Abstract
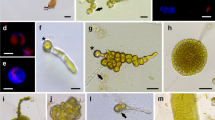
This paper reports the first successful isolation of protoplasts from Gracilariopsis bailiniae and their callus formation. The base solution type, concentration of isolating enzymes, concentration of sorbitol, incubation time, temperature and pH of the enzyme solution were tested to optimize the protoplast yield. The optimized isolation conditions were: 40% base solution 3 (deionized water containing 25 mmol/L MESTris and 25 mmol/L CaCl2·2H2O) and 60% crude Marinomonas sp. YS-70 agarase solution, containing 2% w/v cellulase, 1% w/v macerozyme R-10 and 0.4 mol/L sorbitol, with incubation for 4 h at 28°C and pH 6.5. The highest yield of viable protoplasts, which was obtained in these conditions, was (1.75±0.25)×10 6 cells/g fresh weight. Cell wall regeneration of most protoplasts from G. bailiniae was complete within 60 h and the first division of cells happened after ≥3 days. Two division types were observed in the first division of protoplasts from G. bailiniae— asymmetric division and symmetric division. After the first division, the cells underwent a series of divisions to form callus cell masses.
Similar content being viewed by others
References
Araki T, Lu Z, Morishita T. 1998. Optimization of parameters for isolation of protoplasts from Gracilaria verrucosa (Rhodophyta). Journal of Marine Biotechnology, 6 (3): 193–197.
Baweja P, Sahoo D, García–Jiménez P, Robaina R R. 2009. Review: seaweed tissue culture as applied to biotechnology: problems, achievements and prospects. Phycological Research, 57 (1): 45–58.
Bellanger F, Verdus M C, Henocq V, Christiaen D. 1990. Determination of the composition of the fibrillar part of Gracilaria verrucosa (Gracilariales, Rhodophyta) cell wall in order to prepare protoplasts. Hydrobiologia, 204–205 (1): 527–531.
Björk M, Ekman P, Wallin A, Pedersén M. 1990. Effects of growth rate and other factors on protoplast yield from four species of Gracilaria (Rhodophyta). Botanica Marina, 33 (5): 433–439.
Butler D M, Østgaard K, Boyen C, Evans L V, Jensen A, Kloareg B. 1989. Isolation conditions for high yields of protoplasts from Laminaria saccharina and L. digitata (Phaeophyceae). Journal of Experimental Botany, 40 (11): 1 237–1 246.
Chen Y C, Shih H C. 2000. Development of protoplasts of Ulva fasciata (Chlorophyta) for algal seed stock. Journal of Phycology, 36 (3): 608–615.
Chen Y C. 1998. Development of protoplasts from holdfasts and vegetative thalli of Monostroma latissimum (Chlorophyta, Monostromatacae) for algal seed stock. Journal of Phycology, 34 (6): 1 075–1 081.
Cheney D P, Mar E, Saga N, van der Meer J. 1986. Protoplast isolation and cell division in the agar–producing seaweed Gracilaria (Rhodophyta). Journal of Phycology, 22 (2): 238–243.
Cheney D P. 1990. Genetic improvement of seaweeds through protoplast fusion. In: Yarish C, Penniman C A, Van Patten P eds. Economically Important Marine Plants of the Atlantic: their Biology and Cultivation. Connecticut Sea Grant College Program, Groton, CT, USA. p.15–25.
Compton M E, Saunders J A, Veilleux R E. 2000. Use of protoplast for plant improvement. In: Trigiano R N, Gray D J eds. Plant Tissue Culture Concepts and Laboratory Exercise. CRC Press, Boca Raton, USA. p.249–261.
Davey M R, Anthony P, Power J B, Lowe K C. 2005. Plant protoplasts: status and biotechnological perspectives. Biotechnology Advances, 23 (2): 131–171.
Dipakkore S, Reddy C R K, Jha B. 2005. Production and seeding of protoplasts of Porphyra okhaensis (Bangiales, Rhodophyta) in laboratory culture. Journal of Applied Phycology, 17 (4): 331–337.
Evans D A, Bravo J E. 1983. Protoplast isolation and culture. In: Evans D A, Sharp W R, Ammirato P V, Yamada Y eds. Handbook of Plant Cell Culture, Vol1. Techniques for Propagation and Breeding. Macmillan, New York, USA. p.124–176.
Fujita Y, Saito M. 1990. Protoplast isolation and fusion in Porphyra (Bangiales, Rhodophyta). Hydrobiologia, 204–205 (1): 161–166.
Graham L E, Wilcox L W. 2000. Red algae. In: Graham L E, Wilcox L W eds. Algae. Prentice–Hall, Upper Saddle River, NJ. p.343–396.
Gupta V, Kumar M, Kumari P, Reddy C R K, Jha B. 2011. Optimization of protoplast yields from the red algae Gracilaria dura (C. Agardh) J. Agardh and G. verrucosa (Huds.) Papenfuss. Journal of Applied Phycology, 23 (2): 209–218.
Gupta V, Trivedi N, Kumar M, Reddy C R K, Jha B. 2013. Purification and characterization of exo–β–agarase from an endophytic marine bacterium and its catalytic potential in bioconversion of red algal cell wall polysaccharides into galactans. Biomass and Bioenergy, 49: 290–298.
Huddy S M, Meyers A E, Coyne V E. 2013. Protoplast isolation optimization and regeneration of cell wall in Gracilaria gracilis (Gracilariales, Rhodophyta). Journal of Applied Phycology, 25 (2): 433–443.
Huddy S M, Meyers A E, Coyne V E. 2015. Regeneration of whole plants from protoplasts of Gracilaria gracilis (Gracilariales, Rhodophyta). Journal of Applied Phycology, 27 (1): 427–435.
Hurtado–Ponce A Q. 1992. Rheological properties of agar from Gracilariopsis heteroclada (Zhang et Xia) Zhang et Xia (Gracilariales, Rhodophyta) treated with powdered commercial lime and aqueous alkaline solution. Botanica Marina, 35 (5): 365–370.
Inoue A, Mashino C, Kodama T, Ojima T. 2011. Protoplast preparation from Laminaria japonica with recombinant alginate lyase and cellulase. Marine Biotechnology, 13 (2): 256–263.
Kito H, Kunimoto M, Kamanishi Y, Mizukami Y. 1998. Protoplast fusion between Monostroma nitidum and Porphyra yezoensis and subsequent growth of hybrid plants. Journal of Applied Phycology, 10 (1): 15–21.
Lafontaine N, Mussio I, Rusig A M. 2011. Production and regeneration of protoplasts from Grateloupia turuturu Yamada (Rhodophyta). Journal of Applied Phycology, 23 (1): 17–24.
Liu M Q, Yang X Q, Qi B, et al. 2013. Present situation and prospect of polysaccharide and phycobiliprotein from Gracilaria. Science and Technology of Food Industry, 34 (13): 338–341. (in Chinese with English abstract)
Mantri V A. 2009. Studies on Biology of Gracilaria dura (C. Agardh) J. Agardh. Bhavnagar University, Bhavnagar, India.
Mussio I, Rusig A M. 2006. Isolation of protoplasts from Fucus serratus and F. vesiculosus (Fucales, Phaeophyceae): factors affecting protoplast yield. Journal of Applied Phycology, 18 (6): 733–740.
Pan J Q, Li S D. 2010. Development and utilization of Gracilaria resources. Chinese Journal of Tropical Agriculture, 30 (10): 47–50, 89. (in Chinese with English abstract)
Reddy C R K, Dipakkore S, Kumar G R, Jha B, Cheney D P, Fujita Y. 2006. An improved enzyme preparation for rapid mass production of protoplasts as seed stock for aquaculture of macrophytic marine green algae. Aquaculture, 260 (1–4): 290–297.
Reddy C R K, Gupta M K, Mantri V A, Jha B. 2008. Seaweed protoplasts: status, biotechnological perspectives and needs. Journal of Applied Phycology, 20 (5): 619–632.
Reddy C R K, Gupta V, Jha B. 2010. Developments in biotechnology of red algae. In: Chapman D J, Seckbach J eds. Red Algae in Genomic Age. Springer, New York. p.307–341.
Reddy C R K, Kumar G R K, Siddhanta A K, Tewari A, Eswaran K. 2003. In vitro somatic embryogenesis and regeneration of somatic embryos from pigmented callus of Kappaphycus alvarezii (Doty) Doty (Rhodophyta, Gigartinales). Journal of Phycology, 39 (3): 610–616.
Saminathan K R, Ashok K S, Veeragurunathan V, Mantri V A. 2015. Seedling production in the industrially important agarophyte Gracilaria dura (Gracilariales, Rhodophyta). Journal of Applied Phycology, 27 (4): 1 541–1 548.
Smit A J. 2004. Medicinal and pharmaceutical uses of seaweed natural products: a review. Journal of Applied Phycology, 16 (4): 245–262.
Tang Z X. 2012. Preparation, Purification and Characterization of Agrase from Paenibacillus sp. Zhejiang University of Technology, Hangzhou, Zhejiang, China. (in Chinese with English abstract)
Wang S J. 1994. Seaweed Biotechnology. Shanghai Science and Technology Press, Shanghai, China. p.49–60. (in Chinese)
Wang Z X, Sui Z H, Hu Y Y, Zhang S, Pan Y L, Ju H R. 2014. A comparison of different Gracilariopsis lemaneiformis (Rhodophyta) parts in biochemical characteristics, protoplast formation and regeneration. Journal of Ocean University of China, 13 (4): 671–676.
Yan X H, Wang S J. 1993. Regeneration of whole plants from Gracilaria asiatica Chang et Xia protoplasts (Gracilariaceae, Rhodophyta). Hydrobiologia, 260 (1): 429–436.
Yeong H Y, Khalid N, Phang S M. 2008. Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta). Journal of Applied Phycology, 20 (5): 641–651.
Zablackis E, Vreeland V, Kloareg B. 1993. Isolation of protoplasts from Kappaphycus alvarezii var. tambalang (Rhodophyta) and secretion of i–carrageenan fragments by cultured cells. Journal of Experimental Botany, 44 (9): 1 515–1 522.
Zemke–White W L, Ohno M. 1999. World seaweed utilisation: an end–of–century summary. Journal of Applied Phycology, 11 (4): 369–376.
Zhang S, Liu C, Jin Y M, Chi S, Tang X M, Chen F X, Fang X, Liu T. 2014. Studies on the isolation and culture of protoplasts from Kappaphycus alvarezii. Acta Oceanologica Sinica, 33 (10): 114–123.
Zhao Y, Zhang N, Li B W, Huang Q, Chen Y X. 2005. Comparison on activities of cytodern hydrolase of seaweed from three species of gastropoda. Journal of Xiamen University ( Natural Science ), 44 (2): 276–278. (in Chinese with English abstract)
Zhong Z H, Huang Z J, Chen W Z. 2014. Effects of various environmental factors on growth and biochemical components of Gracilaria bailinae. Progress in fishery sciences, 35 (3): 98–104. (in Chinese with English abstract)
Acknowledgement
The authors thank Professor LIU Tao (Ocean University of China), Professor MEI Zhiping (Shantou University), Dr. WANG Hui (Shantou University) and Ms. WANG Zhongxia (Ocean University of China) for providing much advice and kind assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the China Agriculture Research System (No. CARS-50), the Science and Technology Program of Guangdong Province of China (Nos. 2016A020222023, 2015B090903081), and the Project of Guangdong Province Education Department (No. 2017KCXTD014)
Rights and permissions
About this article
Cite this article
Chen, H., Chen, W., Shi, J. et al. Isolation and callus formation of Gracilariopsis bailiniae (Gracilariales, Rhodophyta) protoplasts. J. Ocean. Limnol. 36, 2268–2277 (2018). https://doi.org/10.1007/s00343-019-7217-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-7217-y