Abstract
Purpose
The Enhanced Recovery After Surgery (ERAS) program aims to combine and coordinate evidence-based perioperative care interventions that support standardizing and optimizing surgical care. In conjunction with its clinical benefits, it has been suggested that ERAS reduces costs through shorter convalescence and reduced morbidity. Nevertheless, few studies have evaluated the cost-effectiveness of ERAS programs. The aim of this systematic review, therefore, is to evaluate the claims that ERAS is cost-effective and to characterize how these costs were reported and evaluated.
Source
The electronic databases, MEDLINE® and EMBASE™, were searched from inception to April 2014.
Principal findings
Seventeen studies met the inclusion criteria and were included for review. Enhanced Recovery After Surgery protocols in various abdominal surgeries have been investigated, including colorectal, bariatric, gynecological, gastric, pancreatic, esophageal, and vascular surgery. All studies reported cost savings associated with hastening recovery and reducing morbidity and complications. All studies included in this review focused primarily on in-hospital costs, with some attempting to account for readmission costs and follow-up services. In all but two studies, the breakdown of cost data for the individual studies was poorly detailed.
Conclusions
In conclusion, ERAS protocols appear to be both clinically efficacious and cost effective across a variety of surgical specialties in the short term. Nevertheless, studies reporting out-of-hospital cost data are lacking. Further research is required to determine how best to evaluate both medium- and long-term costs relating to ERAS pathways while taking quality of life data into account.
Résumé
Objectif
Le programme de Récupération rapide après la chirurgie (RRAC) vise à combiner et coordonner des interventions de soins périopératoires basées sur des données probantes qui soutiennent la standardisation et l’optimisation des soins chirurgicaux. En association avec ses avantages cliniques, il a été suggéré que la RRAC réduisait les coûts grâce à une convalescence raccourcie et une moindre morbidité. Néanmoins, peu d’études ont évalué le rapport efficacité-coût des programmes de RRAC. Le but de cette synthèse systématique est donc d’évaluer l’argument de rentabilité de la RRAC et de préciser dans quelle mesure ces coûts ont été rapportés et évalués.
Sources
Une recherche a été menée dans les bases de données électroniques MEDLINE® et EMBASE™ depuis les origines du concept jusqu’en avril 2014.
Constatations principales
Dix-sept études répondaient aux critères d’inclusion et ont été analysées. Les protocoles de Récupération rapide après la chirurgie concernant différentes interventions chirurgicales abdominales (colorectales, bariatriques, gynécologiques, gastriques, pancréatiques, œsophagiennes et vasculaires) ont été étudiés. Toutes les études rapportaient des économies associées à l’accélération de la récupération et à la réduction de la morbidité et des complications. Toutes les études incluses dans cette synthèse étaient principalement centrées sur les coûts hospitaliers, certaines d’entre elles tentant de prendre en compte les coûts de réadmission et des services de suivi. Hormis dans deux études, la ventilation des coûts était médiocrement détaillée dans chacune de ces études.
Conclusions
En résumé, les protocoles de RRAC semblent efficaces à court terme à la fois sur le plan clinique et sur celui de la rentabilité dans une gamme de spécialités chirurgicales. Néanmoins, il manque dans ces études les données concernant les coûts hors de l’hôpital. D’autres recherches seront nécessaires pour déterminer comment mieux évaluer à la fois les coûts à court et à long terme en rapport avec les programmes de RRAC, tout en prenant en compte les données de qualité de vie.
Similar content being viewed by others
Enhanced Recovery After Surgery (ERAS) programs are evidence-based perioperative care interventions aimed at standardizing and optimizing perioperative care. Henrik Kehlet of Denmark initially established an Enhanced Recovery After Surgery pathway in colorectal surgery, and there is now a large body of evidence that shows significant reductions in hospital length of stay (LOS) and morbidity in patients who undergo surgery within ERAS programs. Although the initial experiences with ERAS programs were described in the setting of colorectal surgery, the principles of ERAS are now utilized in many other surgical specialties, particularly abdominal surgery.
It has been suggested that, along with the established clinical benefits, ERAS programs may be associated with a reduction in costs.1 This is thought to be a result of the reduction in LOS and morbidity. Even so, there are currently very few studies that have investigated the cost-effectiveness of ERAS programs. Two review articles have investigated how cost data are reported and analyzed in colorectal surgery.1,2 Despite suggestions that ERAS programs in colorectal surgery are cost effective, authors from both of these reviews pointed out that differences in the reporting and analysis of cost data mean that these claims must be interpreted with caution. In their systematic review, Lee et al. give an excellent account on how to evaluate studies reporting costs, and the authors of this present review drew on that account as a template for the current review.
When performing an economic evaluation of an intervention, it is important to recognize the perspective by which the costs are determined – direct payer costs or indirect societal costs – as this can influence the study design.3 Evaluating the relative costs (and effectiveness) of an intervention is often measured by the amount of in-hospital resource utilization or the costs incurred during readmissions. Measurement of such direct costs may not account for the costs/benefits to the welfare of the population as a whole. From a societal perspective, many argue that interventions have wider implications outside the immediate hospital admission that influence return to work, mood, and quality of life (QoL). For example, consider a patient who underwent an operation within an ERAS setting and was successfully discharged early from hospital, thus effectively reducing in-hospital costs. Despite an expedited discharge, the patient may still experience fatigue and require additional home support in the form of equipment or caregiver supervision and outpatient services. If these new out-of-hospital costs (i.e., societal costs) are not recognized and detailed in studies, then the cost savings from a ERAS program may be overestimated. In addition, a societal perspective helps to detect cost-shifting between health sectors and is useful for policymaking when allocating resources.3
Thus, the purpose of this systematic review was to identify studies that evaluate the cost-effectiveness of ERAS programs in other abdominal surgeries. We aimed to evaluate claims within the current literature that ERAS is cost effective as well as to characterize how costs within an ERAS pathway are reported and evaluated.
Methods
Search strategy
A comprehensive systematic review was conducted independently by all three authors using search terms described in Table 1. The MEDLINE® and EMBASE™ medical databases were utilized from inception until April 2014. The authors then manually scrutinized reference lists in the recovered articles and relevant abstracts from scientific meetings to identify any further articles.
Study selection
Studies were considered for review if they evaluated the outcomes of patients undergoing elective abdominal surgery within an optimized perioperative care or ERAS program and included perioperative cost data as either a primary or a secondary outcome. For the purposes of this review, we excluded studies that evaluated optimized perioperative care programs within cardiac, emergency, and pediatric surgery, and studies that could not be retrieved in English. There were no exclusions based on study design. The final decisions on article inclusion were made by the senior author (A.G.H.).
For the purposes of this review, all studies incorporating optimized perioperative care or an ERAS program were termed ERAS programs.
Data extraction and synthesis
Data extraction was carried out using pre-designed Microsoft Excel data sheets. The primary outcome of interest was the cost-data analysis. Costs were determined from either a payer (direct) or a societal (indirect) perspective. Other details recorded included sample sizes, study design, country, patient demographics, and surgery type and costs. In addition, using the ERAS Society guidelines (http://www.erassociety.org/index.php/eras-care-system/eras-protocol), each study’s perioperative care protocol, excluding the review articles and abstracts, was assessed against the 20 care elements of an ERAS care system. Realizing disease-specific and surgery-specific distinctions, we made an effort to highlight these differences.
As with a previous review reporting on cost-data analysis in ERAS programs,2 we used the Consensus on Health Economic Criteria (CHEC) tool to assess the quality of the economic evaluation of the included studies.4 The CHEC instrument contains 19 yes/no questions, and where there is insufficient information reported in the study, we allocate a negative response (“no”). Each of the 19 items on the questionnaire was given one point if present, thus giving a possible total quality score of 19 if all items are present. An economic evaluation with a score ≥ 12 is considered to be of “high quality”. A Measurement Tool to Assess Systematic Reviews (AMSTAR) was used to measure the quality of the systematic reviews included in the study.5 The tool uses 11 yes/no items to assess the methodological quality of systematic reviews and has been shown to have good inter-rater agreement, test-retest reliability, and face and construct validity.6 When assessing an individual study, each positive answer is allocated one point. A higher score denotes a more methodologically sound review. Quality assessment was performed by two independent reviewers (M.D.J.S. and D.P.L.), and any disagreement was settled by discussion and then by the senior author (A.G.H.).
Results
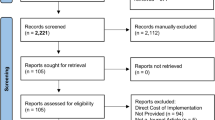
After excluding duplicates, studies that were already covered in a systematic review, and studies not in English, 17 papers or abstracts analyzing ERAS program costs were included in the review (Figure).
Enhanced Recovery After Surgery programs in abdominal surgery have been implemented in many countries around the world. In this review, studies investigating the cost as either a primary or secondary outcome have been conducted in Europe,7-12 Asia,13-18 North America,2,19 USA,20 and Australasia.1,21
Six of the 17 studies were systematic reviews.1,2,7,12,14,15 Three of these, all of which evaluated ERAS in the setting of gastric surgery, performed meta-analysis suggesting cost savings compared with conventional management.7,14,15 The mean quality score of these systematic reviews was 8.75 (range: 7-10 out of 11). Two reviews had insufficient information to perform a quality assessment as they were abstracts only.7,12 Six of the included studies were abstracts.7,10-12,17,19 Outcome measures, including costs and any other relevant information (i.e., method of analysis, study characteristics etc.) were reported if present in the abstract.
Studies assessed for methodological quality using the CHEC tool had scores ranging from 4-11.8,9,13,18,20,21 Thus, no study achieved the minimum score to be considered “high quality”. Three studies in gynecology scored 11 of a possible 19. Relph et al. and Yoong et al. employed the same cost-data (institutional perspective) analysis for their evaluations of ERAS in elective vaginal hysterectomy. Excluding systematic reviews and abstracts, components of ERAS programs in colorectal, gynecology, bariatric, gastric, and vascular surgery are outlined in Table 2, and specialty-specific care elements are also mentioned.
The identified studies evaluated costs across several surgical specialties, including colorectal surgery, gynecological procedures, bariatric surgery, gastric surgery, pancreatic surgery, esophageal surgery, and abdominal vascular surgery (Table 3).
Colorectal surgery
Two recent systematic reviews of the cost-effectiveness of ERAS in colorectal surgery have been published. Lemanu et al. reported on seven studies in colorectal surgery.1 Reporting of cost data was inconsistent across these studies, but all studies showed cost reductions. There was no attempt in any of the studies to quantify costs to the community.
Lee et al. reported on ten studies,2 the majority (eight) of which were performed from an institutional perspective. Five studies provided enough information to calculate incremental cost-effectiveness ratios, and in all cases, ERAS programs were less costly and more effective for reducing LOS. In four studies, ERAS was potentially cost effective, and in one study, ERAS was questionable for a reduction in overall complications. Similar to the previous systematic review, Lee et al. reported that the studies lacked measurements of the indirect costs of the ERAS programs. Most studies did not include costs related to changes in patient productivity and other indirect costs (e.g., caregiver burden).
Both systematic reviews concluded that the quality of the current evidence is limited but tends to support the cost-effectiveness of ERAS programs. There is need for well-designed trials to determine the cost-effectiveness of ERAS programs from both the institutional and societal perspectives.
Only one study specifically addressed ERAS in rectal surgery. Feng et al. randomized 120 patients with rectal cancer to ERAS combined with laparoscopy or conventional perioperative care plus laparoscopy.13 Compared with the conventional perioperative care group, the ERAS protocol significantly reduced the rate of postoperative complications and LOS. The medical cost of hospitalization was also reduced in the ERAS group, with the majority of reported cost savings being attributed to reduced drug use, especially antibiotics.
Bariatric surgery
One study evaluated costs of an ERAS program in bariatric surgery. Lemanu et al. performed a randomized controlled trial comparing patients undergoing laparoscopic sleeve gastrectomy in an ERAS protocol with those receiving conventional care.21 A propensity-matched historical control group was used to assess crossover of ERAS principles to the control group given the difficulties in blinding patients and their caregivers to ERAS interventions.
There were 116 patients included in the analysis; 78 patients were allocated to either the ERAS (40) or the control (38) group, and 38 patients were in the historical group. Length of stay was significantly shorter in the ERAS group (one day) than in the control (two days) and historical (three days) groups. There was no difference in readmission rates, postoperative complications, or postoperative fatigue. The mean cost per patient was significantly higher in the historical group than in the ERAS and control groups.
Gastric surgery
Three systematic reviews assessed ERAS in gastric surgery.7,14,15 Beamish et al. conducted a systematic review and meta-analysis of ERAS programs in gastric surgery.7 The primary outcome measure investigated was postoperative LOS, and secondary outcome measures were selected based on inclusion in two or more studies. Eight studies (five randomized trials) comprising 1,339 patients with gastric cancer were analyzed. Length of stay was significantly shorter in the ERAS group than in the control group. Mortality, morbidity, and readmissions were similar between groups. Costs were significantly decreased with ERAS.
Yu et al. showed that postoperative hospital stay and hospital costs were significantly less for patients in the ERAS program undergoing gastrectomy for gastric cancer, with mean weighted differences of −1.87 days (95% confidence interval [CI] −2.46 to −1.28) and −505.87 USD (95% CI −649.91 to −361.84).15 No differences were found for readmission and total complication rates. Chen et al. also reported significant reductions in day stay and medical costs in both laparoscopic and open gastrectomy when an ERAS pathway was used.14
One randomized controlled trial investigated ERAS in radical total gastrectomy patients. One hundred nineteen patients were randomized to either an ERAS group (n = 59) or a conventional care group (n = 60).16 Investigators reported significant reductions in time to flatus and defecation, pain scores, complications, and costs (P < 0.05).
Gynecology
Yoong et al. described implementation of an ERAS protocol in vaginal hysterectomy.8 After ERAS implementation, the median length of stay was reduced by 51.6% (22.0 hr vs 45.5 hr; P < 0.01), and the percentage of patients discharged within 24 hr was increased five-fold (78.0% vs 15.6%; P < 0.05). Attendance in the Accident and Emergency Department (12% vs 0%; P > 0.05) and inpatient readmission rate (4% vs 0%; P > 0.05) were not statistically different between the groups. Despite having to start a “gynecology school” and employ a specialist enhanced recovery nurse, a cost savings of 9.25% per patient was realized. Relph et al. reported on similar cost data in their study of 100 patients (50 ERAS and 50 control patients).9 Median length of stay was more than halved (22.0 hr vs 45.5 hr, respectively; P < 0.01) with a cost savings of £106.30 per patient.
Kalogera described patients managed in an ERAS program undergoing a variety of different types of gynecologic surgery.20 A total of 241 ERAS cases (81 cytoreduction, 84 staging, and 76 vaginal surgeries) were compared with cases in the control groups. The ERAS program resulted in a four-day reduction in hospital stay with stable readmission rates and a 30-day cost savings of more than $7,600 USD per patient (18.8% reduction). No differences were observed in either the rate or the severity of complications. Similar improvements were observed in the other two cohorts. Ninety-five percent of patients rated their satisfaction with perioperative care as excellent or very good.
Narang et al. 10 and Torbe et al.11 evaluated an ERAS pathway in their hospitals in patients undergoing hysterectomy and myomectomy (open and laparoscopic). Both authors showed a cost savings with a decrease of £342.66 and £197.71 per patient, respectively.
Pancreatic surgery
Coolsen et al. systematically reviewed ERAS programs in pancreaticoduodenectomies. Four of eight studies from the USA included in his review reported on the economic impact of implementing an ERAS program.12 All studies showed a reduction in cost after introduction of their respective pathways. When cost savings were combined, not accounting for inflation and cost differences over time, this equated to a cost savings of $344.57 USD per patient.
Nakamura et al. retrospectively reviewed 283 consecutive patients undergoing a pancreaticoduodenectomy during 1995-2008.17 During 2004-2009, 134 patients were treated in an ERAS protocol and were compared with 149 historical controls. The rates of intra-abdominal complications, including pancreatic fistula, were similar between the two groups. Length of stay was reduced with the fast-track protocol (median 24 vs 45 days, respectively; P < 0.001), with reduced postoperative costs ($8,716 vs $18,511[currency not stated], respectively; P < 0.001).
Esophageal surgery
Lee et al. investigated the utility of an ERAS program in esophagectomy19 with two well-matched groups of patients, 106 in total (47 traditional care patients and 59 ERAS patients). The median [interquartile range] LOS was lower in the ERAS group than in the traditional care group (87-18 days vs 109-18 days, respectively; P = 0.019). There was no difference in 30‐day complication rates and mortality rates. The overall cost savings per patient was $1,472 CAD (weighted average median cost). Using a one‐way sensitivity analysis, they were able to show that the ERAS program was more costly only at extreme values of ward, operating, and intensive care costs.
Vascular surgery
Tatsuishi et al. compared 52 patients undergoing open aortic aneurysm repair in an ERAS program with 75 patients who previously received conventional treatment.18 The time to restart oral consumption and the postoperative LOS were significantly shorter in the ERAS group than in the conventionally managed group, and the in-hospital medical costs for the ERAS group decreased by 8% compared with those for the conventionally managed group.
Discussion
This paper describes economic evaluations in a wide variety of abdominal surgical procedures conducted in an ERAS environment. Almost all studies showed decreases in LOS and costs. Evaluations varied widely, with some including only hospital costs and others attempting to quantify costs more broadly.
Most studies evaluated only hospital-associated costs, with only two studies in gynecology from the same institute detailing cost items preoperatively, perioperatively, and postoperatively.8,9 Even so, very few studies made any attempt to look at cost-transfer from the hospital to the community. This is an important consideration given that any cost reduction shown in the included studies could be tempered by transference of costs to community health services, which could potentially result from a shortened LOS. Similarly, there are few studies reporting on the indirect costs associated with surgery, such as convalescence before resuming employment and a prolonged inability to participate in day-to-day household and community activities.
Interestingly, costs for readmission and morbidity are not consistently included in the cost analyses. Given the increased likelihood that such events would require greater utilization of healthcare resources, readmission and morbidity are likely to inflate costs per patient significantly. While none of the included studies found a significant increase in the incidence of either readmission or morbidity, it is important to report these costs in future studies given that small, even statistically insignificant changes may be significant in terms of cost analyses, particularly if penalties for such events are costly. One approach to address this issue is to develop a universal administrative database that monitors out-of-hospital use of resources. Cheng et al. used such methods to evaluate use of health resources up to one year following discharge after coronary artery bypass graft surgery in the setting of fast-track cardiac anesthesia (FTCA) versus conventional anesthesia.22 Resource use was analyzed as use of hospital and rehabilitation service bed days, physician consult costs, and use of cardiac drugs. They showed a 68% and 50% reduction in health resource costs in the FTCA group at three months and one year, respectively. Obviously, the cost to implement the program and set up such a database would need to be evaluated in order to obtain an accurate record of cost savings, if any.
Only a few studies have looked at QoL with ERAS protocols. Lemanu et al. utilized a surgical recovery instrument but were unable to show any improvements in recovery with an ERAS program in bariatric surgery.21 In a recent systematic review investigating the impact of ERAS programs in orthopedic surgery on patient experience and QoL, only two of eight included studies measured QoL data.23 Improvements in QoL may manifest as an earlier return to work and possibly a reduction in minor complications, which could lead to reduced direct and indirect costs. Tools to evaluate QoL data should be reliable, validated, and applicable across populations.
The Short Health Survey (SF-36®) and the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) are such tools that have been used to evaluate QoL data, with the latter having direct explicit links to the International Classification of Functioning, Disability and Health.24 Like the SF-36, WHODAS 2.0 covers an individual’s functioning in six major life domains: (i) cognition (understanding and communication); (ii) mobility (ability to move and get around); (iii) self-care (ability to attend to personal hygiene, dressing and eating, and to live alone); (iv) getting along (ability to interact with other people); (v) life activities (ability to carry out responsibilities at home, work, and school); (vi) participation in society (ability to engage in community, civil, and recreational activities).24 One important omission from these tools, however, is the impact an intervention has on sleep, which may have important implications on daily functioning. Future studies performing economic evaluations should therefore include such measures as an important surrogate for indirect costs.
This study has some limitations. Most of the included studies were retrospective in nature, which subjects the conclusions of the studies to the biases associated with retrospective study design. There was significant heterogeneity of the included studies with respect to study design, population (including geographical diversity and differences in healthcare systems), and cost-assessment methodology. Pooling data is therefore ineffective, and meta-analysis cannot be performed. Studies varied widely in their definition of ERAS or fast-track; therefore, the composition of the ERAS protocols used differed significantly amongst the studies. As a result, it is difficult to determine if specific care interventions are more effective than others at conferring the benefits of the ERAS pathways. Lastly, all studies report only short-term data, with long-term clinical efficacy and cost-effectiveness remaining unclear.
In conclusion, ERAS protocols appear to be both clinically efficacious and cost effective across a variety of surgical specialties in the short-term. Importantly, investigators evaluating the efficacy of ERAS in future studies should be encouraged to include cost-effectiveness in their outcome measures. This approach will allow for a better grasp on how to evaluate costs relating to ERAS as well as how well cost outcomes correlate with clinical outcomes.
Key points
-
Studies performing economic evaluations of Enhanced Recovery After Surgery (ERAS) protocols across many abdominal surgical specialties appear to be cost-effective. However, cost data reporting is inconsistent with many authors preferring to report direct costs (payer’s perspective) over indirect costs (societal perspective).
-
Identifying key cost savings on specific ERAS items may be useful in the allocation of scarce health resources.
-
Further studies evaluating the cost-effectiveness of ERAS programs are required in order to determine how best to evaluate costs relating to ERAS protocols, including medium- to long-term costs.
References
Lemanu DP, Singh PP, Stowers MD, Hill AG. A systematic review to assess cost effectiveness of enhanced recovery after surgery programmes in colorectal surgery. Colorectal Dis 2014; 16: 338-46.
Lee L, Li C, Landry T, et al. A systematic review of economic evaluations of enhanced recovery pathways for colorectal surgery. Ann Surg 2014; 259: 670-6.
Byford S, Raftery J. Perspectives in economic evaluation. BMJ 1998; 316: 1529-30.
Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005; 21: 240-5.
Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007; 7: 10.
Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009; 62: 1013-20.
Beamish AJ, Chan DS, Blake PA, Karran A, Lewis WG. Systematic review and meta-analysis of enhanced recovery programmes in gastric cancer surgery. Gastroenterology 2013; 1: S220 (abstract).
Yoong W, Sivashanmugarajan V, Relph S, et al. Can enhanced recovery pathways improve outcomes of vaginal hysterectomy? Cohort control study. J Minim Invasive Gynecol 2014; 21: 83-9.
Relph S, Bell A, Sivashanmugarajan V, et al. Cost effectiveness of enhanced recovery after surgery programme for vaginal hysterectomy: a comparison of pre and post-implementation expenditures. Int J Health Plann Mgmt 2013; DOI:10.1002/hpm.2182.
Narang L, Mitchelmore S, Byrne H. Cost reduction and enhanced patient experience following the introduction of enhanced recovery programme in gynaecological surgery. BJOG 2013; 120: 439 (absract).
Torbe E, Louden K. An enhanced recovery programme for women undergoing hysterectomy. Int J Gynecol Obstet 2012; 119: S690.
Coolsen M, Van Dam R, Van Der Wilt A, et al. Enhanced recovery after pancreatic surgery: a systematic review and meta-analysis. HPB 2012; 14(Suppl 2): 218-9 (abstract).
Feng F, Li XH, Shi H, et al. Fast-track surgery combined with laparoscopy could improve postoperative recovery of low-risk rectal cancer patients: a randomized controlled clinical trial. J Dig Dis 2014; 15: 306-13.
Chen ZX, Liu AH, Cen Y. Fast-track program vs traditional care in surgery for gastric cancer. World J Gastroenterol 2014; 20: 578-83.
Yu Z, Zhuang CL, Ye XZ, Zhang CJ, Dong QT, Chen BC. Fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Langenbecks Arch Surg 2014; 399: 85-92.
Feng F, Ji G, Li JP, et al. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol 2013; 19: 3642-8.
Nakamura T, Inoue Y, Ambo Y, et al. Economic impact of fast-track surgery after pancreaticoduodenectomy. Pancreas 2009; 38: 1032 (abstract).
Tatsuishi W, Kohri T, Kodera K, et al. Usefulness of an enhanced recovery after surgery protocol for perioperative management following open repair of an abdominal aortic aneurysm. Surg Today 2012; 42: 1195-200.
Lee L, Li C, Ferri LE, et al. Economic impact of an enhanced recovery pathway for esophagectomy. Gastroenterology 2013; 144: S1099 (abstract).
Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol 2013; 122(2 Pt 1): 319-28.
Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg 2013; 100: 482-9.
Cheng DC, Wall C, Djaiani G, et al. Randomized assessment of resource use in fast-track cardiac surgery 1-year after hospital discharge. Anesthesiology 2003; 98: 651-7.
Jones EL, Wainwright TW, Foster JD, Smith JR, Middleton RG, Francis NK. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl 2014; 96: 89-94.
Ustun TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 2010; 88: 815-23.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stowers, M.D.J., Lemanu, D.P. & Hill, A.G. Health economics in Enhanced Recovery After Surgery programs. Can J Anesth/J Can Anesth 62, 219–230 (2015). https://doi.org/10.1007/s12630-014-0272-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0272-0