Abstract
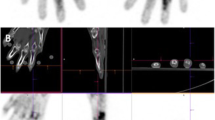
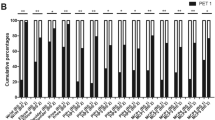
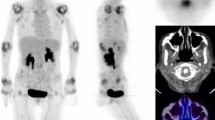
The present retrospective study investigated the relationship between [18F]fluorodeoxyglucose–positron emission tomography (FDG-PET) findings and subsequent progression of joint destruction on plain X-ray. Nineteen rheumatoid arthritis (RA) patients (59 joints) who underwent FDG-PET and whose joints could be evaluated on plain X-ray 5 years later were included in this retrospective investigation. The relationship between the standardized uptake value (SUV) on FDG-PET and Larsen grade progression on plain X-ray was investigated for each joint. Factors related to progression of joint destruction were also investigated. Joints with advanced joint destruction (Larsen grades IV and V) on X-ray imaging at the time of FDG-PET were excluded. On initial plain X-ray images taken at the time of FDG-PET, a significant correlation was observed between the initial SUV of each joint and the progression of joint destruction 5 years later (R = 0.47, P < 0.01). Significant correlations between the SUV and progression of joint destruction were observed in both load-bearing (R = 0.52, P < 0.01) and non-load-bearing joints (R = 0.52, P < 0.01). On logistic regression analysis, higher SUV and lower prednisolone dose were associated with greater risk of progressive joint destruction (P < 0.05). On receiver operating characteristics curve analysis, the optimum threshold for identifying preceding joint destruction was an SUVmean of 1.33. In RA joints, FDG uptake was seen mostly by inflammatory cells; therefore, FDG uptake reflected joint inflammation. Additionally, the activity seen on FDG-PET might be associated with future radiographic changes in RA patients.



Similar content being viewed by others
References
Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, Combe B, Cutolo M, de Wit M, Dougados M, Emery P, Gibofsky A, Gomez-Reino JJ, Haraoui B, Kalden J, Keystone EC, Kvien TK, McInnes I, Martin-Mola E, Montecucco C, Schoels M, van der Heijde D, T2T Expert Committee (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69:631–637. doi:10.1136/ard.2009.123919
Yoshimi R, Hama M, Takase K, Ihata A, Kishimoto D, Terauchi K, Watanabe R, Uehara T, Samukawa S, Ueda A, Takeno M, Ishigatsubo Y (2013) Ultrasonography is a potent tool for the prediction of progressive joint destruction during clinical remission of rheumatoid arthritis. Mod Rheumatol 23:456–465. doi:10.1007/s10165-012-0690-1
Døhn UM, Ejbjerg B, Boonen A, Hetland ML, Hansen MS, Knudsen LS, Hansen A, Madsen OR, Hasselquist M, Møller JM, Ostergaard M (2011) No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann Rheum Dis 70:252–258. doi:10.1136/ard.2009.123729
Ogishima H, Tsuboi H, Umeda N, Horikoshi M, Kondo Y, Sugihara M, Suzuki T, Matsumoto I, Sumida T (2014) Analysis of subclinical synovitis detected by ultrasonography and low-field magnetic resonance imaging in patients with rheumatoid arthritis. Mod Rheumatol 24:60–68. doi:10.3109/14397595.2013.854050
Hama M, Uehara T, Takase K, Ihata A, Ueda A, Takeno M, Shizukuishi K, Tateishi U, Ishigatsubo Y (2012) Power Doppler ultrasonography is useful for assessing disease activity and predicting joint destruction in rheumatoid arthritis patients receiving tocilizumab—preliminary data. Rheumatol Int 32:1327–1333. doi:10.1007/s00296-011-1802-5
Ikeda K, Nakagomi D, Sanayama Y, Yamagata M, Okubo A, Iwamoto T, Kawashima H, Takahashi K, Nakajima H (2013) Correlation of radiographic progression with the cumulative activity of synovitis estimated by power Doppler ultrasound in rheumatoid arthritis: difference between patients treated with methotrexate and those treated with biological agents. J Rheumatol 40:1967–1976. doi:10.3899/jrheum.130556
Beckers C, Jeukens X, Ribbens C, André B, Marcelis S, Leclercq P, Kaiser MJ, Foidart J, Hustinx R, Malaise MG (2006) (18)F-FDG PET imaging of rheumatoid knee synovitis correlates with dynamic magnetic resonance and sonographic assessments as well as with the serum level of metalloproteinase-3. Eur J Nucl Med Mol Imaging 33:275–280
Goerres GW, Forster A, Uebelhart D, Seifert B, Treyer V, Michel B, von Schulthess GK, Kaim AH (2006) F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin Nucl Med 31:386–390
Kubota K, Ito K, Morooka M, Mitsumoto T, Kurihara K, Yamashita H, Takahashi Y, Mimori A (2009) Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann Nucl Med 23:783–791. doi:10.1007/s12149-009-0305-x
Okamura K, Yonemoto Y, Arisaka Y, Takeuchi K, Kobayashi T, Oriuchi N, Tsushima Y, Takagishi K (2012) The assessment of biologic treatment in patients with rheumatoid arthritis using FDG-PET/CT. Rheumatology (Oxford) 51:1484–1491. doi:10.1093/rheumatology/kes064
Okamura K, Yonemoto Y, Okura C, Higuchi T, Tsushima Y, Takagishi K (2014) Evaluation of tocilizumab therapy in patients with rheumatoid arthritis based on FDG-PET/CT. BMC Musculoskelet Disord 15:393. doi:10.1186/1471-2474-15-393
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Watanabe H, Shinozaki T, Yanagawa T, Aoki J, Tokunaga M, Inoue T, Endo K, Mohara S, Sano K, Takagishi K (2000) Glucose metabolic analysis of musculoskeletal tumours using 18fluorine-FDG PET as an aid to preoperative planning. J Bone Joint Surg Br 82:760–767
Larsen A (1975) A radiological method for grading the severity of rheumatoid arthritis. Scand J Rheumatol 4:225–233
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T (1992) Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high uptake in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 33:1972–1980
Matsui T, Nakata N, Nagai S, Nakatani A, Takahashi M, Momose T, Ohtomo K, Koyasu S (2009) Inflammatory cytokines and hypoxia contribute to 18F-FDG uptake by cells involved in pannus formation in rheumatoid arthritis. J Nucl Med 50:920–926. doi:10.2967/jnumed.108.060103
Polisson RP, Schoenberg OI, Fischman A, Rubin R, Simon LS, Rosenthal D, Palmer WE (1995) Use of magnetic resonance imaging and positron emission tomography in the assessment of synovial volume and glucose metabolism in patients with rheumatoid arthritis. Arthritis Rheum 38:819–825
Palmer WE, Rosenthal DI, Schoenberg OI, Fischman AJ, Simon LS, Rubin RH, Polisson RP (1995) Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology 196:647–655
Kirwan JR (1995) The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med 333:142–146
Hickling P, Jacoby RK, Kirwan JR (1998) Joint destruction after glucocorticoids are withdrawn in early rheumatoid arthritis. Arthritis and Rheumatism Council Low Dose Glucocorticoid Study Group. Br J Rheumatol 37:930–936
Hansen M, Podenphant J, Florescu A, Stoltenberg M, Borch A, Kluger E, Sørensen SF, Hansen TM (1999) A randomised trial of differentiated prednisolone treatment in active rheumatoid arthritis. Clinical benefits and skeletal side effects. Ann Rheum Dis 58:713–718
Hall GM, Spector TD, Delmas PD (1995) Markers of bone metabolism in postmenopausal women with rheumatoid arthritis. Effects of corticosteroids and hormone replacement therapy. Arthritis Rheum 38:902–906
Messina OD, Barreira JC, Zanchetta JR, Maldonado-Cocco JA, Bogado CE, Sebastián ON, Flores D, Riopedre AM, Redondo G, Lázaro A (1992) Effect of low doses of deflazacort vs prednisone on bone mineral content in premenopausal rheumatoid arthritis. J Rheumatol 19:1520–1526
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report any conflict of interest with the subject matter of this manuscript.
Rights and permissions
About this article
Cite this article
Yonemoto, Y., Okamura, K., Takeuchi, K. et al. [18F]fluorodeoxyglucose uptake as a predictor of large joint destruction in patients with rheumatoid arthritis. Rheumatol Int 36, 109–115 (2016). https://doi.org/10.1007/s00296-015-3331-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3331-0