Abstract
Background
Inflammation of patients joints with severe disease activity of rheumatoid arthritis (RA) has already been visualized and quantified by 2-[18F]fluoro-2-deoxy-D-glucose positron emission computed tomography ([18F] FDG PET/CT), but little is known about the metabolic status and its relationship with clinical and ultrasonography (US) metrology in patients with low/moderate activity or in remission.
Methods
Clinical assessments [based on 28-joint disease activity score (DAS28-CRP) and Clinical Disease Activity Index (CDAI)], [18F] FDG PET/CT, US and X-ray were performed on 63 RA patients classified into remission or low/moderate or severe disease activity groups. PET/CT was visually and then semi-quantitatively analysed by determining the standardized uptake value (SUV) of positive joints.
Results
Of the 1764 joints, 21.1% were tender only, 13.7% swollen only, 27.6% tender or swollen, 7.3% tender and swollen, 20.5% PET/CT-positive and 8.6% US-positive. PET and US measurements were correlated, albeit with poor concordance. The positive predictive value of PET/CT for clinical evaluation (tender and/or swollen) was low, whereas its negative predictive value was high. Highly significant differences were found with the number of PET/CT-positive joints and with cumulative SUV between “severe” and “non-severe” patients (including those in remission and those with low/moderate activity) and not between those classified as “remission” and “non-remission” or “remission” and “low/moderate activity”. Moreover, the correlation between PET/CT measurements and clinical activity was positive only in the CDAI severe disease group. In patients in remission or with low/moderate activity, only 20–30% of joints were PET/CT-negative. In remission, PET/CT and US were positive in different joints, and PET/CT-positive but US-negative joints mainly exhibited RA (38.1%) or normal (49.2%) and not osteoarthritic (12.7%) X-ray patterns.
Conclusions
[18F] FDG PET/CT was effective at distinguishing patients with severely active disease from other patients. In non-severe RA patients, including those in remission, PET/CT results are discordant from US and clinical observations. A longitudinal analysis is needed to explore the clinical relevance of such infra-clinical disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Since the use of biologic agents in the therapeutic armamentarium against rheumatoid arthritis (RA), low disease activity (LDA) and remission are common goals for better outcomes, including less radiographic progression [1]. In clinical trials, remission is often defined as a 28-joint disease activity score (DAS28) < 2.6. However, this target is achieved in only 30–40% of patients [2, 3]. Within this group, the disease remains active in a significant proportion of patients, as observed in the DREAM registry, where 31.1% of patients had a swollen joint count ≥2 [3] and experienced joint damage progression [4]. Furthermore, imaging studies have shown at least one synovitis in 33–73% of patients in remission by ultrasound (US) and in up to 96% of them by magnetic resonance imaging (MRI) [5]. Surprisingly, even the most stringent remission criteria, such as ACR/EULAR Boolean remission [6], did not decrease the prevalence of US-diagnosed synovitis [7,8,9,10,11]. Although the relevance of US, specifically power Doppler activity, in clinical remission is widely accepted for driving radiologic progression [8,9,10,11,12,13,14] and future clinical flares [7, 10, 13, 15], the relevance of other imaging techniques for assessing synovitis remains poorly understood, and discordance between predictors of clinical and US remission indicates complex interactions between them [9, 16].
We and others [17,18,19,20,21,22,23,24] have shown that 2-[18F]fluoro-2-deoxy-D-glucose positron emission computed tomography ([18F] FDG PET/CT) is able to detect and quantify inflammation in RA synovitis. The number of PET-positive joints among the 28 joints of the DAS and the cumulative standard uptake value (CSUV) of these PET-positive joints were highly correlated with clinical status: number of swollen and tender joints, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) parameters [22, 24] and also with US data such as the number of US-positive joints and the cumulative synovial thickness [22]. Roivanen et al. [21] reported that up to 90% of the joints were rated positively by clinical evaluation (swollen and tender) and also by [18F] FDG PET, whereas a proportion of 75% was quoted by Elzinga [19]. However, in these studies, correlations between PET parameters and DAS28 scores were obtained in RA patients who had for all [20, 22] or a large majority [21, 24] severe disease activity. The main objectives of the present study were (1) to correlate PET/CT parameters to US and clinical measurements among RA patients and (2) to analyse variations in PET/CT parameters based on disease activity (remission, low/moderate activity or severe activity) defined by two classic composite indices, DAS28-CRP and Clinical Disease Activity Index (CDAI) scores.
Methods
Study design and patients
This cross-sectional study, approved by the ethics committee of our hospital (B70720108722), included 63 patients fulfilling the ACR/EULAR 2010 criteria for RA [25] from July 2010 to April 2012. Written informed consent was obtained from each patient. All assessments were performed on the same day by the same independent experienced investigator unaware of the other results: the clinical evaluation and biological test were performed in the morning followed by US and then PET/CT evaluation. X-rays were available as routine controls and were performed at a maximum of 6 weeks after the study day. The patient (PtGA) and the physician (PGA) global assessments were determined using a visual analogue scale (VAS) (0–100 mm) as well as the Health Assessment Questionnaire (HAQ) [26]. Disease activity was evaluated using the DAS28-CRP (without the PGA) [27] and the CDAI (with the PGA and without CRP) [28]. Each patient was categorized as in remission (DAS28-CRP ≤ 2.6 or CDAI≤2.8), in low to moderate disease activity (2.6 < DAS28-CRP ≤ 5.1, 2.8 < CDAI≤22), or in severe disease activity (DAS28-CRP > 5.1, CDAI> 22) [29]. The number of joints that were solely tender (T), solely swollen (S), “tender or swollen” (T/S) and “tender and swollen” (T&S) was recorded.
[18F] FDG PET/CT imaging
The PET/CT studies were performed using a Gemini BigBore scanner (Philips Medical Systems, Cleveland, OH, USA). The patients fasted for 4 h and were injected with [18F] FDG (4 MBq/kg body weight with a maximum of 370 MBq) through an indwelling catheter placed in the median cubital vein and flushed with 5 cc of saline solution afterwards. Blood glucose levels were lower than 140 mg/dl. The uptake time was 60 min, and the image acquisition sequence was as follows: first, a scout view CT, followed by a PET emission study that included the knees, hands, wrists, elbows and shoulders, with 2 min per bed position for a total scanning time that ranged from 14 to 18 min. Finally, a low-dose CT (5-mm slice thickness, tube voltage 120 Kv, tube current–time product 80 mAs) was performed over these joints. Figure 1 presents an exemplative picture of [18F] FDG PET/CT imaging of the knees and hands. The hands and wrists were positioned and fixated, arms down, on a dedicated Plexiglas device to avoid movements between the PET and CT acquisitions. PET images were reconstructed using an iterative list mode time-of-flight algorithm, and corrections for attenuation, dead-time, random and scatter events were applied. The images were first visually analysed, and joints were considered positive for synovitis when the [18F] FDG uptake was increased compared to the background in areas corresponding to joint synovium on CT, i.e., either when thickened synovium was recognized on CT or in locations corresponding anatomically to synovium, excluding uptake in other structures such as muscle and tendons. The [18F] FDG uptake was then quantified using the maximum standardized uptake value (SUVmax). In PET-positive joints according to the visual analysis, the SUVmax was obtained by drawing a region of interest (ROI) over the most active synovial area identified. When no synovitis was identified, ROIs were placed in the corresponding areas on the CT and drawn around the appropriate joint: at the dorsal surface of the radius (on top of the lunate) for the wrists, over the lateral recess at the level of the midpatella for the knees and for the small joints as metacarpophalangeal (MCP) or proximal interphalangeal (PIP) joints. A global metabolic assessment was obtained through the number of PET-positive joints (visual evaluation) and the sum of all SUVmax values from the positive joints (cumulative SUV, CSUV).
Extensive inflammatory changes in several joints of the hand, along with tenosynovitis. a: MIP (maximum intensity projection) of the [18F] FDG PET image. b: CT (upper row), PET (middle row) and fused PET/CT images (lower row) in the coronal, sagittal and transaxial views, respectively. [18F] FDG PET/CT: 18F-fluorodeoxyglucose positron emission computed tomography
Ultrasound and X-ray examinations
US assessments were performed using a B-mode multifrequency 10–14.0 MHz transducer (Logiq 9) (GE Healthcare, Milwaukee, WI, USA). US positioning for the wrists, MCP and PIP joints and for the knees has been described elsewhere [22]. Proximal and distal radiohumeral recesses and posterior recess were studied for the elbows. The glenohumeral joint, with a posterior transverse view, was studied for the shoulders. Synovial measurements were systematically carried out perpendicular to the great axis and at the point of greatest thickness. A cut-off for US positivity was defined as synovitis that was at least 1-mm thick (3 mm for the shoulders) based on US determinations in healthy controls, described elsewhere [22]. In joints where 2 (wrists) or 3 (elbows, knees) scans were obtained, the joint was considered positive if at least one measurement was positive. The cumulative synovial thickness (CST), i.e., the sum of thicknesses of all US-positive joints, is the addition of all (single or multiple) synovial measurements performed. X-rays were obtained for peripheral joints (PIPs, MCPs and wrists).
Statistical analysis
The results are generally expressed as the mean ± standard deviation (SD). Correlation coefficients were calculated to measure the association between PET/CT and clinical or US parameters. Spearman correlations were used for skewed distributions. Concordance between methods was quantified by the intraclass coefficient (ICC). Ordinal logistic regression was used to assess the relationship between disease activity categories based on the DAS28-CRP or CDAI (remission, low/moderate and severe disease activity) and PET/CT number of positive joints and CSUV. A test was performed to determine whether all three disease severity categories were distinguishable. If this was not the case, a classic logistic regression analysis was applied, and optimal Youden cut-off values were determined from the receiver operating characteristic (ROC) curve method. The results were considered significant at the 5% level (p < 0.05). All statistical analyses were performed with SAS (version 9.4).
Results
Patient characteristics
The study patients (42 women and 21 men) had a mean age of 54.8 ± 12.3 years and disease duration of 7.0 ± 6.0 years. IgM rheumatoid factor and anti-citrullinated antibodies were positive in 49.2 and 69.8%, respectively. At baseline, 40 (63.5%) subjects were taking classic disease-modifying antirheumatic drugs (DMARDs); 32 (50.8%), biological agents; 19 (30.2), daily oral prednisolone; and 13 (20.6%), non-steroidal anti-inflammatory drugs. Laboratory, physical examination, disease activity, US and PET/CT results are displayed in Table 1.
Relationships between PET/CT and US measurements
In total, 1764 (63 × 28) joints were analysed. The number of positive joints and the cumulative activity (CSUV) were obtained by PET/CT, and the number of positive joints and the total synovial thickness (CST) were obtained by US. The distributions of PET/CT- and US-positive joints, CSUV and CST are illustrated in Fig. 2. Significant correlations were found between the number of PET/CT-positive joints and CSUV (r = 0.96, P < 0.0001) and between the number of US-positive joints and CST (r = 0.94, P < 0.0001). Significant correlations were also found between the number of PET/CT-positive joints and US measurements (number of PET/CT-positive joints and number of US-positive joints: r = 0.42, P = 0.0005; number of PET/CT-positive joints and CST: r = 0.39, P = 0.0017) and between CSUV and US measurements (CSUV and number of US-positive joints: r = 0.41, P = 0.0009; CSUV and CST: r = 0.39, P = 0.0017). Concordance between the number of PET/CT-positive joints and the number of US-positive joints, however, was poor (ICC = 0.34; 95% ICC 0.13); PET/CT-positive joints were twice as frequent as US-positive joints.
Relationships between PET/CT and clinical measurements
Joint positivity on PET/CT was compared to the clinical evaluation (“tender”, “swollen”, “tender or swollen”, “tender and swollen”) for each joint. The diagnostic efficacy of PET/CT (sensitivity, specificity, and positive and negative predictive values) is presented in Table 2. PET/CT sensitivity was low with respect to clinical measurements: only 58.9% of the joints that were both tender and swollen and only 35.8% of the joints that were tender or swollen were positive on PET/CT. Specificity was higher: 82.6% of the joints that were not tender or not swollen, and 85.4% of the joints that were neither tender nor swollen were PET-negative (Table 2). In accordance with these data, the positive predictive value of PET/CT was low, while the negative predictive value was as high as 96.2% in tender and swollen joints. In other words, when PET/CT was negative, the probability that the articulation was not tender and/or not swollen was high; however, when PET/CT was positive, the probability that this articulation was tender and/or swollen was of poor value.
Relationships between PET/CT parameters and disease activity threshold
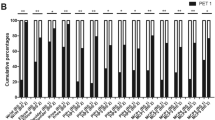
The number of PET/CT-positive joints and CSUV were analysed based on disease activity categories (based on the DAS28-CRP or CDAI) and illustrated in Fig. 3 and Supplementary Table 1 (e.g., there were 3.6 ± 5.4 PET/CT-positive joints in those with DAS28-CRP remission, 4.7 ± 6.7 joints in those with low/moderate activity, and 13.6 ± 11.2 joints in those with severe disease activity). An ordinal logistic regression evidenced a significant relationship between the mean number of PET/CT-positive joints or the CSUV and clinical disease activity (Supplementary Table 1). However, “remission” and “low/moderate” disease activity categories could not be dissociated by PET/CT. Moreover, 27.3% of patients without any metabolic activity were observed in both remission subgroups defined by the DAS28-CRP and CDAI, while 25.8 and 27% of patients were observed in the DAS28-CRP and CDAI low/moderate activity subgroups (p = 0.99 for both DAS28-CRP and CDAI subgroups), indicating that PET/CT was unable to discern remission and low/moderate activity (Table 3).
Number of PET/CT-positive joints (left panels) and CSUV (right panels) across disease activity categories based on the DAS28-CRP (upper panels) or CDAI (lower panels). PET-CT: positron emission computed tomography; CSUV: cumulative standard uptake value; DAS: disease activity score; CDAI: Clinical Disease Activity Index; CRP: C-reactive protein
Thus, the two categories remission and low/moderate activity were merged, and a classic logistic regression analysis was performed between patients with severe and non-severe (including remission and low/moderate activity) disease activity (Table 4): highly significant differences were found in the number of PET/CT-positive joints and in CSUV. The optimal threshold for identifying RA patients with clinically and biologically severe disease was at least 8 PET/CT-positive joints and a CSUV ≥17.8 for the DAS28-CRP and 6.8 and 15.0 for the CDAI, respectively. Disease activity thresholds were also studied by dividing RA patients into remission and non-remission categories (including low/moderate and severe disease), but no significant differences were observed in terms of the number of PET/CT-positive joints and CSUV (data not shown).
Table 3 shows that 16/22 and 8/11 patients in remission (according to DAS28-CRP and CDAI, respectively) were nonetheless PET/CT-positive for at least one joint. Of interest, 12/22 (54.5%) and 6/11 (54.5%) patients also had at least one US-positive joint, despite being in remission (data not shown). As an illustration regarding the patients in remission based on the DAS28-CRP, at the joint level, 75 joints were PET/CT-positive, and 24 were US-positive. Moreover, in the 6 patients with strict Boolean remission (tender and joint score ≤ 1, PGA ≤ 1 (0–10 cm), CRP ≤ 1 mg/dl), 20/168 joints were PET/CT-positive, and 9/168 were US-positive, suggesting a dissociation of PET/CT metrology and the clinical assessment in RA patients in remission. Among the patients in remission based on the DAS28-CRP, only 10 joints were positive for both PET/CT and US (5 wrists, 4 MCPs, and 1 shoulder). The 65 remaining PET/CT-positive joints were distributed as follows: 27 PIPs, 21 MCPs, 8 wrists, 7 knees, 1 elbow and 1 shoulder. Among the 6 patients under Boolean remission, only 4 wrists were both PET/CT- and US-positive, enhancing the lack of concordance between PET/CT and US measures.
Overall, there was a significant correlation between the metabolic measurements (number of positive joints and CSUV) and the clinical assessments (DAS28-CRP and CDAI) (Table 5). However, when classifying patients in remission, low/moderate or severe categories, this significant correlation between PET and clinical assessment was observed only in the subjects with severe activity according to CDAI and not in the RA patients in the low/moderate disease activity or remission groups.
Relationship between X-rays and PET/CT
The 63 peripheral joints (PIPs, MCPs and wrist) that were PET-positive but US-negative in the 16 patients in clinical remission (DAS28-CRP < 2.6) were characterized with X-ray. Features of RA, i.e., symmetrical joint narrowing, bone erosion or demineralization, and of OA, i.e., asymmetrical joint narrowing, subchondral sclerosis, or osteophytes, were recorded. The results were consistent with RA in 24/63 joints (38,1%) and with OA in 8/63 joints (12.7%) with OA signs. In 31/63 joints (49.2%), X-rays were normal. In particular, RA/OA/normal features were described in 7/0/24 of the 31 PIPs, 12/6/5 of the 23 MCPs and 5/2/2 of the 9 wrists. Considering the corresponding clinical status, none of these joints were tender or swollen.
Discussion
In line with previous work [22], we confirmed that the number of PET/CT-positive joints and the CSUV significantly correlated with the number of US-positive joints, synovial thickness and disease activity based on either the DAS28-CRP or CDAI. In addition, PET/CT was quite effective at distinguishing patients with a severely active disease from the others, as a cut-off of 8 for the number of PET-positive joints and 17.8 for the CSUV yielded an area under the curve (AUC) of 0.77 (considering the DAS28-CRP as the clinical gold standard). Although the number of hypermetabolic joints and the cumulative SUV tended to be higher with increased clinical severity of the disease, one notable exception should be mentioned. There was no significant difference with the number of PET-positive joints and their CSUV between patients in clinical remission and those with low/moderate disease activity. In both groups, only 25–27% of the patients presented negative PET/CT findings. Clearly, PET/CT results and clinical assessments diverge in non-severe RA, including remission, in agreement with previous observations made with US and MRI [3,4,5,6,7,8,9].
Comparing the PET and US findings, there were twice as many PET/CT-positive joints as US-positive joints. Furthermore, there was also clear evidence that the PET/CT and US analyses of joints did not concur for those in remission. For example, out of the 22 patients in remission based on the DAS28-CRP, 12 were positive with both PET/CT and US, but at the joint level, only 10 of the 75 PET/CT-positive joints (5 wrists, 4 MCPs and one shoulder) were also US-positive. This divergence was also observed for patients in remission according to the strict Boolean-based definition (which is stricter than the DAS28-CRP-based definition). In other words, PET/CT was positive in a significant number of patients with no or low/moderate disease activity based on current clinical scales and was also positive in a significant number of joints that were not considered inflamed according to clinical and US parameters. Two interpretations are possible for this observation. The first would be a higher sensitivity of the metabolic measurements for identifying subclinical joint inflammation. Indeed, in inflammatory diseases, incidental PET/CT findings due to [18F] FDG accumulation are consistently associated with enhanced glycolytic metabolism in inflammatory cellular infiltrates, including activated macrophages, neutrophils and lymphocytes [30]. We may therefore consider that hypermetabolic joints with normal US appearance are joints with an inflammatory component without proliferating synovitis or with a synovitis < 1 mm thickness, which was the cut-off. In a previous series of RA patients with severe disease activity, only 50% of the PIPs and 62% of the MCPs, both tender and swollen, were US-positive using the same cut-off (data not shown) [22]. PET/CT analysis might therefore exhibit greater sensitivity than US. It is noteworthy that in the current series, the PET/CT-positive but US-negative joints within these 16 patients were mostly PIPs (31 joints in 7 patients) and MCPs (23 joints in 7 patients), which are joints that are typically involved in RA. X-ray analysis supports this hypothesis, as 38% of the joints had signs of RA and 49% were normal. An alternative explanation would be to consider those joints and patients as false positive results of PET/CT. It is indeed possible that the joints actually suffer from secondary (MCPs) or primary (PIPs) osteoarthritis. However, only 8/62 (13%) joints, 6 MCPs in 2 patients and 2 wrists in 2 patients had signs of OA. The radiological analysis is thus in favour of the first hypothesis, but a longitudinal follow-up of the patients would be needed to provide definitive evidence. As a limitation, X-rays were only available for peripheral joints (PIPs, MCPs and wrist) with systematic X-ray realization but not for larger joints (knee, elbow, shoulder). In addition, it should be noted that 30% of the studied patients had daily oral glucocorticoid intake, even at low doses, and that our population was composed of 35% of patients in remission (based on the DAS28-CRP) and only 16% of patients with severe activity; these factors could have influenced our results. A brief report also proposed the use of hybrid PET/magnetic resonance imaging (PET-MRI) to assess inflammatory changes within the synovial tissue in RA [31]. This technique could also be promising to assess remission, mainly because of a higher spatial resolution but also because of the capacity of MRI to detect bone marrow oedema, which is a sign of activity and a risk factor for progression. However, RA was not restricted to the hand, and the use of PET/CT allowed us to obtain a CT view of several other joints, such as the knees, elbows and shoulders.
Conclusion
[18F] FDG PET/CT demonstrated a high specificity and negative predictive value compared to individual clinical evaluation of the joints. Furthermore, PET/CT was effective at differentiating “severe” from “non-severe” patients, although clinical remission was not associated with metabolic remission. Such issues are of high clinical relevance, as PET/CT could possibly identify subclinical and infra-radiological inflammation worthy of treatment to prevent further irrevocable damage to the joints. Further studies are needed to ascertain whether this represents a clinically relevant activity of the disease or secondary degenerative changes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
rheumatoid arthritis
- LDA:
-
low disease activity
- DAS28 :
-
Disease activity score 28-joint
- US:
-
Ultrasonography
- MRI:
-
Magnetic resonance imaging
- 18F-FDG PET/CT:
-
18F-Fluorodeoxyglucose Positron Emission Computed Tomography
- CSUV:
-
Cumulative standard uptake values
- ESR:
-
Erythrocyte sedimentation rate
- CRP:
-
C-reactive protein
- CDAI:
-
Clinical Disease Activity Index
- PtGA:
-
Patient Global Assessment
- PGA:
-
Physician Global Assessment
- VAS:
-
Visual Analogue Scale
- HAQ:
-
Health Assessment Questionnaire
- T:
-
Tender
- S:
-
Swollen
- T/S:
-
Tender or swollen
- T&S:
-
Tender and swollen
- SUVmax:
-
Maximum standardized uptake value
- ROI:
-
Region of interest
- MCP:
-
Metacarpophalangeal
- PIP:
-
Proximal interphalangeal
- CST:
-
Cumulative synovial thickness
- SD:
-
Standard deviation
- ICC:
-
Intraclass correlation coefficient
- ROC:
-
Receiver Operating Characteristic
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ICC95%:
-
95% lower limit confidence
- AUC:
-
Area under the curve
References
Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, et al. Evidence for treating rheumatoid arthritis to target: results of a systemic literature search update. Ann Rheum Dis. 2016;75(1):16–22. https://doi.org/10.1136/annrheumdis-2015-207526.
van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis. Two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:163–74.
de Punder YMR, Fransen J, Kievit W, Houtman PM, Visser H, van de Laar MAFJ, et al. The prevalence of clinical remission in RA patients treated with anti-TNF: results from the Dutch rheumatoid arthritis monitoring (DREAM) registry. Rheumatol. 2012;51(9):1610–7. https://doi.org/10.1093/rheumatology/kes078.
Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the disease activity score in 28 joint and is driven by residual swollen joints. Arthritis Rheum. 2011;63(12):3702–11. https://doi.org/10.1002/art.30634.
Brown AK, Quinn MA, Karim Z, Conaghan PG, Peterfy CG, Hensor E, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission. Evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54(12):3761–73. https://doi.org/10.1002/art.22190.
Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl L, Funovits J, et al. American College of Rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–86. https://doi.org/10.1002/art.30129.
Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Wakefield R, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis. 2011;70(5):792–8. https://doi.org/10.1136/ard.2010.134445.
Zufferey P, Möller B, Brulhart L, Tamborrini G, Scherer A, Finckh A, et al. Persistence of ultrasound synovitis in patients with rheumatoid arthritis fulfilling the DAS28 and/or the new ACR/EULAR RA remission definitions: results of an observational cohort study. Joint Bone Spine. 2014;81(5):426–32. https://doi.org/10.1016/j.jbspin.2014.04.014.
Horton SC, Tan AL, Freeston JE, Wakefield RJ, Buch MH, Emery P. Discordance between the predictors of clinical and imaging remission in patients with early rheumatoid arthritis in clinical practice : implications for the use of ultrasound within a treatment-to-target strategy. Rheumatology. 2016;55(7):1177–87. https://doi.org/10.1093/rheumatology/kew037.
Nguyen H, Ruyssen-Witrand A, Gandjbakhch F, Constantin A, Foltz V, Cantagrel A. Prevalence of ultrasound-detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: a systematic review and meta-analysis. Rheumatology. 2014;53(11):2110–8. https://doi.org/10.1093/rheumatology/keu217.
Sewerin P, Vordenbaeumen S, Hoyer A, et al. Silent progression in patients with rheumatoid arthritis: is DAS28 remission an insufficient goal in RA? Results from the German Remission-plus cohort. BMC Musculoslelet Disord. 2017;18:163.
Conaghan PG, O’Connor P, McGonagle D, et al. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003;48(1):64–71. https://doi.org/10.1002/art.10747.
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2958–67. https://doi.org/10.1002/art.23945.
Foltz V, Gandjbakhch F, Etchepare F, Rosenberg C, Tanguy ML, Rozenberg S, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low level of disease activity. Arthritis Rheum. 2012;64(1):67–76. https://doi.org/10.1002/art.33312.
Scirè CA, Montecucco C, Codullo V, et al. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology. 2009;48(9):1092–7. https://doi.org/10.1093/rheumatology/kep171.
Haavardsholm EAN, Aga AB, Olsen IC, et al. Ultrasound in management of rheumatoid arthritis: ARTIC randomised controlled strategy trial. Br Med J. 2016;354:i4205.
Polisson RP, Schoenberg OI, Fischman A, Rubin R, Simon LS, Rosenthal D, et al. Use of magnetic resonance imaging and positron emission tomography in the assessment of synovial volume and glucose metabolism in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(6):819–25. https://doi.org/10.1002/art.1780380616.
Palmer WE, Rosenthal DI, Schoenberg OI, Fischman AJ, Simon LS, Rubin RH, et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F−18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;196(3):647–55. https://doi.org/10.1148/radiology.196.3.7644624.
Elzinga EH, van der Laken CJ, Comans EFI, Lammertsma AA, Dijkmans BAC, Voskuyl AE. 2-Deoxy-2[F-18] fluoro-D-glucose joint uptake on positron emission tomography images: rheumatoid arthritis versus osteoarthritis. Mol Imaging Biol. 2007;9(6):357–60. https://doi.org/10.1007/s11307-007-0113-4.
Elzinga EH, van der Laken CJ, Comans EFI, Boellaard R, Hoekstra OS, Dijkmans BAC, et al. 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: an explorative study. J Nucl Med. 2011;52(1):77–80. https://doi.org/10.2967/jnumed.110.076711.
Roivainen A, Hautaniemi S, Möttönen T, Nuutila P, Oikonen V, Parkkola R, et al. Correlation of 18F-FDG PET/CT assessments with disease activity and markers of inflammation in patients with early rheumatoid arthritis following the initiation of combination therapy with triple oral antirheumatic drugs. Eur J Nucl Med Mol Imaging. 2013;40(3):403–10. https://doi.org/10.1007/s00259-012-2282-x.
Beckers C, Ribbens C, André B, Marcelis S, Kaye O, Mathy L, et al. Assessment of disease activity in rheumatoid arthritis with 18F-FDG PET. J Nucl Med. 2004;45(6):956–64.
Beckers C, Jeukens X, Ribbens C, André B, Marcelis S, Leclercq P, et al. 18F-FDG PET imaging of rheumatoid knee synovitis correlates with dynamic magnetic resonance and sonographic assessments as well as with the serum level of metalloproteinase-3. Eur J Nucl Med Mol Imaging. 2006;33(3):275–80. https://doi.org/10.1007/s00259-005-1952-3.
Lee SJ, Jeong JH, Lee CH, et al. Development and validation of an 18F-fluorodeoxyglucose-positron emission tomography with computed tomography-based tool for the evaluation of joint counts and disease activity in patients with rheumatoid arthritis. Arthritis Rheum. 2019;71:1232–40.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria : an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. https://doi.org/10.1002/art.27584.
Fries JF, Spitz PW, Young DY. The dimension of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–93.
Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of disease activity score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann Rheum Dis. 2007;66(3):407–9. https://doi.org/10.1136/ard.2006.054205.
Aletaha D, Nell VPK, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. https://doi.org/10.1186/ar1740.
Boyesen P, Machado P. Measuring disease activity and damage in arthritis. In: Bijlsma JWJ, Hachulla E, editors. Textbook on rheumatic diseases. London: BMJ Publishing Group Ltd; 2015. p. 1223–55.
Liu Y, Ghesani NV, Zuckier LS. Physiology and pathophysiology of incidental findings detected on FDG-PET scintigraphy. Semin Nucl Med. 2010;40(4):294–315. https://doi.org/10.1053/j.semnuclmed.2010.02.002.
Miese F, Scherer A. OStendorf et al. hybrid 18F-FDG PET-MRI of the hand in theumatoid arthritis: initial results. Clin Rheumatol. 2011;30(9):1247–50. https://doi.org/10.1007/s10067-011-1777-3.
Acknowledgements
Not applicable.
Funding
This work was supported by academic funding from “Fonds Léon Fredericq”, University of Liège, Belgium. “Fonds Léon Fredericq” had no implication in the acquisition or analyzing of the data.
Author information
Authors and Affiliations
Contributions
CR performed the study, the US data acquisition, analyzed and interpreted the results wrote the manuscript and revised the manuscript critically. PF performed the PET-CT data acquisition, analyzed and interpreted the results and revised the manuscript critically. OM analyzed and interpreted the results, wrote the manuscript and revised the manuscript critically. NC performed the clinical evaluation and revised the manuscript critically. JH performed the X-ray data acquisition and revised the manuscript critically. LS and AA analyzed and interpreted the results, realized all the statistics analyses, and revised the manuscript critically. RH and MM designed the study, analyzed and interpreted the results, wrote the manuscript and revised the manuscript critically. All the authors approved the final manuscript for submission and publication. All co-authors take full responsibility for the integrity of the study and all parts of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethical committee of the university hospital of Liège (B70720108722). Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Association between the number of PET/CT-positive joints or cumulative uptake value and disease activity based either on DAS28-CRP or CDAI by ordinal logistic regression. PET/CT: positron emission computer tomography. CSUV: cumulative standard uptake value. DAS: disease activity score. CRP: C-reactive protein. CDAI: clinical disease activity index.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rinkin, C., Fosse, P., Malaise, O. et al. Dissociation between 2-[18F]fluoro-2-deoxy-D-glucose positron emission computed tomography, ultrasound and clinical assessments in patients with non-severe rheumatoid arthritis, including remission. BMC Rheumatol 5, 31 (2021). https://doi.org/10.1186/s41927-021-00196-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-021-00196-1