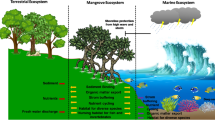
Abstract
Microorganisms inhabiting bulk soil and rhizosphere play an important role in soil biogeochemical cycles leading to enhanced plant growth and productivity. In this context, the role of bacteria is well established, however, not much reports are available about the role archaea plays in this regard. Literature suggests that archaea also play a greater role in nutrient cycling of carbon, nitrogen, sulfur, and other minerals, possess various plant growth promoting attributes, and can impart tolerance to various abiotic stresses (especially osmotic and oxidative) in areas of high salinity, low and high temperatures and hydrogen ion concentrations. Thermoacidophilic archaea have been found to potentially involve in bioleaching of mineral ores and bioremediation of chemical pollutants and aromatic compounds. Looking at immense potential of archaea in promoting plant growth, alleviating abiotic stresses, and remediating contaminated sites, detailed studies are required to establish their role in different ecological processes, and their interactions in rhizosphere with plant and other microflora (bacteria and fungi) in different ecosystems. In this review, a brief discussion on archaea from the agro-ecological point of view is presented.

Similar content being viewed by others
References
Howland JL (2000) The surprising archaea: discovering another domain of life. Oxford University Press on Demand, Oxford
Reysenbach AL, Voytek M, Manicnelli R (2001) Thermophiles: biodiversity. Ecology, and evolution. Kluwer Academic-Plenum Publishers, New York
Amend JP, Shock EL (2010) ChemInform abstract: energetics of overall metabolic reactions of thermophilic and hyperthermophilic archaea and bacteria. ChemInform. https://doi.org/10.1002/chin.200133293
Oren A (2002) Molecular ecology of extremely halophilic archaea and bacteria. FEMS Microbiol Ecol 39:1–7
Andrei AŞ, Banciu HL, Oren A (2012) Living with salt: metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol Lett 330:1–9
Krause S, Bremges A, Münch PC et al (2017) Characterisation of a stable laboratory co-culture of acidophilic nanoorganisms. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-03315-6
Deppenmeier U (2004) The unique biochemistry of methanogenesis. Prog Nucleic Acid Res Mol Biol 71:223–283. https://doi.org/10.1016/s0079-6603(02)71045-3
Delong EF (1992) Archaea in coastal marine environment. Proc Natl Acad Sci 89:5685–5689
Gubry-Rangin C, Nicol GW, Prosser JI (2010) Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol 74:566–574. https://doi.org/10.1111/j.1574-6941.2010.00971.x
Schleper C, Holben W, Klenk HP (1997) Recovery of Crenarchaeotal ribosomal DNA sequences from freshwater-lake sediments. Appl Environ Microbiol 63:321–323
Jurgens G, Lindstro K, Saano A (1997) Novel group within the Kingdom. Microbiology 63:803–805
Yadav AN (2017) Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int J Environ Sci Nat Resour. https://doi.org/10.19080/ijesnr.2017.03.555601
Kumar S, Puniya AK, Puniya M et al (2009) Factors affecting rumen methanogens and methane mitigation strategies. World J Microbiol Biotechnol 25:1557–1566. https://doi.org/10.1007/s11274-009-0041-3
Nkamga VD, Henrissat B, Drancourt M (2017) Archaea: essential inhabitants of the human digestive microbiota. Hum Microbiome J 3:1–8
Makarova KS, Koonin EV (2003) Comparative genomics of archaea: how much have we learned in six years, and what’s next? Genome Biol 4:1–17
Koonin EV, Wolf YI (2008) Genomics of bacteria and archaea: the emerging dynamic view of the prokaryotic world. Nucleic Acids Res 36:6688–6719. https://doi.org/10.1093/nar/gkn668
Gaba S, Kumari A, Medema M, Kaushik R (2020) Pan-genome analysis and ancestral state reconstruction of class halobacteria: probability of a new super-order. Sci Rep 10:1–16. https://doi.org/10.1038/s41598-020-77723-6
Wagner A, Whitaker RJ, Krause DJ et al (2017) Mechanisms of gene flow in archaea. Nat Rev Microbiol 15:492–501
Nath Yadav A, Verma P, Kaushik R et al (2017) Archaea endowed with plant growth promoting attributes. EC Microbiol 8:294–298
Lammel DR, Feigl BJ, Cerri CC, Nüsslein K (2015) Specific microbial gene abundances and soil parameters contribute to C, N, and greenhouse gas process rates after land use change in Southern Amazonian soils. Front Microbiol 6:1–14. https://doi.org/10.3389/fmicb.2015.01057
Karlsson AE, Johansson T, Bengtson P (2012) Archaeal abundance in relation to root and fungal exudation rates. FEMS Microbiol Ecol 80:305–311. https://doi.org/10.1111/j.1574-6941.2012.01298.x
Mamet SD, Lamb EG, Piper CL et al (2017) Archaea and bacteria mediate the effects of native species root loss on fungi during plant invasion. ISME J 11:1261–1275. https://doi.org/10.1038/ismej.2016.205
Chen XP, Zhu YG, Xia Y et al (2008) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ Microbiol 10:1978–1987. https://doi.org/10.1111/j.1462-2920.2008.01613.x
Mohanty S, Kollah B, Chaudhary RS et al (2015) Methane uptake in tropical soybean–wheat agroecosystem under different fertilizer regimes. Environ Earth Sci 74:5049–5061. https://doi.org/10.1007/s12665-015-4603-4
Fox GE, Stackebrandt E, Hespell RB et al (1980) The phylogeny of prokaryotes. Science 209:457–463
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains archaea, bacteria, and eucarya. Proc Natl Acad Sci USA 87:4576–4579
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6:245–252. https://doi.org/10.1038/nrmicro1852
Elkins JG, Podar M, Graham DE et al (2008) A korarchaeal genome reveals insights into the evolution of the archaea. Proc Natl Acad Sci 105:8102–8107. https://doi.org/10.1073/pnas.0801980105
Waters E, Hohn MJ, Ahel I et al (2003) The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism. Proc Natl Acad Sci 100:12984–12988. https://doi.org/10.1073/pnas.1735403100
Nunoura T, Takaki Y, Kakuta J et al (2011) Insights into the evolution of archaea and eukaryotic protein modifier systems revealed by the genome of a novel archaeal group. Nucleic Acids Res 39:3204–3223. https://doi.org/10.1093/nar/gkq1228
Rinke C, Schwientek P, Sczyrba A et al (2013) Insights into the phylogeny and coding potential of microbial dark matter. Nature 499:431–437. https://doi.org/10.1038/nature12352
Petitjean C, Deschamps P, López-Garciá P, Moreira D (2014) Rooting the domain archaea by phylogenomic analysis supports the foundation of the new kingdom Proteoarchaeota. Genome Biol Evol 7:191–204. https://doi.org/10.1093/gbe/evu274
Mayer F, Müller V (2014) Adaptations of anaerobic archaea to life under extreme energy limitation. FEMS Microbiol Rev 38:449–472
Jiao S, Xu Y, Zhang J, Lu Y (2019) Environmental filtering drives distinct continental atlases of soil archaea between dryland and wetland agricultural ecosystems. Microbiome 7:1–13. https://doi.org/10.1186/s40168-019-0630-9
Haroon MF, Hu S, Shi Y et al (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570. https://doi.org/10.1038/nature12375
Zhang R, Neu TR, Li Q et al (2019) Insight into interactions of thermoacidophilic archaea with elemental sulfur: biofilm dynamics and EPS analysis. Front Microbiol 10:1–16. https://doi.org/10.3389/fmicb.2019.00896
Yadav AN, Sharma D, Gulati S et al (2015) Haloarchaea endowed with phosphorus solubilization attribute implicated in phosphorus cycle. Sci Rep. https://doi.org/10.1038/srep12293
Adams MWW (1994) Biochemical diversity among sulfur-dependent, hyperthermophilic microorganisms. FEMS Microbiol Rev 15:261–277. https://doi.org/10.1111/j.1574-6976.1994.tb00139.x
Verhaart MRA, Bielen AAM, Van Der Oost J et al (2010) Hydrogen production by hyperthermophilic and extremely thermophilic bacteria and archaea: mechanisms for reductant disposal. Environ Technol 31:993–1003. https://doi.org/10.1080/09593331003710244
Lecker B, Illi L, Lemmer A, Oechsner H (2017) Biological hydrogen methanation—a review. Bioresour Technol 245:1220–1228. https://doi.org/10.1016/j.biortech.2017.08.176
Lee JC, Kim JH, Chang WS, Pak D (2012) Biological conversion of CO 2 to CH 4 using hydrogenotrophic methanogen in a fixed bed reactor. J Chem Technol Biotechnol 87:844–847. https://doi.org/10.1002/jctb.3787
Pavlov AR, Pavlova NV, Kozyavkin SA, Slesarev AI (2004) Recent developments in the optimization of thermostable DNA polymerases for efficient applications. Trends Biotechnol 22:253–260
Kim DJ, Kim O, Kim HW et al (2009) ATP-dependent DNA ligase from Archaeoglobus fulgidus displays a tightly closed conformation. Acta Crystallogr Sect F Struct Biol Cryst Commun 65:544–550. https://doi.org/10.1107/S1744309109017485
Loder AJ, Zeldes BM, Conway JM et al (2017) Extreme thermophiles as metabolic engineering platforms: strategies and current perspective. Ind Biotechnol Microorg 2:505–580
Counts JA, Zeldes BM, Lee LL et al (2017) Physiological, metabolic and biotechnological features of extremely thermophilic microorganisms. Wiley Interdiscip Rev Syst Biol Med. https://doi.org/10.1002/wsbm.1377
Saxena AK, Kaushik R, Yadav AN et al (2015) Role of Archaea in sustenance of plants in extreme saline environments. In: Proceeding of 56th Annual Conference of Association of Microbiologists of India and International Symposium on “Emerging Discoveries in Microbiology”. https://doi.org/10.13140/RG.2.1.2073.9925
Saxena AK, Shende R, Pandey AK (2005) Culturing of plant growth promoting rhizobacteria. Basic research applications of mycorrhizae. IK International Pvt Ltd, New Delhi, pp 453–474
Mus F, Crook MB, Garcia K et al (2016) Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl Environ Microbiol 82:3698–3710. https://doi.org/10.1128/AEM.01055-16
Boyd ES, Peters JW (2013) New insights into the evolutionary history of biological nitrogen fixation. Front Microbiol 4:1–12. https://doi.org/10.3389/fmicb.2013.00201
Belay N, Sparling R, Daniels L (1984) Dinitrogen fixation by a thermophilic methanogenic bacterium. Nature 312:286–288. https://doi.org/10.1038/312286a0
Murray PA, Zinder SH (1984) Nitrogen fixation by a methanogenic archaebacterium. Nature 312:284–286. https://doi.org/10.1038/312284a0
Leigh JA (2000) Nitrogen fixation in methanogens: the archaeal perspective. Curr Issues Mol Biol 2:125–31
Bae HS, Morrison E, Chanton JP, Ogram A (2018) Methanogens are major contributors to nitrogen fixation in soils of the Florida Everglades. Appl Environ Microbiol 84:1–16. https://doi.org/10.1128/AEM.02222-17
Mirza BS, Potisap C, Nüsslein K et al (2014) Response of free-living nitrogen-fixing microorganisms to land use change in the amazon rainforest. Appl Environ Microbiol 80:281–288. https://doi.org/10.1128/AEM.02362-13
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 27:29–43
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability. Plant Physiol 156:989–996. https://doi.org/10.1104/pp.111.175448
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2:1–14
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448. https://doi.org/10.1111/j.1574-6976.2007.00072.x
White RH (1987) Indole-3-acetic acid and 2-(indol-3-ylmethyl)indol-3-yl acetic acid in the thermophilic archaebacterium Sulfolobus acidocaldarius. J Bacteriol 169:5859–5860. https://doi.org/10.1128/jb.169.12.5859-5860.1987
Dave BP, Anshuman K, Hajela P (2006) Siderophores of halophilic archaea and their chemical characterization. Indian J Exp Biol 44:340–344
Anderson I, Scheuner C, Göker M et al (2011) Novel insights into the diversity of catabolic metabolism from ten haloarchaeal genomes. PLoS ONE. https://doi.org/10.1371/journal.pone.0020237
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42:155–159. https://doi.org/10.1111/j.1472-765X.2005.01827.x
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Chinnadurai C, Balachandar D, Sundaram SP (2009) Characterization of 1-aminocyclopropane-1-carboxylate deaminase producing methylobacteria from phyllosphere of rice and their role in ethylene regulation. World J Microbiol Biotechnol 25:1403–1411. https://doi.org/10.1007/s11274-009-0027-1
Singh RP, Shelke GM, Kumar A, Jha PN (2015) Biochemistry and genetics of ACC deaminase: a weapon to “stress ethylene” produced in plants. Front Microbiol 6:1–14. https://doi.org/10.3389/fmicb.2015.00937
Fujino A, Ose T, Yao M et al (2004) Structural and enzymatic properties of 1-aminocyclopropane-1-carboxylate deaminase homologue from Pyrococcus horikoshii. J Mol Biol 341:999–1013. https://doi.org/10.1016/j.jmb.2004.06.062
Dimkpa C, Weinand T, Asch F (2009) Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694. https://doi.org/10.1111/j.1365-3040.2009.02028.x
Sandhya V, Ali S, Grover M et al (2009) Pseudomonas sp. strain P45 protects sunflowers seedlings from drought stress through improved soil structure. J Oilseed Res 26:600–601
Grover M, Ali SZ, Sandhya V et al (2011) Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol 27:1231–1240. https://doi.org/10.1007/s11274-010-0572-7
Farooq M, Wahid A, Kobayashi N, Fujita DB, Basra SMA (2009) Plant drought stress: effects, mechanisms, and management. In: Lichtfouse E, Navarrete M, Debaeke P, Veronique S, Alberola C (eds) Sustainable Agriculture. Springer, Dordrecht, pp 153–188
Yuwono T, Handayani D, Soedarsono J (2005) The role of osmotolerant rhizobacteria in rice growth under different drought conditions. Aust J Agric Res 56:715–721. https://doi.org/10.1071/AR04082
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166:525–530. https://doi.org/10.1016/j.plantsci.2003.10.025
Afrasayab S, Faisal M, Hasnain S (2010) Comparative study of wild and transformed salt tolerant bacterial strains on Triticum aestivum growth under salt stress. Braz J Microbiol 41:946–955. https://doi.org/10.1590/S1517-83822010000400013
Zhang Y, Zhao L, Wang Y et al (2008) Enhancement of heavy metal accumulation by tissue specific co-expression of iaaM and ACC deaminase genes in plants. Chemosphere 72:564–571. https://doi.org/10.1016/j.chemosphere.2008.03.043
Yadav AN, Gulati S, Sharma D et al (2019) Seasonal variations in culturable archaea and their plant growth promoting attributes to predict their role in establishment of vegetation in Rann of Kutch. Biologia (Bratisl) 74:1031–1043. https://doi.org/10.2478/s11756-019-00259-2
Bini E (2010) Archaeal transformation of metals in the environment. FEMS Microbiol Ecol 73:1–16
Kashefi K, Lovley DR (2000) Reduction of Fe(III), Mn(IV), and toxic metals at 100°C by Pyrobaculum islandicum. Appl Environ Microbiol 66:1050–1056. https://doi.org/10.1128/AEM.66.3.1050-1056.2000
Kashefi K, Holmes DE, Reysenbach AL, Lovley DR (2002) Reductive precipitation of gold by dissimilatory Fe. Appl Environ Microbiol 68:1735. https://doi.org/10.1128/AEM.67.7.3275
Hawkes RB, Franzmann PD, O’hara G, Plumb JJ (2006) Ferroplasma cupricumulans sp. nov., a novel moderately thermophilic, acidophilic archaeon isolated from an industrial-scale chalcocite bioleach heap. Extremophiles 10:525–530. https://doi.org/10.1007/s00792-006-0527-y
Bathe S, Norris PR (2007) Ferrons iron- and sulfur-induced genes in Sulfolobus metallicus. Appl Environ Microbiol 73:2491–2497. https://doi.org/10.1128/AEM.02589-06
Auernik KS, Maezato Y, Blum PH, Kelly RM (2008) The genome sequence of the metal-mobilizing, extremely thermoacidophilic archaeon Metallosphaera sedula provides insights into bioleaching-associated metabolism. Appl Environ Microbiol 74:682–692. https://doi.org/10.1128/AEM.02019-07
Bertrand JC, Almallah M, Acquaviva M, Mille G (1990) Biodegradation of hydrocarbons by an extremely halophilic archaebacterium. Lett Appl Microbiol 11:260–263. https://doi.org/10.1111/j.1472-765X.1990.tb00176.x
Al-Mailem DM, Sorkhoh NA, Al-Awadhi H et al (2010) Biodegradation of crude oil and pure hydrocarbons by extreme halophilic archaea from hypersaline coasts of the Arabian Gulf. Extremophiles 14:321–328. https://doi.org/10.1007/s00792-010-0312-9
Tapilatu YH, Grossi V, Acquaviva M et al (2010) Isolation of hydrocarbon-degrading extremely halophilic archaea from an uncontaminated hypersaline pond (Camargue, France). Extremophiles 14:225–231. https://doi.org/10.1007/s00792-010-0301-z
Al-Mailem DM, Eliyas M, Radwan SS (2012) Enhanced haloarchaeal oil removal in hypersaline environments via organic nitrogen fertilization and illumination. Extremophiles 16:751–758. https://doi.org/10.1007/s00792-012-0471-y
Al-Mailem DM, Eliyas M, Radwan S (2014) Enhanced bioremediation of oil-polluted, hypersaline, coastal areas in Kuwait via vitamin-fertilization. Environ Sci Pollut Res 21:3386–3394. https://doi.org/10.1007/s11356-013-2293-6
Brim H, Osborne JP, Kostandarithes HM et al (2006) Deinococcus radiodurans engineered for complete toluene degradation facilitates Cr(VI) reduction. Microbiology 152:2469–2477. https://doi.org/10.1099/mic.0.29009-0
Lage CAS, Dalmaso GZL, Teixeira LCRS et al (2012) Mini-Review: probing the limits of extremophilic life in extraterrestrial environment-simulated experiments. Int J Astrobiol 11:251–256. https://doi.org/10.1017/S1473550412000316
Zhang CL, Ye Q, Huang Z et al (2008) Global occurrence of archaeal amoA genes in terrestrial hot springs. Appl Environ Microbiol 74:6417–6426. https://doi.org/10.1128/AEM.00843-08
Shen JP, Zhang LM, Di HJ, He JZ (2012) A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front Microbiol 3:1–7
Zhalnina K, Dörr de Quadros P, Camargo FAO, Triplett EW (2012) Drivers of archaeal ammonia-oxidizing communities in soil. Front Microbiol 3:1–9. https://doi.org/10.3389/fmicb.2012.00210
Taffner J, Erlacher A, Bragina A et al (2018) What is the role of archaea in plants? New insights from the vegetation of alpine bogs. mSphere 3:1–14. https://doi.org/10.1128/msphere.00122-18
Müller H, Berg C, Landa BB et al (2015) Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front Microbiol 6:1–9. https://doi.org/10.3389/fmicb.2015.00138
Hinrichs K-U, Hayes JM, Sylva SP (1999) Hinrichs_1999. Lett Nat 398:802–805
Reeburgh WS (2007) Oceanic methane biogeochemistry. Chem Rev 107:486–513. https://doi.org/10.1021/cr050362v
Wang FP, Zhang Y, Chen Y et al (2014) Methanotrophic archaea possessing diverging methane-oxidizing and electron-transporting pathways. ISME J 8:1069–1078. https://doi.org/10.1038/ismej.2013.212
Lin YS, Heuer VB, Ferdelman TG, Hinrichs KU (2010) Microbial CONVERSION of inorganic carbon to dimethyl sulfide in anoxic lake sediment (Plußsee, Germany). Biogeosciences 7:2433–2444. https://doi.org/10.5194/bg-7-2433-2010
Acknowledgements
The authors wish to thank the Department of Biotechnology (project Grant Number: BT/PR6540/BCE/8/917/2012) project for funding the research work.
Author information
Authors and Affiliations
Contributions
Dr RK suggested the idea underlying the manuscript and reviewed and edited the same. MGN has written and compiled the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there is no conflict of interest for authorship of the manuscript in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naitam, M.G., Kaushik, R. Archaea: An Agro-Ecological Perspective. Curr Microbiol 78, 2510–2521 (2021). https://doi.org/10.1007/s00284-021-02537-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02537-2