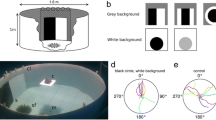
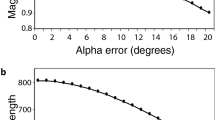
Abstract
A particularly unusual visual system exists in the visually guided aquatic predator, the Sunburst Diving Beetle, Thermonectus marmoratus (Coleoptera: Dytiscidae). The question arises: how does this peculiar visual system function? A series of experiments suggests that their principal eyes (E1 and E2) are highly specialized for hunting. These eyes are tubular and have relatively long focal lengths leading to high image magnification. Their retinae are linear, and are divided into distinct green-sensitive distal and UV and polarization-sensitive proximal portions. Each distal retina, moreover, has many tiers of photoreceptors with rhabdomeres the long axis of which are peculiarly oriented perpendicular to the light path. Based on detailed optical investigations, the lenses of these eyes are bifocal and project focused images onto specific retinal tiers. Behavioral experiments suggest that these larvae approach prey within their eyes’ near-fields, and that they can correctly gauge prey distances even when conventional distance-vision mechanisms are unavailable. In the near-field of these eyes object distance determines which of the many retinal layers receive the best-focused images. This retinal organization could facilitate an unusual distance-vision mechanism. We here summarize past findings and discuss how these eyes allow Thermonectus larvae to be such successful predators.





Similar content being viewed by others
Abbreviations
- PR:
-
Proximal retina
- DR:
-
Distal retina
References
Arikawa K (2003) Spectral organization of the eye of a butterfly, Papilio. J Comp Phys A 189:791–800
Bland K, Revetta NP, Stowasser A, Buschbeck EK (2014) Unilateral range finding in diving beetle larvae. J Exp Biol 214:327–330
Blest AD, Hardie RC, McIntyre P, Williams DS (1981) The spectral sensitivities of identified receptors and the function of retinal tiering in the principal eyes of a jumping spider. J Comp Physiol 145:227–239
Buschbeck EK (2014) Escaping compound eye ancestry: the evolution of single-chamber eyes in holometabolous larvae. J Exp Biol 217:2818–2824
Buschbeck EK, Ehmer B, Hoy R (1999) Chunk versus point sampling: visual imaging in a small insect. Science 286:1178–1180
Buschbeck EK, Sbita SJ, Morgan RC (2007) Scanning behavior by larvae of the predacious diving beetle, Thermonectus marmoratus (Coleoptera: Dytiscidae) enlarges visual field prior to prey capture. J Comp Physiol A 193:973–982
Cartwright B, Collett T (1979) How honey-bees know their distance from a near-by visual landmark. J Exp Biol 82:367–372
Chaudhuri S, Rajagopalan AN (1999) Depth from defocus: a real aperture imaging approach. Springer, Berlin, Heidelberg, New York, Toronto
Chung W-S, Marshall J (2014) Range-finding in squid using retinal deformation and image blur. Curr Biol 24:R64–R65
Clarkson E, Levi-Setti R, Horváth G (2006) The eyes of trilobites: the oldest preserved visual system. Arthropod Struct Dev 35:247–259
Clemente CJ, McMaster KA, Fox E, Meldrum L, Stewart T, Main BY (2010) The visual system of the Australian wolf spider Lycosa leuckartii (Araneae: Lycosidae): visual acuity and the functional role of the eyes. J Arachnol 38:398–406
Collett TS (1978) Peering-a locust behaviour pattern for obtaining motion parallax information. J Exp Biol 76:237–241
Collett TS, Harkness LIK (1982) Depth vision in animals. In: Ingle D, Goodale MA, Mansfield RJW (eds) Analysis of visual behavior. MIT Press, Cambridge, pp 111–176
Collett TS, Land MF (1975) Visual control of flight behaviour in the hoverfly Syritta pipiens L. J Comp Physiol 99:1–66
Cronin TW (2006) Invertebrate vision in water. In: Warrant EJ, Nilsson D-E (eds) Invertebrate vision. Cambridge University Press, New York, pp 211–249
Cronin TW, Marshall J (2011) Patterns and properties of polarized light in air and water. Phil Trans R Soc B 366:619–626
Duelli P (1978) Movement detection in the posterolateral eyes of jumping spiders (Evarcha arcuata, Salticidae). J Comp Physiol 124:15–26
Egri Á, Horváth G (2012) Possible optical functions of the central core in lenses of trilobite eyes: spherically corrected monofocality or bifocality. J Opt Soc Am A 29:1965–1976
Evans AV, Hogue JN (2006) Field guide to beetles of California. University of California Press, Berkeley
Forster L (1979) Visual mechanisms of hunting behavior in Trite planiceps—a jumping spider. N Z J Zool 6:79–93
Forster L (1982) Vision and prey-catching strategies in jumping spiders. Am Sci 70:165–175
Gál J, Horváth G, Clarkson ENK (2000a) Reconstruction of the shape and optics of the lenses in the abathochroal-eyed trilobite Neocobboldia chinlinica. Hist Biol 14:193–204
Gál J, Horváth G, Clarkson ENK, Haiman O (2000b) Image formation by bifocal lenses in a trilobite eye? Vis Res 40:843–853
Goulet M, Campan R, Lambin M (1981) The visual perception of relative distances in the wood-cricket, Nemobius sylvestris. Physiol Entomol 6:357–367
Grenacher H (1879) Untersuchungen über das Sehorgan der Arthropoden: insbesondere der Spinnen. Insecten und Crustaceen. Verlag vonVandenhoeck & Ruprecht, Göttingen
Günther K (1911) Die Sehorgane der Larve und Imago von Dytiscus marginalis. Z Wiss Zool ABT A 100:60–115
Gupta N, Naroo SA, Wolffsohn JS (2009) Visual comparison of multifocal contact lens to monovision. Optom Vis Sci 86:E98–E105
Held RT, Cooper EA, Banks MS (2012) Blur and disparity are complementary cues to depth. Curr Biol 22:426–431
Hofbauer A, Buchner E (1989) Does Drosophila have 7 eyes? Naturwissenschaften 76:335–336
Horváth G, Varjú D (2004) Polarized light in animal vision: polarization patterns in nature. Springer, Berlin, Heidelberg, New York, Toronto
Kral K (1999) Binocular vision and distance estimation. In: Prete FR, Wells H, Wells PH (eds) The praying mantids. JHU Press, Baltimor
Kral K (2009) Comparison of the use of active vision for depth perception in three grasshopper families (Orthoptera: Caelifera). Ann Entomol Soc Am 102:339–345
Kral K (2012) The functional significance of mantis peering behaviour. Eur J Entomol 109:295–301
Land MF (1969a) Movements of the retinae of jumping spiders (Salticidae: Dendryphantinae) in response to visual stimuli. J Exp Biol 51:471–493
Land MF (1969b) Structure of the retinae of the principal eyes of jumping spiders (Salticidae: Dendryphantinae) in relation to visual optics. J Exp Biol 51:443–470
Land MF (1972) Mechanisms of orientation and pattern recognition by jumping spiders (Salticidae). In: Wehner R (ed) Information processing in the visual systems of anthropods. Springer, Berlin, Heidelberg, New York, Toronto, pp 231–247
Land MF (1982) Scanning eye movements in a heteropod mollusc. J Exp Biol 96:427–430
Land MF (1988) The functions of eye and body movements in Labidocera and other copepods. J Exp Biol 140:381–391
Land MF, Nilsson D-E (2012) Animal eyes. Oxford University Press, New York
Land MF, Marshall JN, Brownless D, Cronin TW (1990) The eye-movements of the mantis shrimp Odontodactylus scyllarus (Crustacea: Stomatopoda). J Comp Physiol A 167:155–166
Larson DJ, Alarie Y, Roughley RE (2009) Predaceous Diving Beetles (Coleoptera: Dytiscidae) of the Nearctic Region, with emphasis on the fauna of Canada and Alaska. NRC Research Press, Ottawa
Lee MR, Torney C, Owen AW (2007) Magnesium-rich intralensar structures in schizochroal trilobite eyes. Palaeontology 50:1031–1037
Lewis JE, Maler L (2002) Blurring of the senses: common cues for distance perception in diverse sensory systems. Neuroscience 114:19–22
Maksimovic S, Cook T, Buschbeck EK (2009) Spatial distribution of opsin encoding mRNAs in the tiered larval retinas of the Sunburst Diving Beetle Thermonectus marmoratus (Coleoptera: Dytiscidae). J Exp Biol 212:3781–3794
Maksimovic S, Layne JE, Buschbeck EK (2011) Spectral sensitivity of the principal eyes of Sunburst Diving Beetle, Thermonectus marmoratus (Coleoptera: Dytiscidae), larvae. J Exp Biol 214:3524–3531
Mandapaka K, Morgan RC, Buschbeck EK (2006) Twenty-eight retinas but only twelve eyes: an anatomical analysis of the larval visual system of the diving beetle Thermonectus marmoratus (Coleoptera: Dytiscidae). J Comp Neurol 497:166–181
Mather G (1997) The use of image blur as a depth cue. Reception 26:1147–1158
Mather G, Smith DRR (2000) Depth cue integration: stereopsis and image blur. Vis Res 40:3501–3506
Morgan RC (1992) Natural history, captive management and the display of the Sunburst Diving Beetle Thermonectus marmoratus. In: AAZPA/CAZPA annual conference proceedings, pp 457–464
Nagata T, Koyanagi M, Tsukamoto H, Saeki S, Isono K, Shichida Y, Tokunaga F, Kinoshita M, Arikawa K, Terakita A (2012) Depth perception from image defocus in a jumping spider. Science 335:469–471
Packer M (2011) Multifocal intraocular lens technology: biomaterial, optical design and review of clinical outcomes. Expert Rev Ophthalmol 6:437–448
Patten W (1887) Studies on the eyes of arthropods. 1. Development of the eyes of vespa, with observations on the ocelli of some insects. J Morphol 1:193–226
Patten W (1888) Studies on the eyes of arthropods. II. Eyes of Acilius. J Morphol 2:97–190
Pentland AP (1987) A new sense for depth of field. IEEE Trans Pattern Anal 9:523–531
Poteser M, Kral K (1995) Visual distance discrimination between stationary targets in praying mantis: an index of the use of motion parallax. J Exp Biol 198:2127–2137
Rajkumar P, Rollmann SM, Cook TA, Layne JE (2010) Molecular evidence for color discrimination in the Atlantic sand fiddler crab, Uca pugilator. J Exp Biol 213:4240–4248
Sabbah S, Shashar N (2006) Polarization contrast of zooplankton: a model for polarization-based sighting distance. Vis Res 46:444–456
Sbita SJ, Morgan RC, Buschbeck EK (2007) Eye and optic lobe metamorphosis in the Sunburst Diving Beetle, Thermonectus marmoratus (Coleoptera: Dytiscidae). Arth Struct Dev 36:449–462
Schöne H (1951) Die Lichtorientierung der Larven von Acilius sulcatus L. und Dytiscus marginalis L. Z vergl Physiol 33:63–98
Schwind R (1989) Size and distance perception in compound eyes. In: Stavenga DG, Hardie RC (eds) Facets of vision. Springer, Berlin, Heidelberg, New York, Toronto, pp 425–444
Schwind R (1991) Polarization vision in water insects and insects living on a moist substrate. J Comp Physiol A 169:531–540
Schwind R (1999) Daphnia pulex swims towards the most strongly polarized light—a response that leads to “shore flight”. J Exp Biol 202:3631–3635
Sharkey CR, Roberts NW, Partridge JC (2012) The role of polarized light in prey capture in an aquatic predator. Front behavioural neuroscience conference abstract: tenth international congress of neuroethology
Sharkey CR, Roberts NW, Partridge JC (2013) Dragonfly larval polarization sensitivity as a contrast enhancer in turbid water. Front physiology conference abstract: international conference on invertebrate vision
Shashar N, Hagan R, Boal JG, Hanlon RT (2000) Cuttlefish use polarization sensitivity in predation on silvery fish. Vis Res 40:71–75
Shashar N, Johnsen S, Lerner A, Sabbah S, Chiao C-C, Mäthger LM, Hanlon RT (2011) Underwater linear polarization: physical limitations to biological functions. Philos Trans R Soc B 366:649–654
Sobel EC (1990) The locust’s use of motion parallax to measure distance. J Comp Physiol A 167:579–588
Srinivasan MV, Lehrer M, Zhang SW, Horridge GA (1989) How honeybees measure their distance from objects of unknown size. J Comp Physiol A 165:605–613
Stecher N, Morgan R, Buschbeck EK (2010) Retinal ultrastructure may mediate polarization sensitivity in larvae of the Sunburst Diving Beetle, Thermonectus marmoratus (Coleoptera: Dytiscidae). Zoomorphology 129:141–152
Stowasser A, Buschbeck EK (2012) Electrophysiological evidence for polarization sensitivity in the camera-type eyes of the aquatic predacious insect larva Thermonectus marmoratus. J Exp Biol 215:3577–3586
Stowasser A, Buschbeck EK (2014) Multitasking in a larval eye: how the unusual organization of the principal eyes of Thermonectus marmoratus allows for far and near vision and might aid in depth perception. J Exp Biol 217:2509–2516
Stowasser A, Rapaport A, Layne JE, Morgan RC, Buschbeck EK (2010) Biological bifocal lenses with image separation. Curr Biol 20:1482–1486
Toh Y, Okamura J-Y (2001) Behavioral responses of the tiger beetle larva to moving objects: role of binocular and monocular vision. J Exp Biol 204:615–625
Toh Y, Okamura J-Y (2007) Morphological and optical properties of the corneal lens and retinal structure in the posterior large stemma of the tiger beetle larva. Vis Res 47:1756–1768
Veleri S, Rieger D, Helfrich-Förster C, Stanewsky R (2007) Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J Biol Rhythms 22:29–42
Via SE (1977) Visually mediated snapping in the bulldog ant: a perceptual ambiguity between size and distance. J Comp Physiol A 121:33–51
Warrant EJ, McIntyre PD (1993) Arthropod eye design and the physical limits to spatial resolving power. Prog Neurobiol 40:413–461
Wehner R, Labhart T (2006) Polarisation vision. In: Warrant EJ, Nilsson D-E (eds) Invertebrate Vision. Cambridge University Press, New York, pp 291–348
Wojtusiak R (1929) Über Lichtreaktionen normaler und geblendeter Acilius-larven. Acta Biol Exp Warszawa 3:165–174
Zeil J (1993) Orientation flights of solitary wasps (Cerceris; Sphecidae; Hymenoptera). J Comp Physiol A 172:189–205
Acknowledgments
We thank the Buschbeck lab group for helpful discussions. Drs. Tiffany Cook and Ilya Vilinsky provided valuable intellectual and editorial feedback. This work was supported by the National Science Foundation under Grants IOS0545978 and IOS1050754 to EKB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stowasser, A., Buschbeck, E.K. How aquatic water-beetle larvae with small chambered eyes overcome challenges of hunting under water. J Comp Physiol A 200, 911–922 (2014). https://doi.org/10.1007/s00359-014-0944-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0944-9