Abstract
Key message
An extended version of an intron-containing soybean polyubiquitin promoter gave very high levels of gene expression using three different validation tools.
Abstract
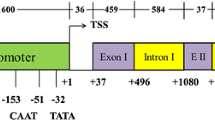
The intron-containing Glycine max polyubiquitin promoter (Gmubi) is able to regulate expression levels five times higher than the widely used CaMV35S promoter. In this study, eleven Gmubi derivatives were designed and evaluated to determine which regions contributed to the high levels of gene expression, observed with this promoter. Derivative constructs regulating GFP were evaluated using transient expression in lima bean cotyledons and stable expression in soybean hairy roots. With both expression systems, removal of the intron in the 5′UTR led to reduced levels of gene expression suggesting a role of the intron in promoter activity. Promoter constructs containing an internal intron duplication and upstream translocations of the intron resulted in higher and similar expression levels to Gmubi, respectively, indicating the presence of enhancers within the intron. Evaluation of 5′ distal extensions of the Gmubi promoter resulted in significantly higher levels of GFP expression, suggesting the presence of upstream regulatory elements. A twofold increase in promoter strength was obtained when Gmubi was extended 1.5 kb upstream to generate GmubiXL (2.4 kb total length). In stably transformed soybean plants containing GFP regulated by CaMV35S, Gmubi and GmubiXL, the GmubiXL promoter clearly produced the highest levels of gene expression, with especially high GFP fluorescence in the vascular tissue and root tips. Use of GmubiXL leads to very high levels of gene expression in soybean and represents a native soybean promoter, which may be useful for regulating transgene expression for both basic and applied research.





Similar content being viewed by others
References
Akua T, Berezin I, Shaul O (2010) The leader intron of AtMHX can elicit, in the absence of splicing, low-level intron-mediated enhancement that depends on the internal intron sequence. BMC Plant Biol 10:93
Bianchi M, Crinelli R, Giacomini E, Carloni E, Magnani M (2009) A potent enhancer element in the 5′-UTR intron is crucial for transcriptional regulation of the human ubiquitin C gene. Gene 448:88–101
Binet MN, Weil JH, Tessier LH (1991) Structure and expression of sunflower ubiquitin genes. Plant Molec Biol 17:395–407
Bourdon V, Harvey A, Lonsdale DM (2001) Introns and their positions affect the translational activity of mRNA in plant cells. EMBO Rep 2:394–398
Chen H, Nelson RS, Sherwood JL (1994) Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 16:664–670
Chiera JM, Finer JJ, Grabau EA (2004) Ectopic expression of a soybean phytase in developing seeds of Glycine max to improve phosphorus availability. Plant Molec Biol 56:895–904
Chiera JM, Bouchard RA, Dorsey SL, Park E, Buenrostro-Nava MT, Ling PP, Finer JJ (2007) Isolation of two highly active soybean (Glycine max (L.) Merr.) promoters and their characterization using a new automated image collection and analysis system. Plant Cell Rep 26:1501–1509
Chiera JM, Lindbo JA, Finer JJ (2008) Quantification and extension of transient GFP expression by the co-introduction of a suppressor of silencing. Transgenic Res 17:1143–1154
Chiu WL, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as vital reporter in plants. Curr Biol 6:325–330
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Chung BYW, Simons C, Firth AE, Brown CM, Hellens RP (2006) Effect of 5′ UTR introns on gene expression in Arabidopsis thaliana. BMC Genom 7:120
Cornejo MJ, Luth D, Blankenship KM, Andersen OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:567–581
Deyholos MK, Sieburth LE (2000) Separate whorl-specific expression and negative regulation by enhancer elements within the AGAMOUS second intron. Plant Cell 12:1799–1810
Finer JJ, McMullen MD (1991) Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell Develop Biol 27P:175–182
Finer JJ, Nagasawa A (1988) Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tiss Org Cult 15:125–136
Finer JJ, Vain P, Jones MW, McMullen MD (1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep 11:323–328
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Garbarino JE, Oosumi T, Belknap WR (1995) Isolation of a polyubiquitin promoter and its expression in transgenic potato plants. Plant Physiol 109:1371–1378
Gidekel M, Jimenez B, Herrera-Estrella L (1996) The first intron of the Arabidopsis thaliana gene coding for elongation factor 1 beta contains an enhancer-like element. Gene 170:201–206
Hernandez-Garcia CM, Finer JJ (2014) Identification and validation of promoters and cis-acting regulatory elements. Plant Sci 217–218:109–119
Hernandez-Garcia CM, Martinelli AP, Bouchard RA, Finer JJ (2009) A soybean (Glycine max) polyubiquitin promoter gives strong constitutive expression in transgenic soybean. Plant Cell Rep 28:837–849
Hernandez-Garcia C, Bouchard RA, Rushton PJ, Jones ML, Chen X, Timko MP, Finer JJ (2010a) High level of transgenic expression of soybean (Glycine max) GmERF and Gmubi gene promoters isolated by a novel promoter analysis pipeline. BMC Plant Biol 10:237
Hernandez-Garcia CM, Chiera JM, Finer JJ (2010b) Robotics and dynamic image analysis for studies of gene expression in plant tissues. J Vis Exp. http://www.jove.com/index/Details.stp?ID=1733
Hess NK, Singer PA, Trinh K, Nikkhoy M, Bernstein SI (2007) Transcriptional regulation of the Drosophila melanogaster muscle myosin heavy-chain gene. Gene Expr Patterns 7:413–422
Joung YH, Kamo K (2006) Expression of a polyubiquitin promoter isolated from Gladiolus. Plant Cell Rep 25:1081–1088
Lu J, Sivamani E, Azhakanandam K, Samadder P, Li X, Qu R (2008) Gene expression enhancement mediated by the 5′ UTR intron of the rice rubi3 gene varied remarkably among tissues in transgenic rice plants. Mol Genet Genomics 279:563–572
Lumbreras V, Stevens DR, Purton S (1998) Efficient foreign gene expression in Chlamydomonas reinhartdii mediated by an endogenous intron. Plant J 14:441–447
Mann DGJ, King ZR, Liu W, Joyce BL, Percifield RJ, Hawkins JS, LaFayette PR, Artelt BJ, Burris JN, Mazarei M, Bennetzen JL, Parrott WA, Stewart CN (2011) Switchgrass (Panicum virgatum L.) polyubiquitin gene (PvUbi1 and PvUbi2) promoters for use in plant transformation. BMC Biotechnol 11:74
Meredith J, Storti RV (1993) Developmental regulation of the Drosophila tropomyosin II gene in different muscles is controlled by muscle-type-specific intron enhancer elements and distal and proximal promoter control elements. Dev Biol 159:500–512
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Plesse B, Durr A, Marbach J, Genshick P, Fleck J (1997) Identification of a new cis-regulatory element in a Nicotiana tabacum polyubiquitin gene promoter. Mol Gen Genet 254:258–266
Plesse B, Criqui MC, Durr A, Parmentier Y, Fleck J, Genschik P (2001) Effects of the polyubiquitin gene Ubi. U4 leader intron and first ubiquitin monomer on reporter gene expression in Nicotiana tabacum. Plant Mol Biol 45:655–667
Ponappa T, Brzozowski AE, Finer JJ (1999) Transient expression and stable transformation of soybean using the jellyfish green fluorescent protein (GFP). Plant Cell Rep 19:6–12
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol Plant 40:1–22
Rasband WS (1997–2006) ImageJ, U.S. National Institutes of Health, Bethesda. http://www.rsb.info.nih.gov/ij/
Rollfinke IK, Silber MV, Pfitzner UM (1998) Characterization and expression of a heptaubiquitin gene from tomato. Gene 211:267–276
Rose AB (2008) Intron-mediated regulation of gene expression. Curr Top Microbiol Immunol 326:277–290
Samadder P, Sivamani E, Lu J, Li X, Qu R (2008) Transcriptional and post-transcriptional enhancement of gene expression by the 5′ UTR intron of rice rubi3 gene in transgenic rice cells. Mol Genet Genomics 279:429–439
Sivamani E, Qu R (2006) Expression enhancement of a rice polyubiquitin gene promoter. Plant Mol Biol 60:225–239
Stark K, Kirk DL, Schmitt R (2001) Two enhancers and one silencer located in the introns of regA control somatic cell differentiation in Volvox carterii. Genes Dev 15:1449–1460
Takimoto I, Christensen AH, Quail PH, Uchimiya H, Toki S (1994) Non-systemic expression of a stress-responsive maize polyubiquitin gene (Ubi-1) in transgenic rice plants. Plant Mol Biol 26:1007–1012
Visel A, Rubin EM, Pennachio LA (2009) Genomic views of distant-acting enhancers. Nature 461:199–205
Vitale A, Wu RJ, Cheng Z, Meagher RB (2003) Multiple conserved 5′ elements are required for high-level pollen expression of the Arabidopsis reproductive actin ACT1. Plant Mol Biol 52:1135–1151
Wang J, Oard JH (2003) Rice ubiquitin promoters: deletion analysis and potential usefulness in plant transformation systems. Plant Cell Rep 22:129–134
Wei H, Wang ML, Moore PH, Albert HH (2003) Comparative expression analysis of two sugarcane polyubiquitin promoters and flanking sequences in transgenic plants. J Plant Physiol 160:1241–1251
Acknowledgments
We wish to acknowledge Robert A. Bouchard for his helpful suggestions and construction development during the early course of this research. We would also like to thank Cheri Nemes for the generation of transgenic plants, Fei-Yi Tsai for the analysis of GFP expression in transgenic plants, Nuananong Semsang for her assistance with the generation of Gmubi derivatives in pCAMBIA1300, and Jenny Tran for the GFP quantification of GmubiM in hairy roots. We appreciate the helpful suggestions from Drs. Eric Stockinger and Leah McHale for their critical reading of this manuscript. Salaries and research support were provided by the United Soybean Board, Bayer CropScience, and by State and Federal funds appropriated to The Ohio State University/Ohio Agricultural Research and Development Center. This research was also supported, in part, through The Consortium for Plant Biotechnology Research, Inc. by DOE Prime Agreement No. DEFG36-02G012026. This support does not constitute an endorsement by DOE or by The Consortium for Plant Biotechnology Research, Inc. of the views expressed in this publication. Mention of trademark or proprietary products does not constitute a guarantee or warranty of the product by OSU/OARDC and also does not imply approval to the exclusion of other products that may also be suitable.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kathryn K. Kamo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De La Torre, C.M., Finer, J.J. The intron and 5′ distal region of the soybean Gmubi promoter contribute to very high levels of gene expression in transiently and stably transformed tissues. Plant Cell Rep 34, 111–120 (2015). https://doi.org/10.1007/s00299-014-1691-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1691-7