Abstract
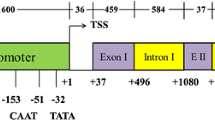
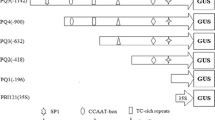
Strong constitutive promoters form a cornerstone for basic and applied research using transgenic plants. GUS (β-glucuronidase) expression levels from constructs containing RUBQ1 or RUB2 rice ubiquitin promoters were 8- to 35-fold higher in transgenic rice [Oryza sativa (L.)] plants, respectively, when compared to the 35S promoter. Deletion analysis of the 5′-upstream region of RUBQ2 revealed a putative enhancer region that produced a 2.4-fold increase in transient GUS expression. Southern blot analysis showed that three to seven copies of the GUS gene were stably inserted into R0 and R1 plants and inherited in a monogenic fashion.




Similar content being viewed by others
Abbreviations
- BAR :
-
Native phosphinothricin acetyltransferase
- GUS :
-
β-Glucuronidase
- HPH :
-
Hygromycin phosphotransferase
- LUC :
-
Luciferase
- PAT :
-
Synthetic phosphinothricin acetyltransferase
- PCR :
-
Polymerase chain reaction
References
Battraw MJ, Hall TC (1990) Histochemical analysis of CaMV35S promoter β-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol 15:527–538
Binet MN, Lepetit M, Weil JH, Tessier LH (1991) Analysis of a sunflower polyubiquitin promoter by transient expression. Plant Sci 79:87–94
Callis J, Raasch J, Vierstra RD (1990) Ubiquitin extension proteins of Arabidopsis thaliana: structure, localization and expression of their promoters in transgenic tobacco. J Biol Chem 265:12486–12493
Chen WP, Gu X, Liang GH, Muthukrishnan S, Chen PD, Liu DJ, Gill BS (1998) Introduction and constitutive expression of a rice chitinase gene in bread wheat using biolistic bombardment and the bar gene as a selectable marker. Theor Appl Genet 97:1296–1306
Christensen AH, Sharrok RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Garbarino JE, Belknap W (1994) Isolation of ubiquitin-ribosomal protein of gene (ubi3) from potato and expression of its promoter in transgenic plants. Plant Mol Biol 24:119–127
Genschik P, Marbach J, Uze M, Feuerman M, Plesse B, Fleck J (1994) Structure and promoter activity of a stress and developmentally regulated polyubiquitin-encoding gene of Nicotiana tabacum. Gene 148:195–202
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Khoury G, Gruss P (1983) Enhancer elements. Cell 33:313–314
Kohli A, Leech M, Vain P, Laurie DA, Christou P (1999) Transgene expression in rice engineered through particle bombardment: molecular factors controlling stable expression and transgene silencing. Planta 208:88–97
Kumpatla SP, Hall TC (1999) Organizational complexity of a rice transgene locus susceptible to methylation-based silencing. IUBMB Life 48:459–467
Kumpatla SP, Teng W, Buchholz WG, Hall TC (1997) Epigenetic transcriptional silencing and 5-azacytidine-mediated reactivation of a complex transgene in rice. Plant Physiol 115:361–373
Kyozuka J, Shimamoto K (1991) Transformation and regeneration of rice protoplasts. In: Plant tissue culture manual, vol. B2. Kluwer, Dordrecht, pp 1–17
Kyozuka J, Olive MR, Peacock WJ, Dennis ES, Shimamoto K (1994) Promoter elements required for developmental expression of the maize adh1 gene in transgenic rice. Plant Cell 6:799–810
McElroy D, Brettel RIS (1994) Foreign gene expression in transgenic cereals. Trends Biotechnol 12:62–68
McElroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–479
Nishizawa Y, Saruta M, Nakazono K, Nishio Z, Soma M, Yoshida T, Nakajima E, Hibi T (2003) Characterization of transgenic rice plants over-expressing the stress-inducible beta-glucanase gene Gns1. Plant Mol Biol 51:143–52
Norris SR, Meyer SE, Callis J (1993) The intron of Arabidopsis thaliana polyubiquitin genes is conserved in location and is a quantitative determinant of chimeric gene expression. Plant Mol Biol 21:895–906
Peterhans A, Datta SK, Datta K, Goodall GJ, Potrykus I, Paszkowski J (1990) Recognition efficiency of dicotyledonous-specific promoter and RNA processing signals in rice. Mol Gen Genet 222:361–368
Register JC, Peterson DJ, Bell PJ (1994) Structure and function of selectable and non-selectable transgenes in maize after introduction by particle bombardment. Plant Mol Biol 25:951–961
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 9.1–9.58
Shimizu M, Kimura T, Koyama T, Suzuki K, Ogawa N, Miyashita K, Sakka K, Ohmiya K (2002) Molecular breeding of transgenic rice plants expressing a bacterial chlorocatechol dioxygenase gene. Appl Environ Microbiol 68:4061–6
Thompson CJ, Movva NR, Tizard R, Crameri R, Devies JE, Lauwereys M, Botterman J (1987) Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J 6:2519–2523
Wang J, Jiang J, Oard J (2000) Structure, expression and promoter activity of two polyubiquitin genes from rice (Oryza sativa). Plant Sci 156:201–211
Weiher H, Konig M, Gruss P (1983) Multiple point mutations affecting the simian virus 40 enhancer. Science 219:626–631
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–5
Zheng Z, Hayashimoto A, Li Z, Murai N (1991) Hygromycin resistant gene cassettes for vector construction and selection of transformed rice protoplasts. Plant Physiol 97:832-835
Acknowledgements
We thank Dr. Peter Quail (USDA Plant Gene Expression Center, Albany, Calif.) for plasmid pAHC25, and Dr. M.C. Rush, Department of Plant Pathology and Crop Physiology, Louisiana State University, Baton Rouge, La., for Taipei 309 suspension cells. This research was funded in part by the Louisiana Rice Research Board.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K.K. Kamo
Approved for publication by the Director of the Louisiana Agricultural Experiment Station as manuscript number 02-14-0709.
Rights and permissions
About this article
Cite this article
Wang, J., Oard, J.H. Rice ubiquitin promoters: deletion analysis and potential usefulness in plant transformation systems. Plant Cell Rep 22, 129–134 (2003). https://doi.org/10.1007/s00299-003-0657-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0657-y