Abstract
Background
Enhanced recovery after surgery (ERAS) programs are well-established, resulting in improved outcomes and shorter length of hospital stay (LOS). Same-day discharge (SDD), or “hyper-ERAS”, is a natural progression of ERAS. This systematic review aims to compare the safety and efficacy of SDD against conventional ERAS in colorectal surgery.
Methods
The protocol was prospectively registered in PROSPERO (394793). A systematic search was performed in major databases to identify relevant articles, and a narrative systematic review was performed. Primary outcomes were readmission rates and length of hospital stay (LOS). Secondary outcomes were operative time and blood loss, postoperative pain, morbidity, nausea or vomiting, and patient satisfaction. Risks of bias was assessed using the ROBINS-I tool.
Results
Thirteen studies were included, with five single-arm and eight comparative studies, of which one was a randomised controlled trial. This comprised a total of 38,854 patients (SDD: 1622; ERAS: 37,232). Of the 1622 patients on the SDD pathway, 1590 patients (98%) were successfully discharged within 24 h of surgery. While most studies had an overall low risk of bias, there was considerable variability in inclusion criteria, types of surgery or anaesthesia, and discharge criteria. SDD resulted in a significantly reduced postoperative LOS, without increasing risk of 30-day readmission. Intraoperative blood loss and postoperative morbidity rates were comparable between both groups. Operative duration was shorter in the SDD group. Patient-reported satisfaction was high in the SDD cohort.
Conclusion
SDD protocols appear to be safe and feasible in selected patients undergoing major colorectal operations. Randomised controlled trials are necessary to further substantiate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced recovery after surgery (ERAS) protocols are a set of evidence-based practices spanning the perioperative period, designed to optimise postoperative outcomes by minimising the physiological stress response and maintaining or rapidly restoring baseline function [1]. The concept that a structured, multimodal, multidisciplinary pathway can result in accelerated recovery after surgery first emerged in the early 2000s amongst patients undergoing colorectal surgery [2,3,4]. ERAS programmes are now well-established in colorectal surgery, with the benefits over traditional care demonstrated by several meta-analyses of randomised controlled trials [5,6,7,8].
In 2018, the international ERAS Society published their fourth set of consensus guidelines concerning perioperative care in elective colorectal surgery [9]. In December 2022, the American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) have also collaborated to report updated clinical practice guidelines [10], which aim to lead international efforts in defining the optimal perioperative approach to colorectal surgery based upon the principles of ERAS.
Continued interest and innovation in the various aspects of ERAS have led to its application, in whole or in part, amongst unconventional patient cohorts. Thus far, the feasibility of ERAS pathways has been demonstrated for emergency/non-elective resections [11, 12], elderly patients [13, 14], low rectal cancer [15], inflammatory bowel disease [16], as well as cytoreductive surgery with hyperthermic intraperitoneal chemotherapy [17]. Regardless of patient profile, ERAS protocol compliance has been well documented as the most essential factor contributing to better postoperative outcomes [18,19,20]. Moreover, addressing clinicians’ resistance to change and rectifying long-standing but non-evidence-based practices are important to ensure successful implementation of ERAS programmes [20].
One of the most striking consequences of improved patient recovery with ERAS is a substantially reduced length of hospital stay (LOS). Various meta-analyses have consistently demonstrated that ERAS decreases the postoperative LOS by an average of two days, as compared to standard perioperative care [5, 6, 21]. There is increasing recognition that same-day discharge (SDD), defined as hospital discharge within 24 h of surgery, or "hyper-ERAS”, may be feasible for a select group of physiologically fit patients.
In 2015, Gignoux et al. reported the first five cases of outpatient colectomy with a postoperative stay of less than 12 h [22]. Thereafter, six prospective and seven retrospective cohort studies have evaluated SDD following colorectal surgery [23,24,25,26,27,28,29,30,31,32,33,34,35]. These studies consistently demonstrate the safety of SDD, without an increase in readmission or complication rates [24, 27, 28, 30]. Enhanced post-discharge patient assessment was often performed, through teleconferencing, mobile applications, or telephone interviews.
Given the growing adoption of SDD as a natural progression of ERAS with an increasing amount of contemporaneous evidence, a systematic review is timely to determine the overall feasibility of “hyper-ERAS” for colorectal surgery and discuss the applications of individual components of SDD.
Methods
Search process
This study protocol was prospectively registered in PROSPERO (394793). This study was performed according to the Cochrane Handbook of Systematic Reviews and Meta-analysis version 6.2 (2021), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement guidelines [36]. In consult with our institution’s librarian, we derived an exhaustive permutation of the following Medical Subject Headings (MeSH) (expanded) terms: “colon surgery”, “colorectal surgery”, “rectal surgery”, “colectomy”, “colon resection”, “ERAS”, “enhanced recovery” “23 h”, “same day discharge”. Using this search strategy, an electronic search was performed on 25 December 2022 in the following databases: Medline (via PubMed), EMBASE, Cochrane databases, and the ClinicalTrials.gov website, to identify all published and indexed studies investigating same-day discharge protocols after colorectal surgery. A manual search of the reference lists of relevant studies was conducted to identify additional studies for potential inclusion. In addition, a search of grey literature (conference proceedings, theses, published abstracts) was performed, as per recommendation in the AMSTAR 2 checklist [37].
Inclusion and exclusion criteria
Both randomised and non-randomised controlled trials were included if outcomes were reported for patients undergoing same-day/23-h discharge protocols after colorectal resection surgeries. Given the relative lack of comparative studies, single-arm studies with reported outcomes and extractable data were included. Non-English studies as well as English studies with no extractable data were excluded as there were no available translators at our institution.
Selection of studies
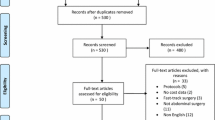
As seen in the PRISMA diagram (Fig. 1), the selection of studies was conducted in two distinct stages. Firstly, two reviewers (VZ, IW) independently screened the studies for preliminary inclusion by their titles and abstracts, and the full text of these studies would then be re-assessed for final inclusion. The senior author would be the arbiter to resolve differences of opinions regarding the studies’ eligibility by consensus.
Outcomes of interest and data extraction
Outcomes of interest included intraoperative and postoperative variables which were extracted independently by two authors using a standardised proforma. Primary outcomes were readmission rates and length of hospital stay. Secondary outcomes were operative time, intraoperative blood loss, conversion to open surgery, postoperative morbidity, pain, nausea and vomiting, patient satisfaction, and protocol compliance. In addition to the outcomes above, we extracted the following data from each study: first author, year, type of publication, age, gender, perioperative protocols, type of surgeries, stage of tumour (when applicable), American Society of Anaesthesiologists (ASA) scores, comorbidities. Data extraction was performed in an independent fashion by VZ and IW, and conflicts were resolved either by consensus or with discussion with the senior author.
Statistical analysis
Where possible, statistical analyses were performed using the RevMan 5.4 software (The Nordic Cochrane Centre, Copenhagen, Denmark), where pooling of risk ratio (RR) was performed for dichotomous variables; and weighted mean difference (WMD) or standardised mean difference (SMD) conducted as the summary statistic for continuous variables. Statistical heterogeneity was assessed using the I2 statistic. A random-effects model was chosen when the I2 statistic was greater than 50%, and a fixed-effects model otherwise. Results were reported with 95 percent confidence intervals (95% CI), and a P value of less than 0.05 was treated as statistically significant.
Pair-wise meta-analyses were not performed given small number of comparative studies, as well as the significant heterogeneity in study protocols and reporting of results. Subgroup analyses were also not performed given the lack of data stratification. Thus, a narrative systematic review was undertaken.
Assessment of bias
The Risk of Bias in Non-randomised Studies – of Interventions (ROBINS-I) tool [39] was utilised to assess the quality and risks of bias from confounding, selection of participants, classification of interventions, deviations from intended intervention, missing data, measurement of outcomes, and selection of reported results. Publication bias was not assessed using the funnel plot and Egger’s regression test since there were less than 10 studies.
Results
Systematic search
Out of a total of 1,185 papers, 29 were included after title and abstract review. These 29 papers were reviewed in entirety and 13 were included in the final systematic review, of which five were single-arm and eight were comparative studies (one randomised controlled trial). The systematic search process and reasons for exclusion are stated in Fig. 1.
The 13 studies comprised a total of 38,854 patients, of whom 1622 received the SDD protocol and 37,232 received conventional ERAS protocol. Of the 1622 patients receiving SDD protocol, 1590 patients (98%) were successfully discharged within 24 h. Gignoux et al. (2015) contributed the least number of patients to the SDD pool (0.3%), while McKenna et al. contributed the most (55.9%). Across the studies, the mean age ranged from 57.4 to 67.0 years in the SDD arm, and 56.5 to 72.6 years in the conventional ERAS arm. The proportion of males ranged from 35 to 80% in the SDD arm, and 40.3% to 62.5% in the conventional ERAS arm.
All procedures were minimally invasive surgeries (MIS), done via laparoscopic or robotic methods. Surgeries performed included colectomies, anterior resections, stoma closures, Hartmann’s reversals and parastomal hernia repairs. The indications for surgery included both benign and malignant conditions. All except one single-arm study [34] reported the indications for surgery. The proportion of patients with malignant indications ranged from 20 to 100% in the SDD arm, and 42% to 100% in the conventional ERAS arm. Patients in most studies were discharged home. Four studies [25, 29, 31, 33] only included patients in the SDD arm if they stayed close to the hospital (less than 30 min drive away). Detailed baseline study characteristics are shown in Table 1. The ERAS and follow-up protocols are reported in Table 2.
Patient selection for SDD and criteria for early discharge
Bednarski et al. [23] randomised patients to the SDD arm. Brandt et al. [24] allowed patient opt-in for SDD. Most prospective studies [22, 25,26,27, 29, 31,32,33, 35] selected patients for SDD based on clinical and social characteristics. Dobradin et al. [28] and McKenna et al. [30] reported retrospective studies without elaboration of SDD selection criteria. For most studies, the final decision for early discharge was made by the surgeon, without a standard discharge checklist. However, McKenna et al. [30], and both studies by Gignoux et al. [22, 35] only discharged patients early if they met an objective set of discharge criteria.
Study quality
Most studies had an overall low risk of bias based on the ROBINS-I tool (Supplementary Fig. 1) and were deemed to be methodologically robust. Three studies, by de Azevedo et al. [27], Popeskou et al. [31], and Tweed et al. [32], had moderate risks of bias, mainly due to missing data and selection of participants.
Primary outcomes
All outcomes are summarised in Table 3.
Readmission rates
The 30-day readmission rate in the intervention arm was low across all studies. Of the five single-arm studies, Campbell et al. [25], Dobradin et al. [28], and Gignoux et al. [22] reported zero readmissions within 30 days. Curfman et al. had a 0.9% (n = 1) readmission rate due to urinary retention [26], while Gignoux et al. had a 5.7% (9/157) readmission rate [35]. Of these nine patients, three had anastomotic leaks, two had small bowel intestinal obstruction and one had omental necrosis [35].
SDD protocols did not lead to increased 30-day readmission rates compared to standard ERAS. Six studies, by Bednarski et al. [23], Brandt et al. [24], Lee et al. [29], Popeskou et al. [31], Tweed et al. [32], and Levy et al. [33], reported no significant differences in 30-day readmission rates between both groups. The remaining two studies did not publish readmission rate data for the control group but reported a 30-day readmission rate of 6.8% and 0% in the SDD group [27, 33].
Length of hospital stay (LOS)
Of the five single-arm studies, three studies, by Gignoux et al. [22], Dobradin et al. [28] and Gignoux et al. [35] reported mean LOS, which ranged from 10 h 24 min [35] to 21 h 47 min [28]. Four comparative studies, by Bednarski et al. [23], Lee et al. [29], Levy et al. [33], Tweed et al. [32] reported mean or median LOS, showing significantly reduced LOS in the SDD group compared to the control group. Bednarski et al. demonstrated that SDD reduced the LOS by 25.6 h as compared to standard ERAS (28.2 h vs 53.8 h, P = 0.002) [23]. Lee et al. reported that the median LOS in the SDD arm was reduced by 2 days as compared to standard ERAS (0 days vs 2 days, P < 0.001) [29]. Levy et al. demonstrated a 2.25 day reduction in median post-operative LOS in SDD versus conventional ERAS arm (0.95 days vs 3.2 days, P = 0.01) [33]. Finally, Tweed et al. showed that majority of their SDD cohort had a median primary LOS of 1 day (80.5%), compared with their ERAS cohort with majority of patients having a median primary LOS of 3 days (50.7%, P < 0.001) [32].
Secondary outcomes
Operative time
Two studies reported significantly quicker operative times with SDD. Brandt et al. reported a 35 min reduction in median operative time in the SDD arm compared to standard ERAS (120 min vs 155 min, P = 0.002), for mostly sigmoid resections and right hemicolectomies [24]. Lee et al. demonstrated a 61 min reduction in mean operative time with SDD versus conventional ERAS (116 min vs 177 min, P < 0.001) [29]. Levy et al. also demonstrated quicker median operative times in the SDD group compared to control (73 min vs 88 min, P = 0.17) [33], although this was not statistically significant. Mckenna et al. showed that median LOS significantly increased as the operative duration increased [30]. Patients discharged POD 0 to 1 had a median operative time of 95 min (IQR 66–141 min), while patients discharged on POD 2 and 3 had significantly longer median operative times of 143 min (IQR 106—185 min) and 159 min (IQR 119—209 min) respectively (P < 0.0001) [30]. This reflects the longer recovery period required for more complex surgery.
Intraoperative blood loss
SDD protocols had similar intraoperative blood loss compared with standard ERAS, demonstrated by two studies; Lee et al. (Median blood loss: 20 ml vs 10 ml, P = 0.498) [29], and Brandt et al. (Median blood loss: 5 ml vs 100 ml, P = 0.089) [24].
Conversion
None of the studies reported conversions from minimally invasive to open surgery.
Postoperative morbidity
Six [23, 24, 29, 30, 32, 33] of the eight comparative studies reported complication rates following both SDD and standard ERAS, while the remaining two [27, 31] did not report complication rates at all. All six studies found similar morbidity rates between the SDD and standard ERAS. Notably, McKenna et al. reported significantly lower superficial infection rates (1.8% vs 2.3%, P < 0.01) in the SDD cohort as compared to those discharged from postoperative day 2 to 5 [30]. Deep surgical site infection rates (0.1% vs 0.3%, P = 0.05) and anastomotic leak rates (0.6% vs 1.2%, P = 0.05) were also lower in the SDD group, although these differences did not reach statistical significance [30].
All five single-arm studies evaluated postoperative morbidity [22, 25, 26, 28, 35]. Campbell et al. reported one patient with nausea and vomiting and one with high ileostomy output [25]. Curfman et al. reported 30-day emergency department visits, including two patients with abdominal pain, and one with urinary retention, one with leg pain and another with diarrhoea [26]. Gignoux’s 2015 study reported one patient with dysuria and one with an incisional hematoma [22]. The prospective study by Gignoux et al. in 2019 reported an overall 30-day morbidity rate of 24.8% with SDD, of which 72% were Clavien-Dindo grade I [35]. Dobradin et al. reported zero complications with SDD [28].
Four [22, 25, 26, 28] of the five single-arm studies reported no high-grade complications requiring reoperation. Gignoux’s 2019 study, comprising 157 patients, reported two patients with rectal bleeding, three with wound abscesses, one with colitis, three with anastomotic leak, two with small bowel intestinal obstruction, and one patient with omental necrosis [35].
Postoperative pain
One comparative study measured postoperative pain using the Brief Pain Inventory (BPI) score [23], with a higher mean discharge pain score in the SDD compared to the control arm (3.23 vs 1.80, P = 0.052), although scores were low in both arms. Furthermore, the authors measured discharge pain scores 1 day earlier in the SDD arm as compared to the control arm. BPI scores were similar post-discharge and at postoperative day 30. Three single-arm studies measured pain using the visual analogue scale rating from 0 to 10 [22, 28, 35]. Dobradin et al. reported a mean postoperative pain score of 1.9 with SDD [28]. In 2015, Gignoux et al. reported mean pain scores for SDD patients on postoperative days 1 (pain score 1.6), 3 (pain score 2.7) and 5 (pain score 1.5), demonstrating low scores throughout [22]. In 2019, Gignoux et al. again reported low mean pain scores amongst patients on SDD protocols across postoperative days 1 (pain score 2.9), 2 (pain score 2.7), and 3 (pain score 2.2) [35].
Postoperative nausea and vomiting (PONV)
PONV rates were generally low amongst studies reporting this outcome. Tweed et al. reported one case of prolonged LOS due to nausea [32]. Campbell et al. had one patient return to the emergency department on postoperative day 1 for PONV [25]. Gignoux et al. (2019) reported two readmissions amongst SDD patients for PONV [35].
Patient satisfaction
Five studies evaluated patient satisfaction [22, 29, 32, 33, 35]. Of these, only one study compared patient satisfaction between SDD and standard ERAS and found no significant differences between the treatment arms for any of the questionnaire items [22]. Nearly all respondents felt that they did not have to be kept in the hospital for a longer period to recover from surgery. In the other four studies, patient satisfaction was only assessed in the SDD arm [22, 29, 32, 33]. All four studies reported that most respondents were highly satisfied with the SDD protocol. Levy et al. [33] and Gignoux et al. [35] reported that most SDD patients would recommend this programme to others.
Protocol compliance
Popeskou et al. reported a 100% adherence rate to the ERAS protocol [31]. No other studies evaluated patient or clinician compliance to individual components of the ERAS or SDD protocols.
Discussion
This is the first systematic review investigating the safety and efficacy of same-day discharge (SDD) protocols, or “hyper-ERAS”, after colorectal surgery, showing a significant reduction in LOS, low risks of readmissions, and comparable risks of morbidity. The benefits of a reduced LOS are numerous and include a reduction in nosocomial infections [36], thromboembolic events [37], healthcare costs [38], and improvement in morbidity and mortality rates while enhancing quality of life and patient satisfaction [37].
The safety and acceptability of SDD protocols depends on a multitude of factors, including patient factors (physiological fitness or presence of comorbidities, disease status, compliance to the pathway), surgical factors (type or complexity of surgery, anaesthetic protocols), and the extent of postoperative assessment or care (teleconsultations, home visitations, etc.). Each of these are key tenets of the reviewed studies and should be considered prior to initial implementation of SDD programmes.
Patient factors
Patients selected to undergo SDD were invariably fitter individuals without serious health problems or poorly controlled chronic medical conditions, which would otherwise have heightened perioperative risks. The high rate of successful SDD amongst patients selected for SDD programmes amongst the reviewed studies reflects the appropriateness of patient selection. Cognitive impairment, high ASA scores, or the presence of significant comorbidities were frequently used exclusion criteria for SDD. Others excluded patients with a history of major or complex abdominal surgeries, or those with prior severe postoperative nausea or vomiting. Patients with poor health literacy, without suitable caregivers, should also be excluded from SDD.
For a more objective evaluation for fitness for discharge, several studies [22, 30, 35] used the Chung criteria [39], consisting of five variables including vital signs (temperature, pulse, respiration), ambulatory status, nausea and vomiting, pain, and surgical bleeding. A cut-off score of less than 9 out of 10 was used for patient exclusion from SDD. Gignoux et al. employed a 2-stage discharge process [22, 35]. First, the Modified Aldrete score [40] was used to discharge patients from the post-anaesthesia care unit. This score consists of aspects of motor activity, breathing, blood pressure, level of consciousness and oxygen saturation. Patients with a score above 9 were transferred from PACU to the ambulatory surgical unit. Patients were subsequently discharged from hospital in the evening if they met the Chung discharge criteria.
Surgical and anaesthetic factors
Patients scheduled for more complex surgeries e.g., multivisceral resections, low rectal resections, resections requiring bowel diversion with ostomy, etc., should not be considered for SDD. Moreover, prolonged surgery, major intraoperative complications or unanticipated difficulties can compromise postoperative recovery and warrant exclusion from SDD protocols at the discretion of the operating surgeon or anaesthetist.
Laparoscopy and other minimally invasive surgery (MIS) options reduce operative trauma to the abdominal wall, reducing postoperative pain, analgesia use [41], improving LOS, as well as decreasing rates of readmission and reoperation [42]. It is unsurprising that MIS techniques have become the cornerstone of colorectal ERAS and SDD protocols. All patients who underwent SDD in this review had MIS colorectal surgery. Several operative variations can further improve patient recovery by minimising operative trauma and pain, including intracorporeal versus extracorporeal anastomosis for laparoscopic right hemicolectomy [43]. Pfannenstiel specimen extraction versus midline specimen extraction [44], or natural orifice specimen extraction versus conventional transabdominal specimen extraction [45, 46]. Individual surgeon expertise and experience are important factors [47, 48], as well as proficiency with the surgical platforms used, including conventional laparoscopy, robotic surgery, or transanal approaches.
The type of anaesthesia and postoperative analgesia administered also influences recovery. Opioid-sparing modalities can reduce postoperative ileus, nausea and urinary retention [9, 10], all of which can prolong LOS. Transversus abdominis plane (TAP) block has been shown to provide effective pain relief resulting in a significant reduction in opioid use following colorectal surgery [49, 50]. This method was employed by five of the reviewed studies [25,26,27, 29, 35]. In a recently reported randomised controlled trial, bilateral erector spinae plane (ESP) block was also demonstrated to reduce pain scores and opioid requirements for 24 h following laparoscopic colorectal surgery [51]. While this method was not employed by any of the reviewed SDD studies, it may be a viable alternative to TAP block for SDD pathways.
The 2022 American ERAS guidelines for colorectal surgery strongly recommend pre-emptive, multimodal antiemetic prophylaxis to reduce PONV [10]. Apart from utilisation of opioid-sparing analgesia and reducing operative time, decreasing inhalational anaesthesia can also reduce the risk of PONV [10]. Propofol-based total intravenous anaesthesia (TIVA) has been shown to lower the risk of PONV, reducing post-extubation pain scores and time spent in the post-anaesthesia care unit, while improving patient satisfaction scores, compared to inhalational anaesthesia [52]. Four of the reviewed studies reported PONV amongst SDD patients [25, 32, 33, 35]. Two studies reported administration of intraoperative droperidol and dexamethasone to reduce the risk of PONV [22, 35]. Tweed et al. initially administered a combination of spinal anaesthesia with bupivacaine-glucose and morphine intrathecally prior to general anaesthesia [32]. However, after the first nine patients, this was switched to 60–80 mg intrathecal prilocaine in combination with TIVA, because of an observed increase in incidence of PONV and urinary retention [32].
Postoperative home monitoring
Home assessment of patients for post-SDD is essential for recognition of serious morbidity which may require urgent interventions. Adequate counselling should be provided to patients or caregivers, particularly advice concerning the early symptoms of potential complications. Additionally, a convenient channel for communication and/or evaluation between patients, caregivers, and trained healthcare professionals during the early post-discharge period allows for reassurance for minor postoperative issues, including expected gastrointestinal symptoms, or outpatient treatment for minor complications instead of hospital readmission. The postoperative complication rate and distribution of Clavien-Dindo grade of severity were similar between SDD and standard ERAS arms in the comparative study by Tweed et al. [32]. However, the readmission rate in the SDD group was more than threefold that of the control group (17.1% vs 5.3%, P = 0.051) [32], with outpatient treatment potentially possible in more than half of SDD readmissions.
Availability of and compliance to remote health assessments, as well as familiarity with digital platforms, are important aspects of SDD pathways. Five studies monitored patients via telephone calls with early clinic follow-up appointments [25,26,27, 32, 33]. Bednarski et al. described a “telerecovery” method, where patients underwent videoconferencing with their physicians following discharge [23]. The study protocol also allowed for outpatient intravenous fluid hydration at an ambulatory infusion centre when the patient was identified to have inadequate oral intake or assessed to be at high risk of dehydration [23]. Lee et al. piloted a mobile application that enabled direct communication between patients and physicians, with the added function of daily patient-reported health questionnaire administration [29].
Assessment of individual comfort level with remote postoperative monitoring, in lieu of physical ward rounds, is required. This will likely vary across geographical locations and healthcare settings, and is further dependent on several factors including patient or caregiver tech-savviness or proximity of their residence to the hospital. Appropriate preoperative counselling and reassurance will likely increase patient confidence with remote monitoring modalities. Two studies instituted early healthcare worker home visitation following discharge [22, 35]. Gignoux et.al. instituted a post-discharge protocol consisting of daily home visits by a nurse for the first 10 postoperative days [35]. These resource-intensive initiatives may not be cost-effective in the long-term and modifications can be made once the feasibility of SDD pathways are demonstrated.
Limitations
The review is limited by the paucity of randomised trials comparing SDD with traditional ERAS. Moreover, there was marked heterogeneity in study designs, with significant variability in inclusion criteria, types of surgery, methods of anaesthesia, and discharge criteria, or other components of SDD protocols. The overall compliance rates to SDD or conventional ERAS protocols was also mostly unreported. In addition, not all studies reported the reasons for failure of SDD amongst patients included within SDD pathways. This may be relevant as discharge delays can often be due to social or non-medical reasons, clinician or patient preferences, as well as private insurance requirements [53].
The strict inclusion criteria for SDD limits its generalisability. Differences in patient demographic, surgical, or disease characteristics can result in confounding. SDD patients were significantly younger than their conventional ERAS counterparts in many studies [24, 27, 32, 33]. Most studies did not adjust for confounders during their analysis. Publication bias may ensue when studies with favourable outcomes are preferred over those with negative or inconclusive findings [54]. Lastly, selective reporting of outcomes, including omitting unfavourable or statistically insignificant outcomes, or publishing only a subset of analysed data, can undermine the validity of results.
Conclusion
SDD protocols appear to be safe and feasible in a select group of patients undergoing major colorectal operations, resulting in reduced LOS with no increased risk of 30-day readmissions and postoperative morbidity compared to traditional ERAS. Randomised controlled trials are necessary to further substantiate these findings.
Availability of data and material
Not applicable.
References
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced Recovery After Surgery: A Review. JAMA Surg 152(3):292–298. https://doi.org/10.1001/jamasurg.2016.4952
Bardram L, Funch-Jensen P, Kehlet H (2000) Rapid rehabilitation in elderly patients after laparoscopic colonic resection. Br J Surg 87(11):1540–1545. https://doi.org/10.1046/j.1365-2168.2000.01559.x
Basse L, Hjort Jakobsen D, Billesbølle P, Werner M, Kehlet H (2000) A clinical pathway to accelerate recovery after colonic resection. Ann Surg 232(1):51–57. https://doi.org/10.1097/00000658-200007000-00008
Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbølle P, Hendel HW, Rosenberg J, Kehlet H (2002) Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg 89(4):446–453. https://doi.org/10.1046/j.0007-1323.2001.02044.x
Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z (2013) Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 56(5):667–678. https://doi.org/10.1097/DCR.0b013e3182812842
Lv L, Shao YF, Zhou YB (2012) The enhanced recovery after surgery (ERAS) pathway for patients undergoing colorectal surgery: an update of meta-analysis of randomized controlled trials. Int J Colorectal Dis 27(12):1549–1554. https://doi.org/10.1007/s00384-012-1577-5
Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP (2011) Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 149(6):830–840. https://doi.org/10.1016/j.surg.2010.11.003
Tan SJ, Zhou F, Yui WK, Chen QY, Lin ZL, Hu RY, Gao T, Li N (2014) Fast track programmes vs. traditional care in laparoscopic colorectal surgery: a meta-analysis of randomized controlled trials. Hepato-gastroenterology 61(129):79–84
Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Ljungqvist O (2019) Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 43(3):659–695. https://doi.org/10.1007/s00268-018-4844-y
Irani JL, Hedrick TL, Miller TE, Lee L, Steinhagen E, Shogan BD, Goldberg JE, Feingold DL, Lightner AL, Paquette IM (2023) Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons. Surg Endosc 37(1):5–30. https://doi.org/10.1007/s00464-022-09758-x
Liska D, Novello M, Cengiz BT, Holubar SD, Aiello A, Gorgun E, Steele SR, Delaney CP (2021) Enhanced Recovery Pathway Benefits Patients Undergoing Nonelective Colorectal Surgery. Ann Surg 273(4):772–777. https://doi.org/10.1097/SLA.0000000000003438
Lohsiriwat V, Jitmungngan R, Chadbunchachai W, Ungprasert P (2020) Enhanced recovery after surgery in emergency resection for obstructive colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 35(8):1453–1461. https://doi.org/10.1007/s00384-020-03652-5
Launay-Savary, M. V., Mathonnet, M., Theissen, A., Ostermann, S., Raynaud-Simon, A., Slim, K., & GRACE (Groupe francophone de Réhabilitation Améliorée après Chirurgie) (2017) Are enhanced recovery programs in colorectal surgery feasible and useful in the elderly? A systematic review of the literature. J Visc Surg 154(1):29–35. https://doi.org/10.1016/j.jviscsurg.2016.09.016
Tan JKH, Ang JJ, Chan DKH (2021) Enhanced recovery program versus conventional care after colorectal surgery in the geriatric population: a systematic review and meta-analysis. Surg Endosc 35(6):3166–3174. https://doi.org/10.1007/s00464-020-07673-7
Karam E, Sindayigaya R, Giger-Pabst U, Gabriel M, Michot N, Courtot L, Tabchouri N, Moussata D, Lecomte T, Chapet S, Calais G, Bourlier P, Salamé E, Ouaissi M (2022) Impact of Modern Management Strategies on the Clinical Outcome of Patients With Low Rectal Cancer – A Retrospective, Monocentric Cohort Study. Anticancer Res 42(4):1949–1963. https://doi.org/10.21873/anticanres.15673
Peng D, Cheng YX, Tao W, Tang H, Ji GY (2022) Effect of enhanced recovery after surgery on inflammatory bowel disease surgery: A meta-analysis. World J Clin Case 10(11):3426–3435. https://doi.org/10.12998/wjcc.v10.i11.3426
Mao F, Huang Z (2021) Enhanced Recovery After Surgery for Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Systematic Review and Meta-Analysis. Front Surg 8:713171. https://doi.org/10.3389/fsurg.2021.713171
Messenger DE, Curtis NJ, Jones A, Jones EL, Smart NJ, Francis NK (2017) Factors predicting outcome from enhanced recovery programmes in laparoscopic colorectal surgery: a systematic review. Surg Endosc 31(5):2050–2071. https://doi.org/10.1007/s00464-016-5205-2
Berian JR, Ban KA, Liu JB, Ko CY, Feldman LS, Thacker JK (2019) Adherence to Enhanced Recovery Protocols in NSQIP and Association With Colectomy Outcomes. Ann Surg 269(3):486–493. https://doi.org/10.1097/SLA.0000000000002566
Seow-En I, Wu J, Yang LWY, Tan JSQ, Seah AWH, Foo FJ, Chang M, Tang CL, Tan EKW (2021) Results of a colorectal enhanced recovery after surgery (ERAS) programme and a qualitative analysis of healthcare workers’ perspectives. Asian J Surg 44(1):307–312. https://doi.org/10.1016/j.asjsur.2020.07.020
Li N, Liu Y, Chen H, Sun Y (2022) Efficacy and Safety of Enhanced Recovery After Surgery Pathway in Minimally Invasive Colorectal Cancer surgery: A Systemic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2022.0349
Gignoux B, Pasquer A, Vulliez A, Lanz T (2015) Outpatient colectomy within an enhanced recovery program. J Visc Surg 152(1):11–15. https://doi.org/10.1016/j.jviscsurg.2014.12.004
Bednarski BK, Nickerson TP, You YN, Messick CA, Speer B, Gottumukkala V, Manandhar M, Weldon M, Dean EM, Qiao W, Wang X, Chang GJ (2019) Randomized clinical trial of accelerated enhanced recovery after minimally invasive colorectal cancer surgery (RecoverMI trial). Br J Surg 106(10):1311–1318. https://doi.org/10.1002/bjs.11223
Brandt E, Poulsen M, Lykke J, Jess P, Ovesen H (2013) A minority of patients discharged within 24 hours after laparoscopic colon resection. Dan Med J 60(7):A4658
Campbell S, Fichera A, Thomas S, Papaconstantinou H, Essani R (2021) Outpatient colectomy-a dream or reality? Proceedings (Baylor University. Medical Center) 35(1):24–27. https://doi.org/10.1080/08998280.2021.1973327
Curfman KR, Poola AS, Blair GE, Kosnik CL, Pille SA, Hawkins ME, Rashidi L (2022) Ambulatory colectomy: a pathway for advancing the enhanced recovery protocol. J Robotic Surg 1–8. Advance online publication. https://doi.org/10.1007/s11701-022-01463-0
de Azevedo JGM, Mendes CRS, Lima MA, Pessoa JCSP, São Julião GP, Perez RO, Vailati BB (2021) Laparoscopic colorectal surgery and discharge within 24 h-who is at risk for readmission? Colorectal Dis 23(10):2714–2722. https://doi.org/10.1111/codi.15791
Dobradin A, Ganji M, Alam SE, Kar PM (2013) Laparoscopic colon resections with discharge less than 24 hours. J Soc Laparoendosc Surg 17(2):198–203. https://doi.org/10.4293/108680813X13654754535791
Lee L, Eustache J, Baldini G, Liberman A, Charlebois P, Stein B, Fiore JF, Feldman LS (2022) Enhanced Recovery 2.0 – Same Day Discharge With Mobile App Follow-up After Minimally Invasive Colorectal Surgery. Ann Surg 276(6):e812–e818. https://doi.org/10.1097/SLA.0000000000004962
McKenna NP, Bews KA, Shariq OA, Habermann EB, Behm KT, Kelley SR, Larson DW (2020) Is Same-Day and Next-Day Discharge After Laparoscopic Colectomy Reasonable in Select Patients? Dis Colon Rectum 63(10):1427–1435. https://doi.org/10.1097/DCR.0000000000001729
Popeskou SG, Christou N, Panteleimonitis S, Langford E, Qureshi T, Parvaiz A (2022) Safety and Feasibility of a Discharge within 23 Hours after Colorectal Laparoscopic Surgery. J Clin Med 11(17):5068. MDPI AG. https://doi.org/10.3390/jcm11175068
Tweed TTT, Sier MAT, Daher I, Bakens MJAM, Nel J, Bouvy ND, van Bastelaar J, Stoot JHMB (2022) Accelerated 23-h enhanced recovery protocol for colon surgery: the CHASE-study. Sci Rep 12(1):20707. https://doi.org/10.1038/s41598-022-25022-7
Levy BF, Scott MJ, Fawcett WJ, Rockall TA (2009) 23-hour-stay laparoscopic colectomy. Dis Colon Rectum 52(7):1239–1243. https://doi.org/10.1007/DCR.0b013e3181a0b32d
Curfman KR, Blair GE, Pille SA, Kosnik CL, Rashidi L (2022) The patient perspective of same day discharge colectomy: one hundred patients surveyed on their experience following colon surgery. Surg Endosc 1–6. Advance online publication. https://doi.org/10.1007/s00464-022-09446-w
Gignoux B, Gosgnach M, Lanz T, Vulliez A, Blanchet MC, Frering V, Faucheron JL, Chasserant P (2019) Short-term Outcomes of Ambulatory Colectomy for 157 Consecutive Patients. Ann Surg 270(2):317–321. https://doi.org/10.1097/SLA.0000000000002800
Rosman M, Rachminov O, Segal O, Segal G (2015) Prolonged patients’ In-Hospital Waiting Period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res 15:246. https://doi.org/10.1186/s12913-015-0929-6
Diwan W, Nakonezny PA, Wells J (2020) The Effect of Length of Hospital Stay and Patient Factors on Patient Satisfaction in an Academic Hospital. Orthopedics 43(6):373–379. https://doi.org/10.3928/01477447-20200910-02
Amin A, Neuman WR, Lingohr-Smith M, Menges B, Lin J (2019) Influence of the duration of hospital length of stay on frequency of prophylaxis and risk for venous thromboembolism among patients hospitalized for acute medical illnesses in the USA. Drugs Context 8:212568. https://doi.org/10.7573/dic.212568
Chung F, Chan VW, Ong D (1995) A post-anesthetic discharge scoring system for home readiness after ambulatory surgery. J Clin Anesth 7(6):500–506. https://doi.org/10.1016/0952-8180(95)00130-a
Twersky RS, Sapozhnikova S, Toure B (2008) Risk factors associated with fast-track ineligibility after monitored anesthesia care in ambulatory surgery patients. Anesth Analg 106(5). https://doi.org/10.1213/ane.0b013e31816a6600
Bastawrous AL, Brockhaus KK, Chang MI et al (2022) A national database propensity score-matched comparison of minimally invasive and open colectomy for long-term opioid use. Surg Endosc 36(1):701–710. https://doi.org/10.1007/s00464-021-08338-9
Ricciardi R, Goldstone RN, Francone T, Wszolek M, Auchincloss H, de Groot A, Shih IF, Li Y (2022) Healthcare Resource Utilization After Surgical Treatment of Cancer: Value of Minimally Invasive Surgery. Surg Endosc 36(10):7549–7560. https://doi.org/10.1007/s00464-022-09189-8
Zhang H, Sun N, Fu Y, Zhao C (2021) Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: updated meta-analysis of randomized controlled trials. BJS Open 5(6):zrab133. https://doi.org/10.1093/bjsopen/zrab133
Lee L, McLemore E, Rashidi L (2022) Same-Day Discharge After Minimally Invasive Colectomy. JAMA Surg 157(11):1059–1060. https://doi.org/10.1001/jamasurg.2022.4123
Chin YH, Decruz GM, Ng CH, Tan HQM, Lim F, Foo FJ, Tai CH, Chong CS (2021) Colorectal resection via natural orifice specimen extraction versus conventional laparoscopic extraction: a meta-analysis with meta-regression. Tech Coloproctol 25(1):35–48. https://doi.org/10.1007/s10151-020-02330-6
Seow-En I, Chen LR, Li YX, Zhao Y, Chen JH, Abdullah HR, Tan EK (2022) Outcomes after natural orifice extraction vs conventional specimen extraction surgery for colorectal cancer: A propensity score-matched analysis. World J Clin Oncol 13(10):789–801. https://doi.org/10.5306/wjco.v13.i10.789
Harmon JW, Tang DG, Gordon TA, Bowman HM, Choti MA, Kaufman HS, Bender JS, Duncan MD, Magnuson TH, Lillemoe KD, Cameron JL (1999) Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 230(3):404–413. https://doi.org/10.1097/00000658-199909000-00013
Prystowsky JB, Bordage G, Feinglass JM (2002) Patient outcomes for segmental colon resection according to surgeon’s training, certification, and experience. Surgery 132(4):663–672. https://doi.org/10.1067/msy.2002.127550
Peltrini R, Cantoni V, Green R, Greco PA, Calabria M, Bucci L, Corcione F (2020) Efficacy of transversus abdominis plane (TAP) block in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 24(8):787–802. https://doi.org/10.1007/s10151-020-02206-9
Kim AJ, Yong RJ, Urman RD (2017) The Role of Transversus Abdominis Plane Blocks in Enhanced Recovery After Surgery Pathways for Open and Laparoscopic Colorectal Surgery. J Laparoendosc Adv Surg Tech A 27(9):909–914. https://doi.org/10.1089/lap.2017.0337
Choi JJ, Chang YJ, Lee D, Kim HW, Kwak HJ (2022) Effect of Erector Spinae Plane Block on Postoperative Pain after Laparoscopic Colorectal Surgery: A Randomized Controlled Study. J Pers Med 12(10):1717. https://doi.org/10.3390/jpm12101717
Schraag S, Pradelli L, Alsaleh AJ, Bellone M, Ghetti G, Chung TL, Westphal M, Rehberg S (2018) Propofol vs. inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anesthesiol 18(1):162. https://doi.org/10.1186/s12871-018-0632-3
Celio DA, Poggi R, Schmalzbauer M, Rosso R, Majno P, Christoforidis D (2019) ERAS, length of stay and private insurance: a retrospective study. Int J Colorectal Dis 34(11):1865–1870. https://doi.org/10.1007/s00384-019-03391-2
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR (1991) Publication bias in clinical research. Lancet (London, England) 337(8746):867–872. https://doi.org/10.1016/0140-6736(91)90201-y
Author information
Authors and Affiliations
Contributions
Zheng V. (co-1st author) made substantial contributions to the conception or design of the work; data acquisition, analysis, and interpretation; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Prepared all tables and figures. IJY Wee. (co-1st author) made substantial contributions to the conception or design of the work; data acquisition, analysis, and interpretation; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Prepared all tables and figures. Abdullah HR. made substantial contributions to the conception or design of the work; revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Tan S. made substantial contributions to the conception or design of the work; revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Tan EKW made substantial contributions to the conception or design of the work; revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Seow-En I. made substantial contributions to the conception or design of the work; data acquisition, analysis, and interpretation; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
This manuscript has not been presented at a podium or poster meeting.
Ethics approval
Not applicable
Patient consent
Not applicable.
Permission to reproduce material from other sources
Not applicable.
Conflicts of interest
All authors disclose no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, V., Wee, I.J.Y., Abdullah, H.R. et al. Same-day discharge (SDD) vs standard enhanced recovery after surgery (ERAS) protocols for major colorectal surgery: a systematic review. Int J Colorectal Dis 38, 110 (2023). https://doi.org/10.1007/s00384-023-04408-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04408-7