conditions [28] or severe osteolysis or the metallosis caused by metal-to-metal reactions [19]. However, Suren, in his paper, reported that patients with metallosis showed calprotectin levels < 14 mg/l, suggesting that the presence of inflammation caused by metallosis might not induce false-positive calprotectin levels [26]. On the other hand, he confirmed that the calprotectin levels could increase in patients with marked osteolysis and wear disease. There is the inflammatory foreign-body reaction to debris particles due to the activation of monocytes and macrophages instead of the neutrophil activation being more predominant in bacterial infection. The positive and negative likelihood ratios of calprotectin were 14.068 (95% CI 10.749–18.414) and 0.080 (95% CI 0.059–0.110), respectively. This finding demonstrated that a positive or negative result for calprotectin indicates an increased or decreased probability of PJI. Moreover, the DOR and AUC in our study support this finding. In our analysis, calprotectin had a high diagnostic utility with elevated discriminatory test performance between patients with and without a PJI, as demonstrated by a DOR of > 1 and an AUC of 0.93 (95% CI 0.91–0.94).
Furthermore, we divided the studies into two groups to distinguish the results between those obtained with LFT test and those with ELISA. We compared the diagnostic accuracy of the ELISA and LFT tests to diagnose TKA and THA infections. Both assays had high diagnostic accuracy, but the analysis of synovial calprotectin with ELISA reported higher diagnostic indices for diagnosing PJI. Even lower results in terms of the pooled sensitivity and specificity were found for the qualitative test. There was a statistical difference between the two accuracy values.
We utilized the fixed-effect model for both tests because we assumed that the only source of variability between the results obtained from the studies is the different sampling that characterizes them. Any differences in observed effects are due to sampling error. Regarding the ELISA, there is no heterogeneity in the studies we pooled (I2 = 0%; p = 0.70), with an overall risk ratio of 24.90 (95% CI 12.06–51.41). For the LFT, there is a degree of heterogeneity in the studies we considered (I2 = 68%; p = < 0.01). Therefore, we performed a subgroup analysis to find the source of heterogeneity. The results of the subgroup analyses suggested that the heterogeneity may result from two main elements: the type of study and the threshold that has been utilized. The heterogeneity is annulled when we proceed to remove the only retrospective study by Trotter et al. [19] and those by Lazic et al., who utilized a different threshold for synovial calprotectin [24, 25, 27] (Fig. 5).
The strengths and potential limitations of this study should be acknowledged. This study is the first meta-analysis on the utility of calprotectin in hip and knee prosthetic infections. We adopted stringent eligibility criteria that led to the exclusion of studies that assessed calprotectin results in patients with PJI that differed from TKA and THA. Studies that reported infections of other joints were excluded only if the specific data (TKA and THA PJI) could not be extrapolated. Another strength of this study is the increased number of included studies on lateral flow test and ELISA compared with that of previous metanalyses. We analyzed fourteen papers in contrast to the 8 of Hantouly et al. [29], 7 of Peng et al. [30], and 7 of Xing et al. [31], respectively.
This study has a few drawbacks. First, the different diagnostic criteria used to rule out PJI and the small number of patients included in the studies may have contributed to the heterogeneity among studies that emerged for some outcomes assessed in the present meta-analysis. Furthermore, many studies do not include in their diagnostic workup for PJI some criteria included in MSIS and modified MSIS criteria. This disparity could alter the ability of these diagnostic criteria to distinguish between septic and aseptic loosening and, consequently, change the accuracy of new diagnostic tests. Lastly, some confounders, such as chronic inflammatory disease, metallosis of patients included, and use of concomitant antibiotic treatment, may be responsible for false results of the calprotectin evaluation and represent another limitation of this study. We know that rheumatic disease is one of the most important reasons for false-positive results, as is the presence of metallosis.
Conclusion
Detection of synovial calprotectin is an accurate test that helps to diagnose hip and knee prosthetic infections. The diagnostic accuracy of the two calprotectin assessment methods analyzed is comparable. Because the results are available within 15 min with the LFT, this test is a valuable and accurate addition to the pre-operative diagnostic workup before arthroplasty exchange, especially in cases where the gold standard results are inconclusive and particularly when we want to rule out the PJI.
References
Elliott Holbert S, Brennan J, Cattaneo S, King P, Turcotte J, MacDonald J (2023) Trends in the reasons for revision total knee arthroplasty. J Orthop Trauma Rehabilitation 0(0). https://doi.org/10.1177/22104917231176573
Balato G, Barbaric K, Bićanić G, Bini S, Chen J, Crnogaca K, Kenanidis E, Giori N, Goel R, Hirschmann M, Marcacci M, Amat Mateu C, Nam D, Shao H, Shen B, Tarabichi M, Tarabichi S, Tsiridis E, Tzavellas AN (2019) Hip and Knee Section, Prevention, Surgical Technique: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. ;34(2S):S301-S307
Karachalios T, Komnos G, Koutalos A (2018) Total hip arthroplasty: Survival and modes of failure. EFORT Open Rev. ;3(5):232–239. https://doi.org/10.1302/2058-5241.3.170068. PMID: 29951261
Kelmer G, Stone AH, Turcotte J, King PJ (2021) Reasons for revision: primary total hip arthroplasty mechanisms of failure. J Am Acad Orthop Surg 29(2):78–87. https://doi.org/10.5435/JAAOS-D-19-00860
Baldini A, Balato G, Franceschini V (2015) The role of offset stems in revision knee arthroplasty. Curr Rev Musculoskelet Med 8(4):383–389
Windisch C, Brodt S, Roehner E, Matziolis G (2017) C-reactive protein course during the first 5 days after total knee arthroplasty cannot predict early prosthetic joint infection. Arch Orthop Trauma Surg 137:1115–1119
Janz V, Wassilew GI, Kribus M, Trampuz A, Perka C (2015) Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch Orthop Trauma Surg 135:1453–1457
Balato G, Franceschini V, Ascione T, Lamberti A, Balboni F, Baldini A (2018) Diagnostic accuracy of synovial fluid, blood markers, and microbiological testing in chronic knee prosthetic infections. Arch Orthop Trauma Surg 138:165–171
Matsen Ko L, Parvizi J (2016) Diagnosis of Periprosthetic Infec- tion: Novel Developments. Orthop Clin North Am 47(1):1–9
Saleh A, George J, Faour M et al (2018) Serum biomarkers in periprosthetic joint infections. Bone Joint Res 7(1):85–93
Balato G, Dall’Anese R, Balboni F, Ascione T, Pezzati P, Bartolini G, Quercioli M, Baldini A (2022) Synovial fluid alpha-defensin in periprosthetic knee infection workup: liquid chromatography-mass spectrometry detection of alpha-defensin in synovial fluid. Bone Joint J 104–B(9):1047–1051
Balato G, de Matteo V, Ascione T, Di Donato SL, De Franco C, Smeraglia F, Baldini A, Mariconda M (2020) Laboratory-based versus qualitative assessment of α-defensin in periprosthetic hip and knee infections: a systematic review and meta-analysis. Arch Orthop Trauma Surg 140(3):293–301
Balato G, Franceschini V, Ascione T, Lamberti A, D’Amato M, Ensini A, Baldini A (2018) High performance of α-defensin lateral flow assay (synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg Sports Traumatol Arthrosc 26(6):1717–1722
Heckmann ND, Wang JC, Liu KC, Won P, Chung BC, Mayer LW, Longjohn DB, Oakes DA, Christ AB, Lieberman JR (2023) Refining the role of routine synovial alpha-defensin in Periprosthetic Joint Infection Following Total Knee Arthroplasty: an analysis of limitations. J Arthroplasty 38(12):2691–2697
Wouthuyzen-Bakker M, Ploegmakers JJW, Kampinga GA, Wagenmakers-Huizenga L, Jutte PC, Muller Kobold AC (2017) Synovial calprotectin: a potential biomarker to exclude a prosthetic joint infection. Bone Joint J 99–B(5):660–665
Wouthuyzen-Bakker M, Ploegmakers JJW, Ottink K, Kampinga GA, Wagenmakers-Huizenga L, Jutte PC, Kobold ACM (2018) Synovial calprotectin: an inexpensive biomarker to exclude a chronic prosthetic joint infection. J Arthroplasty 33(4):1149–1153
Salari P, Grassi M, Cinti B, Onori N, Gigante A (2020) Synovial fluid calprotectin for the preoperative diagnosis of chronic Periprosthetic Joint infection. J Arthroplasty 35(2):534–537
Zhang Z, Cai Y, Bai G, Zhang C, Li W, Yang B, Zhang W (2020) The value of calprotectin in synovial fluid for the diagnosis of chronic prosthetic joint infection. Bone Joint Res 9(8):450–457
Trotter AJ, Dean R, Whitehouse CE, Mikalsen J, Hill C, Brunton-Sim R, Kay GL, Shakokani M, Durst AZE, Wain J, McNamara I, O’Grady J (2020) Preliminary evaluation of a rapid lateral flow calprotectin test for the diagnosis of prosthetic joint infection. Bone Joint Res 9(5):202–210
Grzelecki D, Walczak P, Szostek M, Grajek A, Rak S, Kowalczewski J (2021) Blood and synovial fluid calprotectin as biomarkers to diagnose chronic hip and knee periprosthetic joint infections. Bone Joint J 103–B(1):46–55
Warren J, Anis HK, Bowers K, Pannu T, Villa J, Klika AK, Colon-Franco J, Piuzzi NS, Higuera CA (2021) Diagnostic utility of a Novel Point-of-care test of Calprotectin for periprosthetic joint infection after total knee arthroplasty: a prospective cohort study. J Bone Joint Surg Am 103(11):1009–1015
Grassi M, Salari P, Farinelli L, D’Anzeo M, Onori N, Gigante A (2022) Synovial biomarkers to detect chronic Periprosthetic Joint infection: a pilot study to compare Calprotectin Rapid Test, Calprotectin ELISA Immunoassay and Leukocyte Esterase Test. J Arthroplasty 37(4):781–786
Warren JA, Klika AK, Bowers K, Colon-Franco J, Piuzzi NS, Higuera CA (2022) Calprotectin lateral Flow Test: consistent across criteria for ruling out Periprosthetic Joint infection. J Arthroplasty 37(6):1153–1158
Lazic I, Prodinger P, Stephan M, Haug AT, Pohlig F, Langer S, von Eisenhart-Rothe R, Suren C (2022) Synovial calprotectin is a reliable biomarker for periprosthetic joint infections in acute-phase inflammation - a prospective cohort study. Int Orthop 46(7):1473–1479
Lazic I, Burdach A, Pohlig F, von Eisenhart-Rothe R, Suren C (2022) Utility of synovial calprotectin lateral flow test to exclude chronic prosthetic joint infection in periprosthetic fractures: a prospective cohort study. Sci Rep 12(1):18385
Suren C, Lazic I, Haller B, Pohlig F, von Eisenhart-Rothe R, Prodinger P (2023) The synovial fluid calprotectin lateral flow test for the diagnosis of chronic prosthetic joint infection in failed primary and revision total hip and knee arthroplasty. Int Orthop 47(4):929–944
Lazic I, Stephan M, Pohlig F, Langer S, Eisenhart-Rothe VON, Suren R (2023) C. Synovial Calprotectin for Diagnosing Periprosthetic Joint Infection in Loose Hip and Knee Arthroplasties: A Prospective Cohort Study. In Vivo. Jul-Aug;37(4):1714–1720
Bottagisio M, Viganò M, Pellegrini A, Logoluso N, Zagra L, Prina A, de Girolamo L, De Vecchi E (2023) Evaluation of synovial calprotectin by using a lateral Flow Test for the diagnosis of prosthetic joint infections. Diagnostics (Basel) 13(4):741
Hantouly AT, Salameh M, Toubasi AA, Salman LA, Alzobi O, Ahmed AF, Hameed S, Zikria B, Ahmed G (2022) Synovial fluid calprotectin in diagnosing periprosthetic joint infection: a meta-analysis. Int Orthop 46(5):971–981
Peng X, Zhang H, Xin P, Bai G, Ge Y, Cai M, Wang R, Fan Y, Pang Z (2022) Synovial calprotectin for the diagnosis of periprosthetic joint infection: a diagnostic meta-analysis. J Orthop Surg Res 17(1):2
Xing J, Li J, Yan Z, Li Y, Liu X, He L, Xu T, Wang C, Zhao L, Jie K (2022) Diagnostic accuracy of calprotectin in periprosthetic joint infection: a diagnostic meta-analysis. J Orthop Surg Res 17(1):11
Stríz I, Trebichavský I (2004) Calprotectin - a pleiotropic molecule in acute and chronic inflammation. Physiol Res 53(3):245–253
Ascione T, Balato G, Di Donato SL, Pagliano P, Granata F, Colella G, Ruosi C (2017) Clinical and microbiological outcomes in haematogenous spondylodiscitis treated conservatively. Eur Spine J 26(Suppl 4):489–495
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Dettori JR, Norvell DC, Chapman JR (2022) Fixed-effect vs Random-effects models for Meta-Analysis: 3 points to consider. Global Spine J 12(7):1624–1626
Balato G, Ascione T, Festa E, De Vecchi E, Pagliano P, Pellegrini A, Pandolfo G, Siciliano R, Logoluso N (2023) The combined evaluation of fibrinogen and D-dimer levels are a helpful tool to exclude periprosthetic knee infection. J Orthop Res 41(8):1840–1847
Balato G, Ascione T, Festa E, Di Gennaro D, Pandolfo G, Pagliano P (2023) The diagnostic accuracy of neutrophils to lymphocytes ratio, platelets to lymphocytes ratio, monocytes to lymphocytes ratio, and platelets to Mean platelet volume ratio in diagnosing periprosthetic knee infections. Are gender-specific cutoff values needed? J Arthroplasty 38(5):918–924
Zimmerli W, Moser C (2012) Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 65:158–168
Balato G, Roscetto E, Vollaro A, Galasso O, Gasparini G, Ascione T, Catania MR, Mariconda M (2019) Bacterial biofilm formation is variably inhibited by different formulations of antibiotic-loaded bone cement in vitro. Knee Surg Sports Traumatol Arthrosc 27(6):1943–1952
Balato G, Ascione T, Rosa D, Pagliano P, Solarino G, Moretti B, Mariconda M, RELEASE OF GENTAMICIN FROM, CEMENT SPACERS IN TWO-STAGE PROCEDURES FOR HIP AND KNEE PROSTHETIC INFECTION: AN IN VIVO PHARMACOKINETIC STUDY WITH CLINICAL FOLLOW-UP (2015 Oct-Dec) J Biol Regul Homeost Agents 29(4 Suppl):63–72
Balato G, Rizzo M, Ascione T, Smeraglia F, Mariconda M (2018) Re-infection rates and clinical outcomes following arthrodesis with intramedullary nail and external fixator for infected knee prosthesis: a systematic review and meta-analysis. BMC Musculoskelet Disord 19(1):361
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioan- nidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–34
Funding
No funding source was involved in the conduction of this study.
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Festa, E., Ascione, T., Di Gennaro, D. et al. Synovial calprotectin in prosthetic joint infection. A systematic review and meta-analysis of the literature. Arch Orthop Trauma Surg (2024). https://doi.org/10.1007/s00402-024-05416-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00402-024-05416-0