Abstract
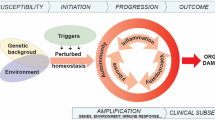
Systemic sclerosis (SSc) is characterized by inflammation, vascular dysfunction, and ultimately fibrosis. Progress in understanding disease pathogenesis and developing effective disease treatments has been hampered by an incomplete understanding of SSc heterogeneity. To clarify this, we have used genomic approaches to identify distinct patient subsets based on gene expression patterns in SSc skin and other end-target organs. Here, we review what is known about the gene expression-based subsets in SSc, currently defined as the inflammatory, fibroproliferative, limited, and normal-like subsets. The inflammatory subset of patients is characterized by infiltrating immune cells that include T cells, macrophages, and possibly dendritic cells, although little is known about the mediators these cells secrete and the pathways that govern cell activation. Prior studies have suggested a role for pathogens as a trigger of immune responses in SSc, and recent data have identified viral and mycobiome components as potential environmental triggers. We present a model based on analyses of gene expression data and a review of the literature, which suggests that the gene expression subsets observed in patients possibly represent distinct, interconnected molecular states of disease, to which an innate immune response is central that results in the generation of clinical disease.


Similar content being viewed by others
References
Milano A et al (2008) Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One 3:e2696
Pendergrass SA, Lemaire R, Francis IP, Mahoney JM, Lafyatis R, Whitfield ML (2012) Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J Invest Dermatol 132:1363–1373
Hinchcliff M et al (2013) Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol 133:1979–1989
Mahoney JM et al (2015) Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput Biol 11:e1004005
Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA (2011) Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum 63:783–794
Christmann RB et al (2014) Association of interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol 66:714–725
Whitfield ML et al (2003) Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci U S A 100:12319–12324
Gardner H et al (2006) Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum 54:1961–1973
Perou CM et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Alizadeh AA et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Garber ME et al (2001) Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A 98:13784–13789
Bhattacharjee A et al (2001) Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A 98:13790–13795
Sorlie T et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100:8418–8423
Lafyatis R et al (2009) B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 60:578–583
Chakravarty EF et al (2015). A pilot randomized placebo-controlled study of abatacept for the treatment of diffuse cutaneous systemic sclerosis. Arthritis Res Ther. In press.
Gordon JK et al (2015). Nilotinib in the treatment of early diffuse systemic sclerosis: an open-label, pilot clinical trial. Arthritis Res Ther. Submitted.
Assassi S et al (2015). Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheum. In Press.
Wong AK, Park CY, Greene CS, Bongo LA, Guan Y, Troyanskaya OG (2012) IMP: a multi-species functional genomics portal for integration, visualization and prediction of protein functions and networks. Nucleic Acids Res 40:W484–W490
Assassi S, Radstake TR, Mayes MD, Martin J (2013) Genetics of scleroderma: implications for personalized medicine? BMC Med 11:9
Taroni J et al (2015). Genome-wide gene expression analysis of systemic sclerosis esophageal biopsies identifies disease-specific molecular subsets. Arthritis Res Ther. In press.
Chung L et al (2009) Molecular framework for response to imatinib mesylate in systemic sclerosis. Arthritis Rheum 60:584–591
Sargent JL et al (2009) A TGFbeta-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol 130:694–705
Greenblatt MB et al (2012) Interspecies comparison of human and murine scleroderma reveals IL-13 and CCL2 as disease subset-specific targets. Am J Pathol 180:1080–1094
Johnson ME et al (2015) Experimentally-derived fibroblast gene signatures identify molecular pathways associated with distinct subsets of systemic sclerosis patients in three independent cohorts. PLoS One 10:e0114017
Bhattacharyya S, Wei J, Tourtellotte WG, Hinchcliff M, Gottardi CG, Varga J (2012) Fibrosis in systemic sclerosis: common and unique pathobiology. Fibrogenesis Tissue Repair 5:S18
Farina A et al (2014) Epstein-Barr virus infection induces aberrant TLR activation pathway and fibroblast-myofibroblast conversion in scleroderma. J Invest Dermatol 134:954–964
van Bon L et al (2010) Distinct evolution of TLR-mediated dendritic cell cytokine secretion in patients with limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis 69:1539–1547
Artlett CM, Sassi-Gaha S, Rieger JL, Boesteanu AC, Feghali-Bostwick CA, Katsikis PD (2011) The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum 63:3563–3574
Arron ST et al (2014) High Rhodotorula sequences in skin transcriptome of patients with diffuse systemic sclerosis. J Invest Dermatol 134:2138–2145
Christmann RB et al (2011) Interferon and alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheum 63:1718–1728
Mathes AL et al (2014) Global chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skin. Ann Rheum Dis 73:1864–1872
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Higashi-Kuwata N et al (2010) Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther 12:R128
Atamas SP, White B (2003) Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev 14:537–550
Chen ZY et al (1984) Immune complexes and antinuclear, antinucleolar, and anticentromere antibodies in scleroderma. J Am Acad Dermatol 11:461–467
Seibold JR, Medsger TA Jr, Winkelstein A, Kelly RH, Rodnan GP (1982) Immune complexes in progressive systemic sclerosis (scleroderma). Arthritis Rheum 25:1167–1173
Hasegawa M, Fujimoto M, Kikuchi K, Takehara K (1997) Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol 24:328–332
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. Faseb J 18:816–827
Varga J, Pasche B (2009) Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 5:200–206
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Li MO, Sanjabi S, Flavell RA (2006) Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25:455–471
Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V (2011) Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 29:163–183
Assassi S et al (2010) Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum 62:589–598
Eloranta ML et al (2010) Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis 69:1396–1402
Kim D et al (2008) Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum 58:2163–2173
van Bon L et al (2014) Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. NEJM 370:433–443
Luzina IG, Atamas SP, Wise R, Wigley FM, Xiao HQ, White B (2002) Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am J Respir Cell Mol Biol 26:549–557
Falanga V, Gerhardt CO, Dasch JR, Takehara K, Ksander GA (1992) Skin distribution and differential expression of transforming growth factor β1 and β2. J Dermatol Sci 3:131–136
Sfikakis PP, McCune BK, Tsokos M, Aroni K, Vayiopoulos G, Tsokos GC (1993) Immunohistological demonstration of transforming growth factor-β isoforms in the skin of patients with systemic sclerosis. Clin Immunol Immunopathol 69:199–204
Higashi-Kuwata N, Makino T, Inoue Y, Takeya M, Ihn H (2009) Alternatively activated macrophages (M2 macrophages) in the skin of patient with localized scleroderma. Exp Dermatol 18:727–729
Pechkovsky DV et al (2010) Alternatively activated alveolar macrophages in pulmonary fibrosis—mediator production and intracellular signal transduction. Clin Immunol 137:89–101
Truchetet M-E, Brembilla NC, Montanari E, Allanore Y, Chizzolini C (2011) Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arth Res Ther 13:R166
Kessel A et al (2004) Increased CD8+ T cell apoptosis in scleroderma is associated with low levels of NF-kappa B. J Clin Immunol 24:30–36
Mathian A et al (2012) Activated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesions. Ann Rheum Dis 71:1227–1234
Murata M et al (2008) Clinical association of serum interleukin-17 levels in systemic sclerosis: is systemic sclerosis a Th17 disease? J Dermatol Sci 50:240–242
Radstake TR et al (2009) The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFβ and IFNγ distinguishes SSc phenotypes. PLoS One 4:e5903
Rodríguez-Reyna TS et al (2012) Th17 peripheral cells are increased in diffuse cutaneous systemic sclerosis compared with limited illness: a cross-sectional study. Rheumatol Int 32:2653–2660
Deleuran B, Abraham DJ (2007) Possible implication of the effector CD4+ T-cell subpopulation TH17 in the pathogenesis of systemic scleroderma. Nat Clin Pract Rheum 3:682–683
Axtell RC, Raman C (2012) Janus-like effects of type I interferon in autoimmune diseases. Immunol Rev 248:23–35
Brand S (2009) Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 58:1152–1167
Camporeale A (2012) IL-6, IL-17, and STAT3: a holy trinity in auto-immunity? Front Biosci 17:2306–2326
Kimura A, Kishimoto T (2011) Th17 cells in inflammation. Int Immunopharmacol 11:319–322
Zheng SG (2013) Regulatory T cells versus Th17: differentiation of Th17 versus Treg, are they mutually exclusive? In: IL-17, IL-22 and their producing cells: role in inflammation and autoimmunity: springer., pp 91–107
Steinman L (2007) A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med 13:139–145
Marangoni RG et al (2015) Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol 67:1062–1073
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on Immunopathology of Systemic Sclerosis - Guest Editors: Jacob M. van Laar and John Varga
Rights and permissions
About this article
Cite this article
Johnson, M.E., Pioli, P.A. & Whitfield, M.L. Gene expression profiling offers insights into the role of innate immune signaling in SSc. Semin Immunopathol 37, 501–509 (2015). https://doi.org/10.1007/s00281-015-0512-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0512-6